Review Article Open Access
Fungal Lipase Production by Solid State Fermentation-An Overview
Devarai Santhosh Kumar1* and Suman Ray2*
1Department of Chemical Engineering, Ordnance Factory Estate, Yeddumailaram, Indian Institute of Technology Hyderabad, Telangana, India
2CSIR-National Institute of Science Technology and Development Studies, Pusa Gate, K.S. Krishnan Marg, New Delhi, India
- *Corresponding Author:
- Devarai Santhosh Kumar
Department of Chemical Engineering
Ordnance Factory Estate, Yeddumailaram
Indian Institute of Technology
Hyderabad, Telangana, India.
Suman Ray
Scientist, Room No:206
CSIR-National Institute of Science
Technology and Development Studies (CSIR-NISTADS)
Pusa Gate, K.S. Krishnan Marg, New Delhi, India
Tel: +91 011 2584-6064/3227
Fax: +91 11 25846640;
E-mail: sumanitrc@gmail.com
Received Date: October 27, 2014; Accepted Date:December 12, 2014; Published Date: December 17, 2014
Citation: Kumar DS, Ray S (2014) Fungal Lipase Production by Solid State Fermentation-An Overview. J Anal Bioanal Tech 5:230. doi: 10.4172/2155-9872.1000230
Copyright: © 2014 Kumar DS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Importance of enzymes is ever-growing specifically microbial lipases which are of great industrial significance because of their applications in detergent, food, pharmaceutical, chemical and leather industry. Solid state fermentation (SSF) is an economical alternative for large scale production of enzymes that are produced by fungi. Therefore, production of lipases by solid state fermentation is a good and preferred option than submerged fermentation (SmF). The important factors in fermentation are carbon concentration, nitrogen concentration, pH, growth temperature, fermentation time and moisture content. This review mainly focuses on production of fungal lipase by solid state fermentation using various fungal strains, substrates and fermentation conditions. Enzyme characteristics, industrial application and assay methods of lipase, biomass estimation, enzyme extraction methods and engineering aspects of fermentation are also dealt with briefly. The main aim of the review is to give an overview of advancements in solid state fermentation for production of fungal lipase hitherto.
Keywords
Lipase; Solid State Fermentation (SSF); Submerged Fermentation (SmF); Fungal Sp; Agricultural Substrates
Introduction
(SSF) is an economical alternative technique to submerged fermentation (SmF) for large scale production of industrial enzymes, where raw materials and processing is cheap with reduced chemical cost and less processing risk. It is defined as ‘the growth of microorganisms (mainly fungi) on moist solid materials in the absence of free flowing water’ [1]. This technique uses agricultural substrates with various carbohydrates, nitrogen sources, salts as well as nutrients like starch, cellulose, pectin, lignin, fibers and minerals. SSF technique has the potential to produce desired microbial products more efficiently than submerged fermentation (SmF) and has practical and economical advantages over SmF [2]. It has been reported that SSF with fungal strains results in greater productivity than submerged fermentation [3,4]. Substrates that have been traditionally fermented by solid state fermentation for enzyme production include agricultural wastes such as rice husk, wheat bran, beans, corn steep dry, sugar cane bagasse, coffee pulp, lemon peel and apple pomace [5-11]. Currently, major countries in the world are implementing this technique for large scale production of enzymes due to its higher titer values [12]. The substrates used for SSF can be classified as (a) agricultural raw materials and waste products (b) industrial wastes and (c) synthetic materials [13].
Lipase
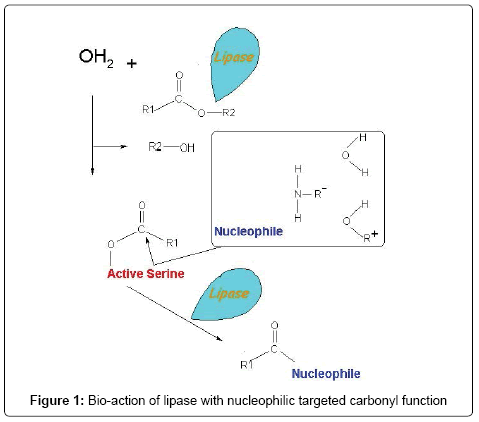
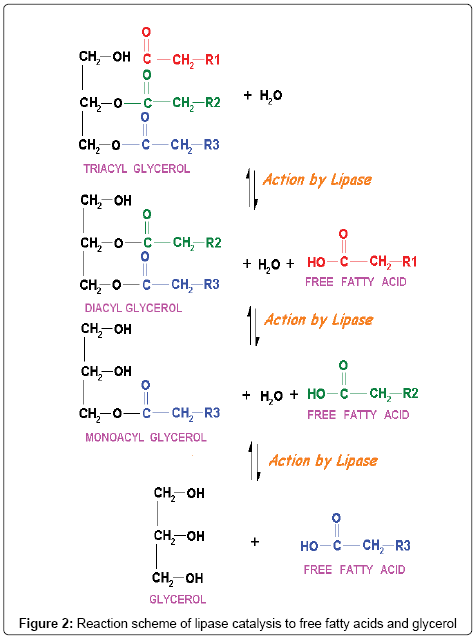
Lipases (triacylglycerol acylhydrolases, EC 3.1.1.3) (Figure 1) are a group of enzymes which catalyze the hydrolysis of triglycerides to diglycerides, and monoglycerides which are further hydrolyzed to glycerol and free fatty acids (Figure 2). Although there is lack of knowledge of the intrinsic kinetics of microbial growth, lipases are assumed to interact at oil-water interface. However, the kinetics regarding the adsorption processes is known [14,15]. Lipases occur widely in bacteria [16-18], yeasts [19,20] and fungi [21,22]. These enzymes also display catalytic activity towards a large variety of alcohols and acids in ester synthesis provided that the water activity is low. They can hydrolyze the ester bonds, trans-esterified triglycerides, resolve raceme mixture and are used in the synthesis of ester bonds in nonaqueous media [23-25]. Fungi are widely recognized as the best lipase sources and used preferably for industrial applications. A few of the general fungal genus include Rhizopus, Aspergillus, Pencillium, Mucor, Ashbya, Geotrichum, Beauveria, Hunicola, Rhizomucor, Fusarium, Acremonium, Alternaria, Eurotrium and Ophiostoma. Fungal species which produces lipases are Candida rugosa, Candida Antarctica, T. lanuginosus, R. miehei, Pseudomonas, Mucor and Geotrichu. Fungal lipases are often reported as Aspergillussp, Candida rugosa, Candida antarctica, Thermomyceslanuginosus, Rhizomucormiehei, Mucor and Geotrichum [26-28]. Lipases play an important role in lipid metabolism in eukaryotes at various stages like fat digestion, reconstitution, adsorption, and lipoprotein metabolism. Lipases are also found in energy tissues of plants and the mechanism of their interactions with lipids at the interface is still a subject of investigation [29]. Lipases provide improved worldwide attention due to their diverse industrial applications and versatility in nature. The world’s enzyme demand is met by 12 major producers and 400 minor suppliers across the globe, 60% of the world’s supply of industrial enzymes is from Europe alone and strikingly 75% of these industrial enzymes include lipases [26,30]. The importance of lipase as biocatalyst is well understood. It is used to catalyze several unnatural and remarkable reactions in non-aqueous media that include bio-fuel production, production of value added products like esters, organic acids, food, beverage, cosmetics, and pharmaceutical materials [13].
Fungal Strains Producing Lipase
Several lipase producing fungal strains have been used for lipase production by SSF which include Rhizopus rhizopodiformis A13 and Rhizomucor pusillus A16 obtained from ORSTOM culture collection [31]. Other important fungi used are Aspergillus niger MTCC2594 [32], Penicillium restrictum [33], Rhizopus oligosporous [34], A.niger NCIM 1207 [35], Rhizopus delemer [36] and Aspergillus oryzae [37], Among these strains, Aspergillus niger is found to produce significant quantities of enzyme and is regarded as GRAS by Food and Drug Administration (FDA). A. niger MTCC 2594 cultured on gingelly oil cake has a lipase activity of 363.6 μ/g dry substrate [32], whereas maximum lipase activity of 630 IU/g dry solid substrate was observed using A.niger NCIM 1207 [35]. Fungal strains producing lipases by SSF are tabulated in detail in Table 1.
| Microorganisms | Solid Substrate | Inducers | Fermentation conditions (hours) | Lipase Activity Units | References |
|---|---|---|---|---|---|
| Fusarium oxysporum | Wheat bran | Cetyl trimethylammonium bromide | 96 h, pH-8.5, 40°C, high MC | 111.48 U/ml | [66] |
| Aspergillus flavus | Wheat bran and Castor oil cake | - | 96 h, pH-7.0, 30°C, MC 64% w/w | 121.35 U/gds | [60] |
| Aspergillus niger | Rice bran | - | pH-6.87, 30.3°C | 121.53 (U/g dss) | [62] |
| Colletotrichum gloeosporioides | Sunflower oil | Triton X-100 | pH-6.5, 25°C | 2560 U/g DM | [63] |
| Yarrowia lipolyticaNCIM 3589 | Palm Kernal cake | - | MC 70% v/w, 96 h | 18.58 U/ gds | [64] |
| Aspergillus niger J-1 | Olive oil and Glucose | - | pH-7.0, 30°C for 7 days | 9.14 IU/g dss | [61] |
| Aspergillus niger MTCC 2594 | Wheat bran, Coconut oil cake and Wheat rawa | Olive oil, Sesame oil and Sunflower oil | 72h, 30°C, pH-7.0, MC 60% v/w | 745.7 ± 11 U/gds | [65] |
| Rhizomucar rhizopodiformis | Sugar cane bagasse and Olive oil cake | - | 20 h-24 h | 79.60 U/gds | [31] |
| Aspergillus nigerMTCC 2594 | Gingelly oil cake | - | 250 ml EF, 30°C, 120 h, pH-7.0, MC 60% | 363.6 /gds | [32] |
| Penicillium restrictum | Babassu oil cake | Peptone, Oliveoil and Starch | EF, 30°C, 15-65 h, | 30.3 U/g | [33] |
| Rhizopus oligosporousNCIM 1207 | Wheat bran and Olive oil | Nacl, Triton X-100 | 500 ml EF, 45°C, pH-2.5, 24 h, MC 71.4% | 630 IU/gds | [35] |
| Aspergillus.sps | Wheat rawa | - | 250 ml EF, 30°C, pH-7.0, 96 h | 1934 U/g | [38] |
| Penicillium restrictum | Babassu oil cake and Olive oil | - | Tray reactor, 37°C, 24 h, pH-7.0 | 5.8 U/ml | [107] |
| Rhizopus oryzae PTCC 5176 | Bagasse and Urea | Olive oil and Canola oil | Tray-bioreactor, pH 8.0, 35°C, MC 80% | 229.355 U/gds | [108] |
| Candida rugosa | Coconut oil cake | Urea, Peptone and Maltose | 96 h | 87.76 U/g ds | [109] |
| Candida rugosaDSM-2031 Yeast Species |
Coconut oil cake and Wheat bran Deoiled rice bran, Rice bran oil and Mineral salts. |
- - | 28°C 96 h, 30°C | 48.61 U/ml 58 LU/gm dry bran |
[82] [76] |
| Aspergillus Terreus |
Mustard oil cake | - | 30°C, 96 h, pH- 6.0 |
1566.67 ± 133.33 U/ml | [106] |
| Aspergillus nigerIIT 53A14 | Wheat bran and Olive oil | - Soap stock | 32°C, 48 h, pH 6.3-6.6 32°C, 48 h, pH 6.3-6.6 | 48.6 U/gds 62.5 U/gds | [110] |
| Aspergillus niger | Shea butter cake | Tween 20 | 30°C, 7 days, pH 7.0 | 3.35 U/g | [111] |
| Aspergillus. sp | Wheat bran | - | 30°C, 96 h, pH 7.0 | 13.1 U/ml | [112] |
| Aspergillus niger | Soya bean and Rice husk | - | 37°C, pH 7.7, 96 h | 4.23 U/ml | [113] |
EF: Erlenmeyer flask; MC: Moisture content; gds: gram dry substrate; LU; lipase units; ds: dry substrate; dss: dry solid substrate; DM: dry matter; U or IU or u: The amount of enzyme that catalyzes the conversion of 1 μmoles of substrates per minute.
Table 1: Comparison of lipase production in various fungal microorganisms
Substrates used for lipase
Different types of agricultural wastes and oily substrates are used for the lipase because of their lipolytic nature. This includes rice bran, wheat bran, gingelly oil cake, almond meal, mustard oil cake, neem oil cake, groundnut oil cake, gingerly seed and groundnut kernel that are used routinely for lipase production by SSF. Lipase production from Aspergillus niger by SSF using gingelly oil cake as substrate is reported [32]. The feasibility of obtaining lipase with Rhizopus delemar growing on a polymeric resin has also been investigated [36]. Babassu oil cake is used as a substrate for the lipase production by Penicillium restrictum in SSF [33]. Among these substrates, almond meal is found to be the potential solid substrate [34], whereas wheat bran with olive oil as lipid substrates is suitable for the production of acidic lipase in SSF with maximum productivity [35].
Fermentation Conditions for Lipase Production in SSF
There have been numerous reports on the optimization of culture medium, agricultural substrates and nutrition factors for lipase production in SSF [38]. Production of lipases depends on different parameters like carbon and nitrogen concentrations, pH of the medium, temperature growth, moisture content and fermentation time required [39].
Substrates used for lipase production
Rice bran: In the case of Candida rugosa, addition of urea to rice bran enhanced lipase production during optimization [40]. Although addition of carbohydrates increase the production, but it causes contamination and this leads to overall reduction in the efficiency [32]. It has been reported that using maltose had only a marginal effect on lipase production with rice bran as substrate [41]. Fermentation time of 48 h at 30°C with an inoculum concentration of 1ml/g bran conducted by rice bran with a particle size of less than 250 μm results in a higher lipase production [42].
Wheat bran: Fungal Mucor javanicus IAM 6089 with wheat bran as substrate showed an activity of about 52 U/g dry bran after 6 days [43]. In the N-rich medium, lipase activity was increased by 4.3 times when it is compared to the basal medium [37]. This study further suggested that enriching the substrates had significant influence on lipase production compared to C/N ratio.
Almond meal: Ikram ul-Haq et al. [34] screened ten moulds to observe maximum lipase activity. Maximum activity of 48.0 ± 2.1 U/g was shown with almond meal, which contains required amount of carbon, nitrogen, sucrose, gum and proteins. In addition, a combination of 50% olive oil cake and sugarcane bagasse as substrate also showed improved activity. Rhizopus rhizopodiformis demonstrated a high activity of 79.6 U/g dry matter (DM) equivalent to 43.04 U/ml whereas, Rhizomucor pusillus showed an activity of 20.24 U/g DM equivalent to 10.83 U/ml [34].
Olive oil: Venkat et al. [40] showed that oil content significantly affects the lipase yield. It has been demonstrated that 2% of olive oil as an enhancer showed a maximum lipase activity of about 30.30 U/g initial dry weight after 24 h of cultivation, whereas with peptone showed a activity of 27.80 U/g of initial dry weight [40]. Earlier investigations showed that an optimum growth of the fungal Rhizopus oligosporous GCBR-3 had maximum activity of 30 ± 2.1 U/g and use of Rhizopus strains also enhanced activity [44,45]. These fungal strains also showed greater activity with other cultures for lipase production [31,46].
Substrates with supplements/inducers: Aspergillums niger NCIM1207 illustrated higher levels of extracellular enzyme at 45°C. The maximum lipase activity of 630 IU/gdss was observed with wheat bran as substrate and the enzyme extraction was done with NaCl by supplementing Triton X-100 [35]. However, in the case of Rhizopus, Rhodotorula and Aspergillus, the lipase production seems to be independent of substrate type [47-49]. Extracellular lipase production from different microorganisms using lipids, carbohydrates and also a mixture of both has been reported on enzyme production [48-51]. The use of surfactants like Triton X-100 during SSF helps to increase enzyme production, with up to three fold increase in enzyme production both in SmF and SSF. Wheat bran has sufficient nutrients and remains loose even in moist conditions, thereby providing a large surface area for the inoculums to give maximum activity [52].
In another report, lipase production by mutant Aspergillus niger 11T53A14 using wheat bran with the fermentation medium containing 100 g of wheat bran grain (size ≤ 5 mm), humidified with a solution containing nitrogen source (ammonium sulfate) and inducer (sunflower soap stock) in a concentration of 3% (w/w) is demonstrated. The activity was tested for different nutrient compositions at pH 7.0, temperature 32°C, and 78 h and the maximum enzyme activity was observed to be 153.2 U/gdm at 0.6 % (w/w) nitrogen concentration [53].
Olive oil cake and Sugar cane bagasse: Cordova et al. [31] reported lipase production with an equal combination of olive oil cake and sugarcane bagasse as substrates. The lipase production was carried out using thermo stable fungal cultures of Rhizomucor pusillus and Rhizopus rhizopodiform is subjected to solid state fermentation. A mixture of 50% each substrate has shown an enzyme activity of about 43.01 U/ml. It was observed that under standard conditions, bagasse has low protein, less fat content and high level of cell wall component whereas olive oil cake possess high protein, fat and higher amount of lignin content [31].
Oily substrates: Out of 34 fungal species isolated from different oil substrates, nine exhibited lipolytic activities [54-56]. Maximum lipase production of 1152 μ/g was obtained after 96 h of incubation by Aspergillus species [38], whereas 8 days of incubation using A.niger at 80% moisture content attained a high enzyme titer of 1216.0 U/g [57]. Increase in the moisture level is believed to reduce the porosity of the wheat rava, thus limiting oxygen transfer [52]. At the same time low moisture content causes reduction in the solubility of nutrients of the substrates and low degree of swelling [58]. Different optimized factors, including wheat rawa with olive oil 1%, corn steep liquor 1% and 80% moisture content increase the solubility of the nutrient with the productivity of lipase in SSF 1.94 times higher than that in SmF [38].
Regiane et al. [59] used mutant strain of Aspergillus niger 11T53A14 for fractional factorial design to optimize lipase production. The soap stocks which are produced during the processing of canola, sun flower and corn has rich amount of lipid by products as inducers. The maximum lipase activity of 201.5 U/gds was observed for sun flower soap stock of 0.5% nitrogen and 3% inducers, when compared with canola and corn respectively [59].
Comparison of lipase activity with substrates
Comparison studies using fungal stains of Penicillium chrysogenum, Trichoderma harzianum and Aspergillus flavus was carried out through solid state fermentation using agro-industrial residues as substrates for optimum extracellular lipase production. For all three strains, the growth temperature was 29 ± 1°C, and 65% w (g/gds) moisture content. The effect of fermentation conditions on production are; initial pH (6.0 and 7.0), time of fermentation (72 h, 96 h and 120 h), and type of mixed substrate (wheat bran-olive oil, and wheat bran-castor oil cake). The Aspergillus flavus showed maximum lipase production of 121.35 U/gds which is five and nine times more than Trichoderma harzianum and Penicillium chrysogenum at pH-7.0 for 30.3°C having 96 h fermentation time [60].
Aspergillus niger J-1 has a maximum enzyme activity of 9.14 IU/g of dry solid substrate equivalent to 4.8 IU/ml of lipase activity using the solid state fermentation techniques with wheat bran as substrate having 0.75% ammonium sulphate, 0.34% urea and nitrogen source as the medium composition at pH-6.0 for 40°C within 24 h [61]. In another report, rice bran as substrate for Aspergillus niger has a maximum lipase production of 121.53 U/gdss at 30.3°C. These activities can further be improved by optimizing process variables such as oil concentration, glucose concentration and humidity ratio [62]. It has also been reported that endophytic fungal strain Colletotrichum gloeosporioides possess more potential for producing extracellular lipolytic enzymes in SSF with sunflower oil as substrate with a production of 2560 U/g DM at 25.0°C [63]. Extracellular lipase production using Yarrowia lipolytica NCIM 3589 with palm kernal cake as substrate alone has a maximum lipase activity of 18.58 U/gds at 96 h [64].
Scale up studies for the production of lipase using Aspergillus niger MTCC 2594 was reported with a maximum activity of 745.7 U/ gds with tri-fermented substrates and suggested that these techniques have a wide potential in cost-effective biofuel production [65]. It has been reported that fungal stain Fusarium oxysporum produced serine peptidase and lipase in alkaline condition with higher enzyme stability. Wherein, wheat bran alone showed a high activity of 228.88 U/ml for proteolytic activity and 111.48 U/ml for lipolytic activity. Study of enzyme production by varying temperature and pH suggested that these enzymes are used for application in the detergent industry [66].
Stability of lipase for different substrates
In Cordova et al. [31] study, finely ground sugar cane bagasse sample was screened to 1mm particle size and assayed for components like minerals, fat, total nitrogen, cell wall components, total fibers, lingo cellulose and lignin, fat and ash whose presence improves the enzyme production [31,52,67,68]. They observed that Rhizomucor pusillus and Rhizopus rhizopodiformis showed maximum lipase production of 1.73 U/ml and 0.97 U/ml at 16 h and 13 h respectively, whereas bagasse and olive oil cake showed a maximum production of 10.83 U/ml and 43.04 U/ml respectively. They showed that olive oil cake has more protein content than bagasse. Kamini et al. (1998) [32] showed that Aspergillus niger MTCC 2594 with gingelly oil cake had a good stability of enzyme at pH of 4.0-10.0 and temperature of 4-50°C. The results were compared with submerged fermentation, where lipase showed low thermal stability and specific activity of 57% at 60°C and 69% activity at 60°C which makes the enzyme stable in all detergents [32]. Similar results have been reported for lipase production from C.cylindracea, A.species and Rhizopus species [69,70].
Gombert et al. [33] investigated three enzymes i.e. lipase, glucoamylase and protease and observed that of three enzymes, maximum activity was obtained with media of C/N ratio [33]. A cake with 1% peptone lipase showed an activity of 4.3 times in the basal medium, whereas protease and gluco amylase showed 1.2 and 1.5 times the activity, respectively. High nitrogen concentration in culture media is effective in enhancing the production of lipase by micro-organisms [37,50,71]. Different nitrogen sources for lipase production by Penicillium strain in laboratory-scale fermentor have also been explored [71,72]. The basal medium rich in protein content as co-factors, amino acids which match P.restrictum physiological characteristics is beneficial for lipase biosynthesis. Previous investigations involving SmF showed that P. restrictum lipase is not stable at alkaline pH levels with a decrease in the lipase activity but a serine protease inhibitor (PMSF) when added at later fermentation stages showed improved activity [72,73]. The accumulation of proteases in SSF affecting the stability of lipases was also observed with fungus and with different species of Penicillium [46].
Ikram ul-Haq et al. [34] studied parameters like temperature, inoculum size etc., and showed that either lower or higher temperature result in increased energy requirements and hence, decrease the product yield [34]. Several investigations have been carried out at different cultivation temperatures and higher values as units per liter are reported [74-78]. Increase in mycelia mass, production of enzyme was shown to decline due to exhaustion of nutrients in the fermentation mash. Enzyme extraction was found to be maximum using phosphate buffer, because of permeability of membrane. Rate of enzyme synthesis using R. oligosporous showed maximum results at 48h with maximum enzyme synthesis at 2.16 u/g substrate per hour, whereas by using optimum concentration of substrate was found to be 0.0245-0.65 U/g per hour, 1.95 U/g per hour, 0.91 U/g per hour and 5.05 U/g per hour [46,79].
It is reported that the enzyme requires an energy of 114.25 and 116 KJ at lower and higher temperatures respectively for conversion of 1 mole substrate with the maximum activity at 45°C and pH 7.0- 8.5 [34]. Moisture content is a crucial factor in determining the process and physical properties of the solid substrate [52,80]. Whereas higher moisture content lead to decreased porosity and thus lowering oxygen transfer, lower moisture decrease the solid solubility, degree of swelling and produces a higher water tension. Maximum lipase production was obtained with wheat bran and synthetic oil based medium (SOB) medium in the ratio of 1:2.5 [35]. A. niger strains are known to be active at pH 4-7 and at temperatures of 40°C and 55°C. Lipase from A. niger NICM 1207 was found to be active at extreme acidic pH of 1.5-2.0 and over a broad pH range of 2.5-9.0 for up to 24 h at room temperature.
The optimum temperature of lipase production from A. niger NCIM 1207 is at 50°C and is stable at 60°C for 5 h. Addition of water to A. niger NCIM 1207 reduces the synthesis of esters shifting the equilibrium of the reaction towards hydrolysis, producing fatty acids. Thus, transesterification becomes dominant only when the availability of water is restricted. This enzyme also showed similar optimum pH in organic solvent. This phenomenon was well explained as pH memory and also reported in several literatures [81]. Characterization and partial purification of iso-lipases (Lip A, Lip B, Lip C) for biomedical application in pharmaceutical industry through SSF is well explained in detail by other investigators [82].
Assays for Determining Lipase Activity
Various techniques have been used to determine the lipase enzyme activity. The commonly used techniques are turbidimetry, interfacial tensiometry, atomic force microscopy, infrared spectroscopy, titrimetry, colorimetry, flourimetry, chromatography, electron microscopy and immunodetection. Colorimetry is one of the known methods used for determining the enzyme activity. In this technique the substrates used are lipids, olive oil emulsions and para-nitrophenyl esters. The principle involved in lipid water interface oil emulsion is absorbance. Safranin absorbance change is due to the net negative charge at the lipid water interface and the product analyzed with this principle is safranin. The olive oil emulsion with copper reagent as substrate involves the estimation of copper complex spectrophotometrically at 440 nm. The complex develops a pink colour absorbance at 513 nm and the product analysed is rhodamine G-FFA complex. Another method of identifying the lipase activity is using the yellow coloured product which can be measured at 410 nm with para-nitrophenyl esters as substrate to analyze the product [83-86].
Titrimetry is the easiest way of identifying the activity of the enzyme. To identify the lipase enzyme activity involves use of stirred emulsion of TAG, tributyrin, olive oil emulsified with gum Arabic as substrates and the principle involved is pH stat-method-Neutralization of released FFAs using titrated NaOH. This is the most common procedure and is sensitive to within 1 μmol fatty acid released per min [87-88].
Turbidimetry assay is a method used for determining the concentration of a substance in a solution by the degree of cloudiness or by the degree of clarification [89]. The principle used in this technique is- increase in optical density of the sample at 500 nm due to precipitation in the form of calcium salts and the substrate used is tween20 in the presence of CaCl2, product analysed being is free fatty acid. Electron microscopy applies the principle of detecting fatty acids by using lipids as substrates [90]. This is simple but, initial cost of the equipment is high for this assay technique. Atomic force microscopy uses lipid bilayer supported on mica as a substrate and involves the principle of lipid dissolution which forms holes on the bilayer with time and it monitored by real time images [91]. Its main advantage is that it is applicable even for the micro as well as nano-size particles. Similarly, other assay techniques like interfacial tensiometry, infrared spectroscopy, flourimetry, chromatography, immuno detection are practiced based on the substrate availability the product is analyzed. Interfacial tensiometry, infrared spectroscopy, atomic force microscopy techniques are highly expensive and sophisticated in practice. The different methods for estimating the lipase activities by quantitatively and qualitatively are given in Table 2.
| Assay | Substrate used | Product analyzed | Principle | Remarks | Reference |
|---|---|---|---|---|---|
| Turbidimetry assay | Tween20 in the presence of CaCl2 | Released fatty acids | Optical density increase at 500 nm due to precipitation in the form of calcium salts | Simple and quantitative but Tweens are not specific substrates for lipases | [89] |
| Interfacial tensiometry | Lipid monolayer spread on surface of aqueous phase | Fatty acids | Monitoring of surface pressure change due to dissolution of lipids using electro microbalance and Teflon barrier | Highly sensitive reliable measurements, | [114] [115] |
| Oil water interface | Fatty acids | Tensiometers for oil drop method | Low amounts of lipids used, but requires very sophisticated equipments | [116] | |
| Atomic force microscopy | Lipid bilayer supported on Mica | Fatty acids | Lipid dissolution forms holes in the bilayer an the increase in area of holes with time is monitored using real time images | Nano scale assay and hence requires very sophisticated instruments | [91] |
| Infrared spectroscopy | Triacyl glycerols (TAG) |
Free fatty acids and Fatty acid esters | In the Fourier transform IR spectrum Fatty acid esters peak at 1751 cm-1 and FFAs at 1715 cm-1 and hence can be quantitated on the basis of molar extinction coefficients | Expensive and sophisticated equipments required | [117] |
| Titrimetry | Stirred emulsion of TAG, tributyrin, Olive oil emulsified with gum Arabic | Fatty acids | pH stat method –Neutralization of released FFAs using titrated NaOH | Most common procedure, sensitive to within 1 μmol fatty acid released per min, disadvantage if FFAs are not fully ionized | [87] [88] |
| Spectrophotometry (Colorimetry) | Lipid at lipid water interface Olive oil emulsion Olive oil emulsion in presence of copper | Safranin Free fatty acids converted to | Absorbance change of Safranin due to change in net negative charge at the lipid water interface Formation of a copper soap. The copper complex is estimated spectrophotometrically at 440 nm |
Lipase activities as low as 50 mU can be detected Sensitivity and efficacy improved for specific | [83] [84] |
| reagent | copper soaps. Rhodamine G-FFA complex | The complex develops a pink color. Absorbance read at 513 nm | purposes by many researchers Reproducibility is difficult |
[85] | |
| Para-nitrophenly esters | Para nitro phenol | Yellow coloured product which is measured at 410 nm | Convenient and quick method, used commonly. These esters are liable to spontaneous hydrolysis and also by non-specific estetrases | [86] | |
| Flourimetry | TAG with alkyl groups substituted with fluorescent group (Pyrenic acylglycerol derivatives) | Free fatty acids | Shift in fluorescence wavelength after hydrolysis | Rapid assay but expensive substrate, Chemically modified Tag is poorly hydrolyzed | [118] [88] |
| Triaclyglycerol in the presence of fatty acid binding protein conjugated to an acrylodan fluorophore | Free fatty acids | Fluorescence emission wavelength changes from 432 nm to 505 nm upon binding | Detection of concentration as low as 1 nM. Kit commercially available | [119] | |
| Phosphatidylch online containing naturally fluorescent parinaric acid | Parinaric acid | Detection of parinaric acid. Excitation and emission wavelengths of parinaric acid -324 nm and 420 nm | Low quantities can be detected | [120] | |
| Chromatography | Lipids, TAGs | Fatty acids | Thin layer chromatography and quantititative analysis of FFAs by densitometric or auto radiographic methods when TAGs are labeled | Detection of as small as a few pmoles of fatty acids. Time consuming and not continuous | [121] |
| Electron microscopy | Lipids | Fatty acids | Electron microscopy detection of fatty acids | ---------------- | [90] |
| Immunodetection | ----------------- | Lipase | ELISA using monoclonal antibodies specific to antigens on Lipase | Detection of both active and inactive form | [122] |
Table 2: Different assay methods used for Lipase activity
Applications of Lipases
Lipases are one of the most versatile enzymes that are extensively used in hydrolysis of fats and oils, dairy industry, detergents and degreasing formulations, anti-asthma drug, biodiesel production, oleo chemical industry, pharmaceutical industry, cosmetic industry, region selective vacillations, pulp and paper industry and flavor enhancement [92,93]. It also has medical applications like modification of castarospermine (a promising drug for the treatment of AIDS), antihypertensive agents such as angiotonsin-converting enzyme (ACE) inhibitors and synthesis of calcium channel blocking drugs such as diltiazem. Detail industrial applications of lipases are dealt in Table 3.
| Industries | Action | Application |
|---|---|---|
| Detergents industry | Hydrolysis of fats | Remove oil strain from fabrics |
| Food industry | Flavour improvement, Aroma, quality improvement, Transesterification, Hydrolysis of fats, modification of butter fat | Shelf-life prolongation, Fat removal, Whippings, health foods |
| Chemical industry | Transesterification, Hydrolysis, Enantioselectivity, Synthesis. | Chiral building blocks, Triacylglycerides to mono- and diglycerides, Cosmetics, digestive aids |
| Leather industry | Softening, Quality improvement | Tanning |
| Pulp and paper industry | Hydrolysis | Improve Quality |
| Cleaning | Hydrolysis | Removal of fats |
| Bioconversion | Hydrolysis | Aqueous media Organic media nonaqueous biotransformation |
| Biomedical | Treatment | Pharmaceutical |
Table 3: Industrial application of Lipase [27]
Engineering Aspects of Solid State Fermentation
Several bioreactors have been traditionally used in SSF processes. These can be primarily classified as: packed-beds, rotating drums, perforated drums, horizontal paddle mixers, gas-solid fluidized beds, stirred aerated beds, rocking drums and tray bioreactors. A rotating drum bioreactors, as an horizontal cylinder, with mixing provided by the tumbling motion of the solid medium aided by the baffles on the inner wall of the rotating drum (perforated or unperforated) [4]. However, in all of these reactors, the mixing is less efficient than with a paddle mixer [94]. Practically, SSF processes could be operated in batch, fed-batch or continuous modes, although batch processes are the most common [95]. This is due to the fact that uninoculated substrate particles require interparticle colonization which is slow in fed batch or continuous operation. In many reactors, in-situ sterilization is facilitated. A wide variety of solid substrates are employed in SSF, which vary in composition, size, mechanical resistance, porosity and water holding capacity [96].
The SSF bioreactors should be anticorrosive, non-toxic to the process organisms as well as facilitate processing aspects like substrate preparation, sterilization, loading and product recovery. Loading and unloading of substrates is accomplished using pneumatic conveying. A novel and efficient design of integrated solid matrix bioreactor called the PLAFRACTORTM has been recently reported which has computercontrolled compact device wherein all the operations described above are made possible [97]. The entry of contaminants into the process as well as the uncontrolled release of the process organisms into the environment must be avoided by using filters on both the inlet and outlet air streams. Other factors that affect the bioreactor design are: the morphology of the fungus, its resistance to mechanical agitation and the necessity to have a sterile process.
The four major types of bioreactors used by SSF [96] are:
• Reactors without forced aeration (Tray reactor)
• Unmixed reactors with forced aeration (Packed bed)
• Continuously mixed reactors with circulation (Rotating drums)
• Intermittently mixed bed bioreactors with forced aeration
The transfer of heat into or out of the SSF system is closely related with the aeration of fermentation system. The temperature of the substrate is also very critical in SSF as it ultimately affects the growth of the micro-organism, spore formation, germination, and product formation. High moistures results in decreased substrate porosity, which in turn prevents oxygen penetration. This may help to avoid bacterial contamination. On the other hand, low moisture content may lead to poor accessibility of nutrients resulting in poor microbial growth [98]. Aeration is achieved in the reactors by blowing air through the substrate or flowing air around a static bed. Aeration rate depends on the rate of heat removal. Water transfer, air supply and heat removal are achieved simultaneously through proper aeration. In the same way, maintenance of uniformity within the substrate bed could be as effective as possible. The effect of the shear forces generated by mixing of both the substrate and the microorganism should be also considered. In this way, it has been observed that these forces can damage the penetrative A SSF bioreactor is being manufactured and marketed by M/s Fujiwara, Japan [99] which consists of a rotating bed in the form of a bucket with the provision of substrate mixing by ribbon-shaped baffles. Operations like substrate sterilization and inoculation are automated in this equipment. Slow rotation of the helical screws ensures minimal damage to growing hyphae along with an effective mixing of the substrate bed. This coupled with a slow rotation of the basket allows the elimination of temperature gradients within the substrate bed. The forced aeration of humid air from the bottom allows the alleviation of oxygen gradients without lowering the moisture content of the bed [26].
Enzyme Extraction and Purification
The usual method used to extract this enzyme is homogenization of the substrate or mixing it in a rotary shaker with one of the various solvents including water, NaCl, (NH4)2 SO4 and NaCl with tween 80, triton X-100. The temperature at which the mixing is done is kept high enough for maximum extraction of the enzyme. However, there is no marked difference in the recovery of enzyme using various solvents just by themselves [35]. The mixture is then filtered using a double layer muslin cloth and the filtrate is centrifuged at around 5000g for 20 min at low temperature of around 4°C to prevent enzyme denaturation [35]. The clear supernatant is used as the extracellular enzyme. Supplementation of the solvent with surfactants like Triton X-100 increases recovery by increasing the membrane permeability. Phosphate buffer is also used for extraction of these enzymes with suitable pH values [34].
Biomass estimation
Biomass estimation in SSF is done indirectly using certain indicators including (a) biomass constituents such as glucosamine and ergosterol when the media constituents are same (b) carbohydrate or any substrate consumption during growth. Glucosamine content is determined using Ride and Drysdale method [100]. The fungal wall chitin is completely hydrolyzed to N-acetyl glucosamine in concentrated KOH by autoclaving [101]. The resulting chitosan suspension is precipitated and washed with progressive dilutions of ethanol in water which is then deaminated and solubilized with nitrous acid. Glucosamine is calorimetrically assayed using 3-methyl-2-benzothiazolone hydrazone hydrochloride (MBTH) and FeCl3. Ergosterol is the predominant sterol of most fungi which can be measured using gas liquid chromatography on the basis of its characteristic UV absorbance [102]. This is a sensitive measurement for the initial stages of colonization.
Sucrose consumption is also used as an estimator of biomass [102]. Dry sample is agitated with distilled water and centrifuged to remove all cell material. The sucrose remaining is hydrolyzed to glucose using β-fructosidase and glucose is estimated by enzymatic method using hexokinase and glucose-6-phosphate dehydrogenase where the absorbance of NADPH is measured at 340 nm. Glucose can also be assayed using DNS reagent (3, 5-dinitrosalicylic acid in NaOH with sodium potassium tartrate) or GOD-POD method [103]. Similarly nitrogen can be estimated using Kjeldahl method and used to estimate biomass growth [104]. Indirect measurements of growth like CO2 production can be done using Infrared analyzer. Infra analyzer works on the principle of reflected light by the matrix surface at specific wavelengths. The amount of the measured component can be expressed as follows [105].
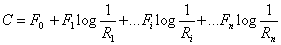
Where C is the amount of component; Fo to F are the calculation constants;
R1 to Rn are the reflection values; 1 to n is the filter numbers. Infrared (IR) estimation of cell components (glucosamine and ergosterol) and medium residues (sucrose and nitrogen) can be done directly on solid medium.
Challenging Aspect of SSF
Solid state fermentation has achieved more importance as the substrates used are agricultural wastes for enzyme production. The detailed applications to the recent technology with a little improvement in the design made the process more desirable and acceptable compared to submerged fermentation.
The major challenges in SSF are the scale up problems, recovery and purification of the end products, separation of biomass which is followed by disposal of biomass. Among several critical challenges, water activity and nature of the solid substrate plays a major role in fermentation process. Heat removal is typically a major concern and it is difficult to remove the waste metabolic heat from the solid bed in which the inter-particle phase is occupied by air in a continuous phase. The reasons could be thermal conductivity and heat capacity of liquid water is superior to those of moist bed with inter-particle air. Mixing greatly promotes heat removal by bringing the medium into contact with the cooling surfaces within the bioreactor. The process of mixing and also the sensitivity of the fungal to withstand the mixing is also a problem during SSF. In addition, it showed major problem in transport phenomena, heat and mass transfer are greater challenges in SSF.
These challenges can be addressed by designing the bioreactor to control the conditions within the bed, such as the temperature and water activity, at the optimum condition for growth and product formation. The growth of the organism on substrate causes deviations as the fermentation process starts where it release of waste metabolic heat and the consumption of O2. The other challenging aspects to run SSF processes in a continuous mode is to maintain a uniform temperature, optimal agitation rate and homogeneity of the carbon, energy source in the SSF substrate [13].
Conclusions
This review mainly highlights the lipase production by SSF using fungal species. It is reported that the fungal species have an excellence in obtaining extracellular enzymes with higher activities [13]. The microorganisms like Aspergillus species, Candida species and Penicillium species are known to produce lipases in SSF when oily substrates are used. Lipase is extracted from the fermented substrate using solvents like distilled water, NaCl, phosphate buffer etc. Lipase can be assayed by using titrimetry, fluorimetry, turbidimetry, electron microscopy and surface tension techniques. It has been observed that wheat bran and mustard oil cake has potential to give high enzyme activity when subjected to SSF with small amount of soap stocks as inducers [35,60,66,106]. Biomass estimation involves methods which estimate substrate consumption or glucosamine of chitin cell wall. Further, it is a complete utilizing process where it produces enzyme with high titer value and the biomass can be utilized for animal feed, fuel generation and fertilization. Different kinds of bioreactors used for SSF include rotary drum, packed bed, tray fermentor and gassolid fluidized bed with mixing or without mixing. The SSF reactors are usually operated in batch mode for better enzyme yields. Microbial lipases have an upper hand due to their specificity, availability of raw materials, economic feasibility and biocompatibility to the environmental. These microorganisms are generally recognized as safe (GRAS) for food, brewing and pharmaceuticals in which lipases have good potential to replace chemical processes. Use of fungal genera for production of lipases may be beneficial for the removal of hazardous materials from the environment since SSF is being used to detoxify chemical from industries. Thus, improvements in SSF in future for fungal lipase production will aid in speeding up the challenges which can bring about higher titer values at lower cost.
Acknowledgements
The author sincerely thanks Director Indian Institute of Technology Hyderabad for their continued encouragement and support. DSK thanks Krishnaveni, Lavanya and Meduri Praveen for critical comments and valuable suggestion. DSK also gratefully acknowledge IIT Hyderabad for funding the research project by the grant from Institute SEED GRANT-2014. Additionally, the authors would like to sincerely thank to CSIR-National Institute of Science Technology and Development Studies for constant support and valuable suggestions in completing this manuscript.
References
- Guerra NP, Agrasar AT, Macias CL, Pastrana L (2003) Main characteristics and application of solid state fermentation. EJEAFChe 2: 343-350.
- Raimbault R (1998) General and microbial aspects of solid substrate fermentation. EJB 1: 1-15.
- Hesseltine CW (1972) Biotechnology report. Solid state fermentations. Biotechnol Bioeng 14: 517-532.
- Lonsane BK, Ghildyal NP, Budiatman S, Ramakrishna SV (1985) Engineering aspects of solid state fermentation. Enz Microb Technol 7: 258-265.
- Pandey A, Soccol CR (1998) Bioconversion of biomass: a case study of ligno-cellulosics bioconversions in solid-state fermentation. Braz Arch Biol Technol 41: 379-390.
- Pandey A, Soccol CR, Nigam P, Soccol VT (2000) Biotechnological potential of agro-industrial residues. I. Sugarcane bagasse. Biores Technol 74: 69-80.
- Pandey A, Soccol CR, Nigam P, Soccol VT, Vandenberghe LPS, et al. (2000) Biotechnological potential of agro-industrial residues. II. Cassava bagasse. Biores Technol 74: 81- 87.
- Pandey A, Selvakumar P, Soccol CR, Nigam P (1999) Solid-state fermentation for the production of industrial enzymes. Curr Sci 77: 149-162.
- Pandey A, Soccol CR (2000) Economic utilization of crop residues for the value addition: a futuristic approach. J Sci Ind Res 59: 12-22.
- Pandey A, Soccol CR, Mitchell F (2000) New developments in solid-state fermentation. I. Bioprocesses and products. Process Biochem 35: 1153-1169.
- Soccol CR, Vandenberghe LPS (2003) Overview of applied solid-state fermentation in Brazil. Biochem Eng J 13: 205-218.
- Suryanarayan S (2003) Current industrial practice in solid state fermentations for secondary metabolite production: the Biocon India experience. Biochem Eng J 13: 189-195.
- Kumar A, Kanwar SS (2012) Lipase production in solid-state fermentation (SSF): recent developments and biotechnological applications. Dynamic Biochemistry, Process Biotechnology and Molecular Biology 6: 13-27.
- Balcao VM, Vieira MC, Malcata FX (1996) Adsorption of protein from several commercial lipase preparations onto a hollow-fiber membrane module. Biotechnol Prog 12: 164-172.
- Verger R, Rivière C, Moreau H, Gargouri Y, Rogalska E, et al. (1991) Enzyme kinetics of lipolysis. In: Alberghina L, Schmid RD, Verger R (eds.) Lipases: Structure, mechanism and genetic engineering, VCH, Weinheim, Germany 105-116.
- Jaeger KE, Dijkstra BW, Reetz MT (2000) Bacterial Biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications. Ann Rev Microbiol 53: 315-351.
- Large KP, Mirjalili N, Peacock MOLM, Zormpaidis V, Walsh M, et al. (1999) Lipase activity in Streptomycetes. Enz Microb Technol 25: 569-575.
- Gaoa X, Cao S, Zhang K (2000) Production, properties and application to nonaqueous enzymatic catalysis of lipase from a newly isolated Pseudomonas strain. Enzyme Microb Technol 27: 74-82.
- Rapp P, Backhaus S (1992) Formation of extracellular lipases by filamentous fungi, yeasts and bacteria. Enz Microb Technol 14: 938-943.
- Dalmau E, Montesinos JL, Lotti M, Casas C (2000) Effect of different carbon sources on lipase production by Candida rugosa. Enzyme Microb Technol 26: 657-663.
- Costa MA, Peralta RM (1999) Production of lipase by soil fungi and partial characterization of lipase from a selected strain (Penicillium wortmanii). J Basic Microbiol 39: 11-15.
- Jaeger KE, Reetz MT (1998) Microbial lipases form versatile tools for biotechnology. Trends Biotechnol 16: 396-403.
- Macrae AR, Hammond RC (1985) Present and future applications of lipases. Biotech Genetic Eng Rev 3: 193-218.
- Kirchner G, Scollar MP, Klibanov AM (1985) Resolution of racemic mixtures via lipase catalysis in organic solvents. J Am Chem Soc 107: 7072-7076.
- Walde P (1989) Lipases as examples for enzymes in reverse micelles. Reactions in Compartmentalized Liquids 11-19.
- Muralidhar RV, Marchant R, Nigam P (2001) Lipases in racemic resolutions. J Chem Tech & Biotech 76: 3-8.
- Sharma R, Chisti Y, Banerjee UC (2001) Production, purification, characterization, and applications of lipases. Biotechnol Adv 19: 627-662.
- Gordillo MA, Montesinos JL, Casas C, Valero F, Lafuente J, et al. (1998) Improving lipase production from Candida rugosa by a biochemical engineering approach. Chem Phys Lipids 93: 131-142.
- Balashev K, Jensen TR, Kjaer K, Bjørnholm T (2001) Novel methods for studying lipids and lipases and their mutual interaction at interfaces. Part I. Atomic force microscopy. Biochimie 83: 387-397.
- Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62: 597-635.
- Cordova J, Nemmaoui M, Ismaili-Alaoui M, Morin A, Roussos S, et al. (1998) Lipase production by solid state fermentation of olive cake and sugarcane bagasse. J Mol Catal B Enz 5: 75-78.
- Kamini NK, Mala JGS, Puvanakrishnan R (1998) Lipase production from Aspergillus niger by solid-state fermentation using gingelly oil care. Process Biochem 33: 505-511.
- Gombert AK, Pinto AL, Castilho LR, Freire DMG (1999) Lipase production by Penicillium restrictum in solid-state fermentation using babassu oil cake as substate. Process Biochem 35: 85-90.
- ul-Haq I, Idrees S, Rajoka MI (2002) Production of lipases by Rhizopus oligosporous by solid-state fermentation. Process Biochem 37: 637-641.
- Mahadik ND, Puntambekar US, Bastawde KB, Khire JM, Gokhale DV (2002) Production of acidic lipase by Aspergillus niger in soild state fermentation. Process Biochem 38: 715-721.
- Christen P, Angeles N, Corzo G, Farres A, Revah S (1995) Microbial lipase production on a polymeric resin. Biotechnol Tech 9: 597-600.
- Ohnishi K, Yoshida Y, Sekiguchi J (1994) Lipase production of Aspergillus Oryzae. J Ferment Bioeng 77: 490-495.
- Adinarayana K, Raju KVVSNB, Zargar IM, Devi RB, Lakshmi PJ, et al. (2004) Optimization of process parameters for production of lipase in solid-state fermentation by newly isolated Aspergillus species. Indian J Biotechnol 3: 65-69.
- Elibol M, Ozer D (2001) Influence of oxygen transfer on lipase production by Rhizopus arrhizus. Process Biochem 36: 325-329.
- Venkata RP, Kunthala J, Lakshmanan CM (1993) Production of lipase by Candida rugosa in solid state fermentation 2: Medium optimization and effect of aeration. Process Biochem 28: 391-395.
- Venkata Rao P, Jayaraman K, Lakshmanan CM (1993) Production of lipase by Candida rugosa in solid state fermentation 1: Determination of significant process variables. Process Biochem 28: 385-389.
- Venkata RP, Lakshmanan CM (1991) Lipase enzyme technology and its potential applications in the oils and fats industry. Indian ChemEng 33: 7-29.
- Saiki T, Narosaki T, Aramati K, Tamura G, Arima K (1968) Studies on the lipoprotein lipases of micro-organisms. Part III. Effect of culture conditions on the production of lipoprotein lipase by Mucor javanicus IAM 6108. Agric Biol Chem 32: 1458-1463.
- Toshiko K, Mari T, Ishii T, Ikoth Y, Kirimura K, et al. (1989) Production of lipase by Rhizopus oligosporous. A newly isolated fungus. J Hokoku Waseda 50: 61-65.
- Korn MS, Fujio Y (1997) Effect of enzyme on the degree of moceration of soyabean fermented by Rhizopus strains. J Fac Agri 41: 231-237.
- Rivera-Munoz G, Tinoco-Valencia JR, Sanchez S, Farres A (1999) Production of microbial lipases in a solid-state fermentation system. Biotechnol Lett 13: 277-280.
- Salleh AB, Musani R, Basri M, Ampon K, Yunus WMZ, et al. (1993) Extra and intracellular lipases from a thermophilic Rhizopus oryzae and factors affecting their production. Can J Microbiol 39: 978-981.
- Papaparaskevas D, Chistakopoulos P, Kekos D, Macris BJ (1992) Optimizing production of extracellular lipase from Rhodotorula glutinis. Biotechnol Lett 14: 397-402.
- Pokorny D, Friedrich J, Cimerman A (1994) Effect of nutritional factors on lipase biosynthesis by Aspergillus niger. Biotechnol Lett 16: 363-366.
- Petrovic SE, Skrinjar M, Becaveric A, Vujicic IF, Branka L (1990) Effect of various carbon sources on microbial lipases biosynthesis. Biotechnol Lett 12: 299-304.
- Macris JB, Kourentzi E, Hatzinkokiou DG (1996) Studies on localization and regulation of lipases production by Aspergillus niger. Process Biochem 31: 807-812.
- Feniksova RV, Tikhomirova AS, Rakheleeva BE (1960) [Conditions of amylase and proteinase formation in a surface culture of Bacillus subtilis]. Mikrobiologiia 29: 745-748.
- dos Santos RR, Muruci LNM, Damaso MCT, Lima da Silva JP, Santos LO (2014) Lipase Production by Aspergillus niger 11T53A14 in Wheat Bran Using Experimental Design Methodology. Journal of Food and Nutrition Research 10: 659-663.
- Lima N, Teixeira JA, Mota M (1991) Deep agar-diffusion test for preliminary screening of lipolytic activity of fungi. J Microbiol Methods 14: 193-200.
- Fryer TF, Lawrence RC, Reiter B (1967) Methods for isolation and enumeration of lipolytic organisms. J Dairy Sci 50: 477-484.
- Alford JA, Steinle EE (1967) A double layered plate method for the detection of microbial lipolysis. J Appl Bacteriol 30: 488-494.
- Olama ZA, el-Sabaeny AH (1993) Lipase production by Aspergillus niger under various growth conditions using solid state fermentation. Microbiologia 9: 134-141.
- Zadrazil F, Brunnert H (1981) Investigation of physical parameters important for the solid state fermentation of straw by white rot fungi. Eur J Appl Microbiol Biotechnol 11: 183-188.
- dos Santos RR, Muruci LNM, Santos LO, Antoniassi R, Lima da Silva JP, et al. (2014) Characterization of Different Oil Soap stocks and their Application in the Lipase Production by Aspergillus niger under Solid State Fermentation. Journal of Food and Nutrition Research 2: 561-566.
- Toscano L, Montero G, Stoytcheva M, Gochev V, Cervantes L, et al. (2013) Lipase production through solid state fermentation using agro-industrial residues as substrates and newly isolated fungal strains. Biotechnol & Biotechnol Eq 27: 4074-4077.
- Falony G, Armas JC, Mendoza JCD, Hernández JLM (2006) Production of extracellular lipase from Aspergillus niger by solid state fermentation. Food Technol Biotechnol 44: 235-240.
- Hosseinpour MN, Najafpour GD, Younesi H, Khorrami M, Vaseghi Z (2012) Lipase Production in Solid State Fermentation Using Aspergillus niger: Response Surface Methodology. IJE TRANSACTIONS B: Applications 25: 151-159.
- Balaji V, Ebenezer P (2008) Optimization of Extracellular Lipase Production in Colletotrichum Gloeosporioides by Solid State Fermentation. Ind J Sci Tech 1: 1-7.
- Sarat BI, Sita KK, Hanumantha RG (2010) Optimization of media constituents for the production of lipase in solid state fermentation by Yarrowia lipolytica from palm Kernal cake (Elaeis guineensis). Adv Biosci Biotech 1: 115-121.
- Edwinoliver NG, Thirunavukarasu K, Naidu RB, Gowthaman MK, Kambe TN, et al. (2010) Scale up of a novel tri-substrate fermentation for enhanced production of Aspergillus niger lipase for tallow hydrolysis. Bioresour Technol 101: 6791-6796.
- Ângelo T, da Silva RR, Cabral H (2014) Concomitant Production of Peptidases and Lipases by Fungus Using Agro-industrial Residue in Solid-state Fermentation. Int J Curr Microbiol App Sci 3: 810-823.
- Soxhlet A, Normes A (1968) Meat, Meat Products and Fishery Products - Determination of Free Fat Content. NF-V-04-403.
- Van Soest PJ (1982) Books (Eds) Nutritional Ecology of the Ruminant: Ruminant Metabolism, Nutritional Strategies, the Celluloytic Fermentation and the Chemistry of Forages and Plant Fibers. O & B Books.
- Fujii T, Tatara T, Minagawa M (1986) Studies on applications of lipolytic enzyme in detergency I. Effect of lipase from Candida cylindracea on removal of olive oil from cotton fabric. J Am Oil Chem Soc 63: 796-799.
- Ardree H, Muller WR, Schmid RD (1980) Lipases as detergent components. J Appl Biochem 2: 218-229.
- Sztajer H, Maliszewska J (1989) The effect of culture conditions on lipolytic productivity of Penicillium citrinum. Biotechnol Lett 11: 895-898.
- Freire DM, Teles EMF, Bon EPS, Sant’Anna GL (1997) Lipase production byPenicillium restrictum in a bench-scale fermenter. Appl Biochem Biotechnol 63: 409-421.
- Freire DM, Gomes PM, Bon EPS, Sant’Anna GL (1997) Lipase production by a new promising strain of Penicillium restrictum. Rev Microbiol (J.Braz Soc Microbiol) 28: 6-12.
- Pirt SJ (1975) Principles of microbe and cell cultivation. Blackwells scientific pulications, London.
- Pandey A, Benjamin S, Soccol CR, Nigam P, Krieger N, et al. (1999) The realm of microbial lipases in biotechnology. Biotechnol Appl Biochem 29: 119-131.
- Bhushan B, Dosanjh NS, Hoondal GS (1994) Lipase production from an alkalophilic yeast sp. by solid-state fermentation. Biotechnol Lett 16: 841-842.
- Lin SF, Lee JC, Chiou CM (1996) Purification and characterization of a lipase from Neurospora sp. TT-241. J Am Chem Soc 73: 739-745.
- Chandrasekaran M (1997) Industrial enzymes from marine microorganism. J Mar Biotechnol 5: 86-89.
- Benjamin S, Pandey A (1997) Coconut cake: A potent substrate for the production of lipases by Candida rugosa in solid-state fermentation. Acta Biotechnol 17: 241-251.
- Pokorny D, Cimerman A, Steiner W (1997) Aspergillus niger lipases: Induction, isolation and characterization of two lipases from MZKI, A116 strain. J Mol catal B: Enzyme 2: 215-222.
- Berkman-Dik T, Ozilgen M, Bozoglu TF (1992) Salt, EDTA and PH effects on rheological behaviour of mold suspensions. Enz Microb Technol 14: 944-948.
- Benjamin S, Pandey A (2001) Isolation and Characterization of Three Distinct forms of Lipases from Candida rugosa Produced in Solid State Fermentation. Braz arch boil technol 44: 2.
- Rawyler A, Siegenthaler PA (1989) A single and continuous spectrophotometric assay for various lipolytic enzymes, using natural, non-labelled lipid substrates. Biochim Biophys Acta 1004: 337-344.
- Duncombe WG (1963) The colorimetric determination of long chain fatty acids in the 0.05–0.5? mole range. Biochem J 88: 7.
- Autryve PV, Ratomahenina R, Riaublanc A, Mitrani C, Pina M, et al. (1991) Spectrophotometry assay of lipase activity using Rhodamine 6G. Oleagineux 46: 29-31.
- Margesin R, Feller G, Hämmerle M, Stegner U , Schinner F (2002) A colorimetric method for the determination of lipase activity in soil. Biotechnol Lett 24: 27-33.
- Desnuelle P, Constantin MJ, Baldy J (1955) [Potentiometric technic for the measurement of pancreatic lipase activity]. Bull Soc Chim Biol (Paris) 37: 285-290.
- Brockman HL (1981) Triglyceride lipase from porcine pancreas. Methods Enzymol 71 Pt C: 619-627.
- Tietz NW, Shuey DF, Astles JR (1987) Turbidimetric measurement of lipase activity--problems and some solutions. Clin Chem 33: 1624-1629.
- Nagata T (1974) Lipases. In: Hayat MA (ed) Electron microscopy of enzymes. Van Nordstand-Reimboldt, New York 132-148.
- Nielsen LK, Risbo J, Callisen TH, Bjørnholm T (1999) Lag-burst kinetics in phospholipase A(2) hydrolysis of DPPC bilayers visualized by atomic force microscopy. Biochim Biophys Acta 1420: 266-271.
- Bevilaqua JV, Pinto JC, Lima LM, Barreiro EJ, Alves TLM, et al. (2004) Enzymatic hydrolysis by immobilized lipase applied to a new prototype anti-asthma drug. Biochem Eng J 21: 103-110.
- Du W, Xu Y, Liu A, Zeng J (2004) Comparative study on lipase- catalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J Mol Catal B Enzym 30: 125-129.
- Mitchell DA, Lonsane BK, Durand A, Renaud R, Almanza S, et al. (1992) General principles of reactor design and operation for solid substrate cultivation. In: Doelle HW, Mitchell DA, Rolz CE (Eds.) Solid-State Fermentation Bioreactors: Fundamentals of Design and Operation. Elsevier Applied Sci 115-139.
- Raghavarao KSMS, Ranganathan TV, Karanth NG (2003) Some engineering aspects of solid state fermentation. Biochem Eng J 13: 127-135.
- Durand A (2003) Bioreactor designs for solid state fermentation. Biochem Eng J 13: 113-125.
- Srikumar S (2001) Proceedings of the International Conference on New Horizons in Biotechnology, Trivandrum.
- Pandey A (2003) Solid state fermentation. Biochem Eng J 13: 81-84.
- Fujiwara fermentor manual (2001) Serial No.P100086.
- Ride JP, Drysdale RB (1972) A rapid method for the chemical estimation of filamentous fungi in plant tissue. Physiol Plant Pathol 2: 7-15.
- Matcham SE, Jordan BR, Wood DA (1985) Estimation of fungal biomass in a solid substrate by three independent methods. Appl Microbiol Biotechnol 21: 108-112.
- Desgranges C, Vergoignan C, Georges M, Durand A (1991) Biomass estimation in solid state fermentation. Appl Microbiol Biotechnol 35: 200-205.
- Miller GL (1972) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31: 426-428.
- Kjeldahl J (1883) New method of determining nitrogen in organic compounds. Anal Chem 22: 366-382.
- Desgranges C, Vergoignan C, Georges M, Durand A (1991) Biomass estimation in solid state fermentation. (Online measurements). Appl Microbiol Biotechnol 35: 206-209.
- Sethi BK, Rout JR, Das R, Nanda PK, Sahoo SL (2013) Lipase production by Aspergillus terreus using mustard seed oil cake as a carbon source. Ann Microbiol 63: 241-252.
- Castilho LR, Polato CMS, Baruque EA, Sant’Anna Jr GL, Freire DMG (2000) Economic analysis of lipase production by Penicillium restritum in soild state fermentations. Biochem Eng J 4: 239-247.
- Vaseghi Z, Najafpour GD, Mohseni S, Mahjoub S, Hosseinpour MN (2012) Lipase Production in Tray-Bioreactor via Solid State Fermentation under Desired Growth Conditions. Iranica Journal of Energy & Environment 3: 76-82.
- Benjamin S, Pandey A (2004) Coconut cake- a potent substrate for the production of lipase by Candida rugosa in soild state fermentation. Acta Biotechnologica 17: 241-251.
- Damaso MCT, Passianoto MA, de Freitas SC, Freire DMG, Lago RCA, et al. (2008) Utilization of agro industrial residues for lipase production by Solid-state fermentation. Braz J Microbiol 39: 676-681.
- Salihu A, Bala M, Bala SM (2013) Application of Plackett-Burman Experimental Design for Lipase Production by Aspergillus niger using Shea Butter Cake. ISRN Biotechnology: 1-5.
- Fleuri LF, de Oliveira MC, Arcuri MLC, Capoville BL, Pereira MS, et al. (2014) Production of Fungal Lipases Using Wheat Bran and Soybean Bran and Incorporation of Sugarcane Bagasse as a Co-substrate in Solid-state Fermentation. Food Sci Biotechnol 23: 1199-1205.
- Colla LM, Ficanha AMM, Rizzardi J, Bertolin TE, Reinehr CO, et al. (2014) Production and Characterization of Lipases by Two New Isolates of Aspergillus through Solid-State and Submerged Fermentation. Biomed Research International 1-10.
- Laurent S, Ivanova MG, Pioch D, Graille J, Verger R (1994) Interactions between beta-cyclodextrin and insoluble glyceride monomolecular films at the argon/water interface: application to lipase kinetics. Chem Phys Lipids 70: 35-42.
- Ransac S, Ivanova M, Panaiotov I, Verger R (1999) Monolayer techniques for studying lipase kinetics. Methods Mol Biol 109: 279-302.
- Nury S, Piéroni G, Rivière C, Gargouri Y, Bois A, et al. (1987) Lipase kinetics at the triacylglycerol-water interface using surface tension measurements. Chem Phys Lipids 45: 27-37.
- Walde P, Luisi PL (1989) A continuous assay for lipases in reverse micelles based on Fourier transform infrared spectroscopy. Biochem 28: 3353-3360.
- Negre AE, Salvayre RS, Dagan A, Gatt S (1985) New fluorometric assay of lysosomal acid lipase and its application to the diagnosis of Wolman and cholesteryl ester storage diseases. Clin Chim Acta 149: 81-88.
- Hendrickson HS, Rauk PN (1981) Continuous fluorometric assay of phospholipase A2 with pyrene-labeled lecithin as a substrate. Anal Biochem 116: 553-558.
- Wolf C, Sagaert L, Bereziat G (1981) A sensitive assay of phospholipase using the fluorescent probe 2-parinaroyllecithin. Biochem Biophys Res Commun 99: 275-283.
- Ruiz-Larrea MF, Galdiz-Valdovinos B, Rodríguez-Fernández C (1982) Kinetic study of hepatic triglyceride lipase from rat liver soluble fraction. Enzyme 27: 215-219.
- Grenner G, Deutsch G, Schmidtberger R, Dati F (1982) [A highly sensitive enzyme immunoassay for the determination of pancreatic lipase]. J Clin Chem Clin Biochem 20: 515-519.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 27658
- [From(publication date):
February-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 21552
- PDF downloads : 6106