Review Article Open Access
Functional Virus-Like Particles Production Using Silkworm and Their Application in Life Science
Tatsuya Kato1, Vipin Kumar Deo2 and Enoch Y Park1,2*1Department of Applied Biological Chemistry, Faculty of Agriculture, Shizuoka University, 836 Ohya Suruga-ku, Shizuoka 422-8529, Japan
2Graduate School of Science and Technology, Shizuoka University, 836 Ohya Suruga-ku, Shizuoka 422-8529, Japan
- Corresponding Author:
- Enoch Y Park
Graduate School of Science and Technology, Shizuoka University
836 Ohya Suruga-ku, Shizuoka 422-8529, Japan
E-mail: acypark@ipc.shizuoka.ac.jp
Received date: December 30, 2011; Accepted date: February 06, 2012; Published date: February 08, 2012
Citation: Kato T, Deo VK, Park EY (2012) Functional Virus-Like Particles Production Using Silkworm and Their Application in Life Science. J Biotechnol Biomaterial S9:001. doi:10.4172/2155-952X.S9-001
Copyright: © 2012 Kato T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Keywords
Virus-like particles; Vaccine; Baculovirus; Silkworm; Insect cell
Introduction
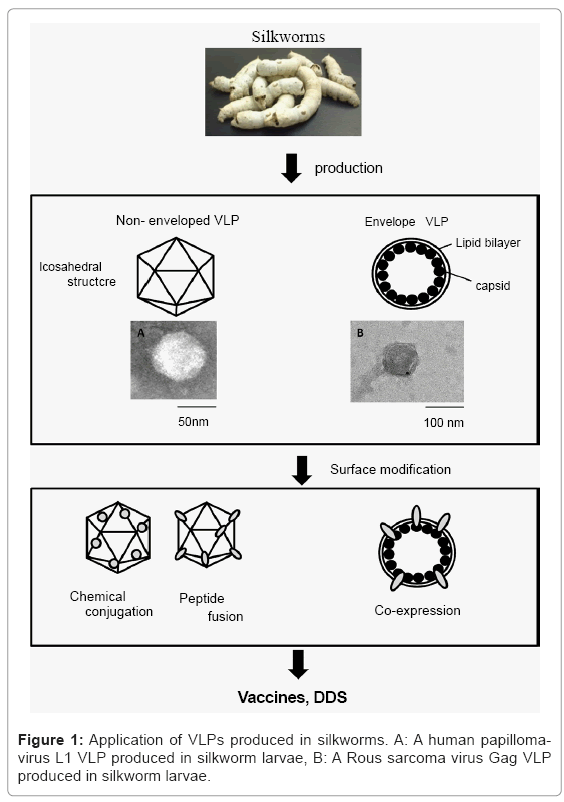
Silkworm larvae and pupae have been used as biofactories for large-scale protein production due to their high capacity for protein production, easy scale-up and low-cost performance. Silkworm expression systems have been also used to produce Virus-like particles (VLPs) efficiently and the surface of VLPs can be modified by several methods, irrespective of non-enveloped and enveloped VLPs (Figure 1). VLPs are composed of virus-derived capsid or envelope proteins and have empty shells similar to native viruses [1,2]. VLPs do not have genetic material and are no longer infectious, indicating that VLPs are safer than live attenuated vaccines and inactivated vaccines. VLPs are still able to enter target cells and are released from cells and can induce cellular and humoral immune responses without any adjuvants, compared to subunit vaccines. Commercial human papilloma virus (HPV) vaccines, Gardasil and Cervarix, are composed of HPV-VLPs and adjuvants, Alum or Alum and monoposphoryl lipid A, which increase vaccine-induced antibody titers [3]. VLPs are now attractive tools for vaccine strategies in terms of safety and efficacy. Moreover, VLPs have considerable medical and clinical impacts and they have also been applied to drug delivery systems (DDSs) to targeted cells and tissues as chemical, drug and vaccine carriers. VLPs as functional biomolecules have been produced in bacteria, yeasts, plants and insect and mammalian cells. Recently, production of VLPs including several subunit vaccines has been investigated in silkworms and other insect larvae. This review gives an overview of VLP production in silkworm with various expression systems and evaluation of targeting for valuable biomaterials.
Virus-like Particles
VLPs as vaccines
Vaccines are powerful means of preventing virus infections and their spread in humans and animals. Vaccines for various virus infections have been developed using inactivated vaccines including protein subunits, peptides and DNA, inactivated and live attenuated viruses, recombinant viruses and VLPs. Although many vaccines are commercially available for human and animal use, protective vaccines against several pathogens, for example, HIV, respiratory syncytial virus and dengue virus, have not yet been developed in spite of enormous efforts (Table 1). A replicating virus infection can elicit a strong immune response in the host and the immune response may last for several years. In the case of live attenuated vaccines, heat and chemicals, which partially denature surface antigens, attenuate virus replication. Alternatively, viral variants that lack replication capacity and deleterious effects and are non-pathogenic can be used as vaccines. A reduced capacity for replication may cause reduced immunogenicity. However, attenuated viruses still have superior capacities to enhance immunogenicity compared to non-replicating and inactivated vaccines, including subunit proteins and DNA vectors. Live attenuated viruses reach the host immune system and are taken up by APCs, including dendritic cells (DCs) and elicit immune responses similar to those induced by natural viruses [4]. However, live attenuated viruses have the possibility to revert to virulent forms and evolve to virulent viruses by recombination with endemic viruses [5]. Intra-dermal injection of non-replicating and inactivated vaccines, including subunit proteins and DNA vectors, induces protective immune responses [6,7]. Nonreplicating and inactivated vaccines have a safety advantage compared to live attenuated vaccines, but are less effective processing and presentation to the immune system because only humoral immune response is induced by non-replicating and inactivated vaccines. Various adjuvants, mineral salts, emulsions and microbial derivatives have been utilized together with non-replicating vaccines to activate the innate and adaptive immune systems [8]. Some viruses can be used as virus-vector-based vaccines when antigen-encoding genes of infectious viruses are heterologously inserted. Vaccinia virus, human adenovirus (AdV) and lentiviruses have been used as virus-vectorbased vaccines [9,10]. Recombinant AdV can prime and boost T cell and B cell responses. VLPs have been utilized as vaccines owing to their capability of stimulating strong cellular and humoral responses as direct immunogens [1]. VLPs mimic the structure of native viruses similarly. Therefore, they can enhance the production of neutralizing antibodies against viruses. VLPs represent a safe alternative vaccine to attenuated live virus vaccines because they cannot replicate and are noninfectious. Most VLPs can stimulate potent immune responses without adjuvants. VLPs are 20–200 nm in diameter and can be taken up by DCs via macro pinocytosis and endocytosis [11]. VLPs induce CD4 proliferative responses and cytotoxic T lymphocyte (CTL) responses, in addition to B-cell-mediated humoral responses [2].
| Virus | Disease |
|---|---|
| Human immunodeficiency virus (HIV) | Acquired immunodeficiency syndrome |
| Respiratory syncytial virus(RSV) | Respiratory infection |
| Hepatitis B | Liver cancer |
| Hepatitis C | Cirrhosis/cancer |
| Epstein barr virus | Lymphomas, nasopharyngeal carcinoma |
| Human papilloma virus (HPV) | Cervical cancer |
| Measles | Pneumonia (infants) |
| Influenza | Pneumonia |
Table 1: List of prominent virus causing epidemic in recent human history.
To enhance the immune response by VLPs, chimeric VLPs have been constructed by the fusion of heterologous epitopes with VLP proteins or incorporation of heterologous protein in VLPs. Insertion of the V3 loop domain of HIV gp120 to p24 domain in HIV gag protein or at the HIV gag protein C terminus enhances a strong V3 domain-specific cytolytic CD8 CTL reactivity [12]. This chimera gag protein forms HIV-like particles in insect cells. Chimeric rabbit hemorrhagic disease viruses VLPs have been produced by expression of VP60 fused with hemagglutinin helper T cell epitope (HAT) at its N-terminus in insect cells and enhance the activation and proliferation of T cells [13]. Chimeric HPV-VLPs composed of L1 capsid protein fused with T-cell epitopes of HPV E6 and E7 proteins at its C-terminus have been produced in tomato plants and induce humoral and CTL responses [14]. This fusion strategy is applicable to enveloped and nonenveloped VLPs and permits heterologous antigens that cannot selfassemble into VLPs to be incorporated into VLPs. Alternatively, in the case of enveloped VLPs, incorporation of foreign proteins into VLPs is achieved by co-expression of gag proteins with foreign proteins; mostly trans-membrane proteins. Foreign proteins are imbedded into VLP envelopes during budding and are displayed on the surface of the VLPs. HIV-VLPs displaying Env glycoprotein (gp120) have been produced in insect cells and induce humoral and cellular immune responses [15,16]. HIV-1-specific CTLs and cross-clade neutralizing antibodies have been detected in immunized mice. The trans-membrane domain of Epstein–Barr virus gp220/350 enhances gp120 expression on the surface of HIV VLPsin insect cells [17]. Moreover, trans-membrane and cytoplasmic domains of mouse mammary tumor virus (MMTV) envelope glycoprotein, influenza virus HA and baculovirus gp64 also enhance gp120 expression on the surface of VLPs, indicating that transmembrane and cytoplasmic domains play an important role in determining the gp120 expression on the surface of VLPs [18]. Incorporation of CD40L into Simian immune-deficiency virus (SHIV) VLPs induces DC activation and enhances humoral and cellular immune responses in mice [19]. These CD40-incorporated VLPs are increased in HIV Env-specific IgG production and CTL activity compared with SHIV-VLPs in mice.
VLPs as carriers for DDSs
Non-enveloped VLPs: Normally, non-enveloped VLPs are composed of virus-derived capsid proteins. To modify the surface of VLPs, chemical and genetic engineering methods have been adopted [20]. Lysine, cysteine, glutamate and aspartate have reactive side chains. These amino acid residues in VLPs can be conjugated with peptides, oligonucleotides, carbohydrates and fluorescent molecules using N-hydroxysuccinimidyl ester (NHS) and maleimide, therefore, functional groups can be displayed on the surface of VLPs. These functionalized VLPs have been utilized for various studies on vaccines, drug carriers and imaging probes. To insert some peptide sequences into capsid proteins, of which VLPs are composed and display these peptide sequences on the VLP surface, genetic engineering methods have been used. Functional moieties can be displayed on the surface of VLPs through these peptide sequences. In this case, peptide sequences are fused into VLPs and then chemical reactions, which often are harsh, can be omitted. HBsAg protein particles (bionanoparticles) displaying HER2 antibody genetically deliver encapsulated molecules to HER2- expressing cells (SK-BR-3 cells) [21]. However, these bio-nanoparticles have lipid bi-layers from host endoplasmic reticulum membranes. Simian virus 40 VP1 VLPs display RDF-motif bound to integrin or cells in an RGD-dependent manner [22]. HBV core protein can incorporate whole proteins (e.g. GFP, Borreliaburgdorferi outer surface protein A) into itself and form VLPs [23]. This indicates that whole proteins can display on the surface of VLPs and this method could lead to the development of novel vaccines and drug carriers.
Envelope VLPs: VLPs are merely basic envelope proteins of the virus, which have the unique ability to self-assemble and form VLPs [24,25]. The VLPs self-assembly theory can be explained by a wellknown viral capsid protein gag and its assembly on the membrane using the lipid raft mobility mechanism. This process occurs efficiently in cells in which a continuous flow in anterograde and retrograde directions takes place, transporting proteins in transport machinery. The gag proteins “hitch a ride” on these lipid rafts and in theory, any protein that binds to these rafts is destined for the plasma membrane. This property makes them an ideal candidate for presenting antigenic proteins in a form that closely resembles the native state and thus provides a method to produce vaccines [26]. Gag proteins usually form VLPs of fixed size but this shape and size can be manipulated by addition of spacer peptides or deletion of specific regions [27]. Almost all the proteins have to interact with cell membranes and many are associated with membranes. To study membrane proteins, it is difficult to express and purify in sufficient quantity trans-membrane and complex proteins. Enveloped VLPs provide an answer to this problem as VLPs formed from enveloped virus structural proteins are the most suited for display of proteins [26]. They have a lipid bi-layer, thus they can provide the support required for the membrane proteins [28]. VLPs aggregate on the plasma membrane, which supports the theory that the VLPs accumulate on the plasma membrane where they are selfassembled (Figure 1). When a sufficient number of gag monomers has accumulated, the decrease in surface tension causes pinching of the VLPs from the surface of the plasma membrane. As a result of this, the VLPs can be easily collected from the supernatant [29]. Using this approach, many proteins that are difficult to express and study are being pseudo typed on VLPs [30]. This has led to development of many vaccine candidates undergoing clinical trials [31]. VLPs are emptycage- like proteins that have the potential to serve as carriers. The inner core of the VLPs is protein with a lipid envelope, which can be used for packaging. These approaches have to date been at the conceptual level. VLP-based DDSs are unique because they can provide target-specific delivery mechanisms. VLPs can be easily pseudo typed with markers specific for affected regions and packaged with drugs.
Production of VLPs
Until now, various kinds of VLPs have been produced in bacteria, yeasts, insect cells, plants and mammalian cells. In particular, VLPs composed of human HPV L1 protein in the baculovirus expression system and Saccharomyces cerevisiae have been approved for marketing as Gardasil and Cervarix, respectively. Hepatitis B virus (HBV) VLPs produced in S. cerevisiae and CHO cells are also available as Recombivax-HB, Engerix-B and Sci-B-Vac.Other VLPs as vaccine candidates are in clinical trials or preclinical stages [1].
Bacteria
Most recombinant proteins including some VLPs have been produced in Escherichia coli. E.coli expression systems have several advantages for protein expression, availability of many commercial expression vectors for high-level expression, ease of scaling-up and highdensity cultivation using bioreactors that permit control of cultivation conditions (pH, dissolved oxygen, culture mode), less expensive production cost. Murine polyomavirus VP1 capsid protein has been produced in E.coli as a glutathione S-transferase (GST) fusion protein on a largescale using bioreactors [32]. In this case, in vitro assembly has yielded murine polyomavirus VP1VLPs that have a diameter of ~50 nm after GST cleavage. Porcine circovirus type 2 capsid protein fused with small ubiquitin like modifiers (SUMOs) is self-assembled in vitro after SUMO cleavage [33]. Porcine circovirus type 2-capsid protein can also be assembled in E.coli [34]. However, the E.coli expression system also has several disadvantages. The most important problem is endotoxin contamination. Removal of endotoxin in VLPs originated from E.coli is needed for its vaccine application [35]. No E.coli-derived VLP vaccine has reached the market yet [36]. Alternatively, a food-grade bacterium, Lactococcuslactis, has been used as a vehicle for the production and oral delivery of HPV L1 protein [37]. HPV L1 capsid protein can be assembled in Lactobacilluscasei [38]. Gram-positive lactic acid bacteria (LAB) are normally known as a safety because of the common use in the food industry and do not have any endotoxins. However, to use LAB as a delivery vector, more efforts to improve the efficiency of gene delivery are still required for human clinical trials [39].
Yeast
Yeast expression systems have been used for the production of eukaryotic proteins because yeasts permit several protein modifications, including glycosylation and phosphorylation. Moreover, recombinant proteins from yeasts are free of pyrogens, toxins and infectious viruses. A prophylactic quadrivalentHPV L1-VLP vaccine, Gardasil, has been produced and highly purified in S. cerevisiae. This vaccine is conjugated with a proprietary amorphous aluminum hydroxyphosphate sulfate adjuvant [40]. HBV core protein (HBc)-VLPs have been produced in methylotrophic yeast Pichia pastoris on a large scale using a fermentor [41]. In this case, the endotoxin level of the purified HBc-VLPs is lower than that of VLPs from E.coli. HBV surface antigen (HBsAg) vaccines from S. cerevisiae are commercially available [1]. Production of HBsAg- VLPs has been performed using P. pastoris [42,43]. Apart from virus VLPs, theTy1 and Ty3 particles were observed intracellularly in yeast and these particles have a diameter of 40–50 nm [44]. The particles are from transposons in yeast, which permit adaptation to extreme environments by giving opportunities for genetic modifications. These particles can be used as vaccine adjuvants because an antigen-presenting cell (APCs) takes up them. Now four yeast-based VLP vaccines have been already approved for commercialization [36].
Insects
Insect cells have the capability to modify recombinant proteins similar to mammalian cells and correctly folded VLPs can be obtained more efficiently and sufficiently than by using microbial expression systems. Moreover, insect cells are amenable to scaling up for mass VLP production [45]. Insect cells have been widely used for VLP production and commercial HPV L1-VLP vaccine, CERVARIX, has been produced in Trichoplusia ni cells using a recombinant baculovirus. Insect cells allow VLP production, irrespective of the type of viruses (enveloped and non-enveloped). One enveloped virus protein, human immunodeficiency virus (HIV) type 1 gag protein, cannot assemble efficiently in yeast but in spheroplasts [46] and the formation of HIV type 2 virus gag protein VLPs has also failed in yeast [47]. However, in insect cells, HIV 1 gag protein VLPs can be formed and secreted into culture medium efficiently using baculovirus expression systems [15,16] and stably transformed cell systems [48]. Another enveloped virus protein, influenza A virus matrix protein (M1), can be assembled in insect cells and its VLPs are produced rapidly and easily in sufficient amounts [49] using Spodopterafrugiperda 9 (Sf-9) and High Five cells.
Insect cells are also useful for non-enveloped virus VLP production. Enhanced production yield of HPV-57L1-VLPs has been achieved by using two L1 expression cassettes under the control of polyhedrin and p10 promoter independently [50]. Multiple capsid proteins can be expressed simultaneously in insect cells using a single recombinant baculovirus containing multiple gene expression cassettes. Triplelayered rotavirus VLPs composed of capsid proteins, VP2, VP6 and VP7,have been produced in Sf-9 and Sf-21 cells using a recombinant baculovirus containing atricistronicgene expression cassette [51]. Double-layered rotavirus VLPs are also produced in stably transformed Drosophila melanogaster S2 cells using encephalomyocarditis-virusderived internal ribosomal entry site element [52]. Improved production of enterovirus 71 VLPs was performed by co-expression of P1 and 3CD protease under the control of polyhedrin and cytomegalovirus immediate early promoters, respectively, in a single recombinant bacmid [53]. Insect larvae have also been used for large-scale VLP production. A baculovirus expression system using insect larvae is described afterward (VLPs production using silkworm larvae and their applications as subunit vaccine). Baculovirus, expression systems are also suitable for the production of subunit vaccines and VLPs (Table 2). CERVARIX is the first human commercial product to be produced in a baculovirus expression system and FluBlok is now in phase III trials as an influenza type a vaccine [54]. Many vaccines produced by baculovirus expression system will be on the market for human and veterinary use in the future. However, in baculovirus expression system, baculovirus particles are also co-produced and purified together with expressed VLPs. It is difficult to separate baculovirus particles and expressed VLP completely. For clinical trials, chemical inactivation of contaminating baculovirusesin VLPs expressed and purified in baculovirus expression system has to be performed [55]. Otherwise baculovirus-free expression system is required to avoid baculovirus contamination.
| Conventional vaccinology | Reverse vaccinology | VLPs vaccinology | References | |
|---|---|---|---|---|
| Essential features |
|
|
|
[80] |
| Advantages |
|
|
|
[25]
[24]
[80] [26] |
| Disadvantages |
|
|
[26] |
Table 2: VLPs their advantages and disadvantages.
Plants
Recombinant protein production in plants has several merits compared to other expression systems; its low production cost, safety and scalability [56]. The most important merit is the possibility of oral therapy by feeding with edible plants expressing vaccines or antigens [57]. Especially, regarding mucosal vaccines, merely minimal processing of plant tissues expressing VLPs is needed as an oral immunization and plant expression system provide a less expensive alternative compared to the conventional vaccines and compete with microbial expression system. For recombinant protein expression in plants, stable transformation method of the nuclear or chloroplast genomes and transient virus infection methods have been performed. Transgenic plants have the merit of easy large-scale protein production with its low cost. Transgenic potato plants expressing HPV 16 L1-VLPs have been generated and feeding transgenic potato tubers to mice orally induces an anti-HPV 16 L1 antibody responses, but this is mostly transient [58]. HIV1/HBV-VLPs expressed in Nicotianatabacum and Arabidopsis thaliana have been orally administered to mice to elicit anHIV1- specific cellular immune response [59]. HIV1 gag-VLPs transgenically produced in tobacco plastids are formed with the same shape as those produced in baculovirus expression systems [60]. However, VLP production in plants needs to be optimized and developed to stabilize VLP production and enhance yield.
Mammalian cells
Most of therapeutic proteins arrived to the market are produced in mammalian cells [61]. Mammalian cells have host-specific glycosylation of virus antigens, which is different from that in insect cells, plants and yeasts. Especially, glycosylation (N- and O-glycosylation) is important for protein function because glycan that attached to proteins has the effects on functionality, immunogenicity of proteins and half-life of proteins in serum. Baculovirus expression systems are superior to mammalian cells with regard to VLP production yield. However, authentic VLPs cannot be obtained in baculovirus expression systems and immature HIV1-VLPs are released from Sf-9 cells [62]. Mammalian influenza a virus VLPs composed of four virus proteins, M1 matrix protein (M1), M2 matrix protein (M2), hemagglutinin (HA) and neuraminidase (NA) have been produced in Vero cells [63]. These VLPs mimic authentic virions in terms of their morphology and HA function and glycosylation. M1 has only limited budding in human embryonic kidney (HEK-293T) cells and co-expressed NA enhances secretion of influenza virus (pandemic H5N1) M1-VLPs from cells, indicating that NA is a major factor in virus budding [64]. However, mammalian cell culture requires tedious culture adaptation before doing suspension cell culture and addition of serum, which may be adventitious viruses and pathogens, into culture medium.
VLPs Production Using Silkworm Larvae and their Applications as Subunit Vaccine
Silkworms have a high capacity for producing recombinant proteins and can produce human therapeutic proteins. Moreover, silkworms make the easy and inexpensive scale-up of protein production possible. Several studies have demonstrated that insect larvae [silkworms, T.ni (cabbage looper)] are useful as living biofactories for the inexpensive production of recombinant antigens and vaccines [65]. Insect larvae have also been used for large-scale VLP production. Rotavirus and HPV VLPs have been produced in S. frugiperda and T. ni larvae [66,67]. This baculovirus expression system using larvae leads to VLP vaccine production at a low cost compared to other expression systems. In particular, silkworms have been used widely for the production of recombinant proteins because the cost is low and the expression protocol is easy. Two decades ago, protein expression in silkworms was difficult and time-consuming, because of cultured insect cells were mainly used as baculovirus hosts for protein production. However, Bombyx mori nucleopolyhedrovirus (BmNPV) bacmid (a baculovirus shuttle vector), which can be replicated in E. coli and generate the recombinant baculovirus DNA by site-specific transposition in E.coli, has been developed [68] and many reports on recombinant protein expression in silkworms are being published [69].
Many types of VLP have been expressed in cultured insect cells, but in silkworms only a few reports were published. Human HBsAg has been expressed in silkworm larvae using recombinant BmNPV (a traditional method of protein expression in silkworms) and purified as VLPs, which have a diameter of 22 nm [70]. Beet western yellow luteovirus capsid protein has also been expressed in silkworm larvae using recombinant BmNPV and purified from fat bodies as VLPs [71]. Recently, canine parvovirus capsid protein, VP2, has been expressed as VLPs in silkworm larvae and pupae using BmNPV bacmid [72]. Regarding enveloped VLPs, Rous sarcoma virus gag protein has been expressed in silkworm larvae using BmNPV bacmid and enveloped gag VLPs have been purified from hemolymph [73,74]. When human (pro) renin receptor (hPRR), which has one trans-membrane domain at its Cterminus, is co-expressed with this gag protein, hPRRis displayed on the surface of gag VLPs. Foot-and-mouth disease virus (FMDV) capsid protein (P1-A2, 3C) has been expressed in silkworm larvae using recombinant BmNPV and its diluted hemolymph is used as a vaccine. In this case, specific antibody against FMDV has been induced in vaccinated animals and four of five animals were completely protected from virus challenge [76]. Alternatively to VLPs, FMDV capsid proteins have been expressed in silkworm larvae and expressed capsid proteins have been used as a subunit vaccine to immunize cattle [75]. However, this capsid forms VLPsin silkworms. In this case, expressed capsid proteins are used as a VLP vaccine rather than a subunit vaccine. Four of five cattle were completely protected against the challenge with a virulent virus after vaccine immunization. In another study, capsid proteins from other FMDV expressed in silkworm larvae were used to immunize cattle [76]. In both cases, hemolymph containing expressed capsid proteins was used as a crude vaccine solution. In the cabbage looper system, when HAfrom A/PR/8/34 influenza virus (H1N1) expressed in Cabbage looper larvae was immunized into mice using its hemolymph as a crude vaccine solution, anaphylaxis was not observed in the immunized mice [65]. These results suggest that hemolymph that contains subunit vaccines or antigens could be used to vaccinate cattle with an inexpensive formulation. Antigen-fusion proteins have also been produced in silkworm larvae as subunit vaccines. Classical swine fever virus envelope glycoprotein, E2, fused with polyhedron from baculovirus has been expressed in silkworm larvae and E2 has been purified by solubilization of recombinant polyhedra. Virus-neutralizing activity is induced when purified E2 is used to immunize mice [77]. In this case, virus-neutralizing activity is induced after immunization with recombinant polyhedra. Oral administration of a cholera toxin B subunit–insulin fusion protein produced in silkworm larvae, using its hemolymph, reduces pancreatic islet inflammation and delays progression of diabetes in non-obese diabetic mice [78]. Alternatively, BmNPV displaying HA from A/Zhejiang 16/06 (H5N1) influenza virus produced in silkworm pupae has been used to immunize rhesus monkeys, which produce virus-neutralizing antibody and protection against influenza virus challenge [79]. BmNPV displaying antigens produced in silkworms can also be used as a vaccine.
Conclusion
VLPs are potential candidate vaccines and are used as carriers of DDSs. Silkworm expression systems are used to produce VLPs efficiently and the surface of VLPs can be modified by several methods, irrespective whether they are enveloped or not (Figure 1). Some commercial vaccines have been manufactured by insect cell technology. Bacteria, especially E.coli, can produce a large amount of recombinant proteins, but cannot perform most of co- and post-translational modifications, phosphorylation, glycosylation and processing. Alternatively, silkworms have a high capacity for producing recombinant proteins, compared to insect cell culture and low-cost productivity comparable to E.coli expression system Silkworms make the easy and inexpensive scale-up of protein production possible. Moreover, hemolymph containing VLPs or antigens can be directly used as a veterinary vaccine formulation without purification; indicating silkworm hemolymph does not cause any anaphylactic reaction in immunized animals, though further purification is undoubtedly needed for human use. Silkworm’s have contributed to the textile industry in the past, but will contribute in the future to prevention of infectious diseases.
References
- Grgacic EV, Anderson DA (2006) Virus-like particles: Passport to immune recognition. Methods 40: 60-65.
- Ludwig C, Wagner R (2007) Virus-like particles-universal molecular toolboxes. Curr Opin Biotechnol 18: 537-545.
- Chen J, Ni G, Liu XS (2011) Papillomavirus like particle-based therapeutic vaccine against human papillomavirus infection related diseases: Immunological problems and future directions. Cell Immunol 269: 5-9.
- Bachmann MF, Zinkernagel RM, Oxenius A (1998) Immune responses in the absence of costimulation: viruses know the tricks. J Immunol 161: 5791-5794.
- Chong YL, Padhi A, Hudson PJ, Poss M (2010) The effect of vaccination on the evolution and population dynamics of avian paramyxovirus-1. PLoS Pathog 8: e1000872.
- Mikszta JA, Dekker JP 3rd, Harvey NG, Dean CH, Brittingham JM, et al. (2006) Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infect Immun 74: 6806-6810.
- Van Damme P, Oosterhuis-kafeja F, Van der Wielen M, Almagor Y, Sharon O, et al. (2009) Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine 27: 454-459.
- Pashine A, Valiante NM, Ulmer JB (2005) Targeting the innate immune response with improved vaccine adjuvants. Nat Med 11: S63-S68
- Draper SJ, Heeney JL (2010) Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol 8: 62-73.
- Richardson JS, Dekker JD, Croyle MA, Kobinger GP (2010) Recent advances in Ebolavirus vaccine development. Hum Vaccine 6: 439-449.
- Gamvrellis A, Leong D, Hanley JC, Xiang SD, Mottram P, et al. (2004) Vaccines that facilitate antigen entry into dendritic cells. Immunol Cell Biol 82: 506-516.
- Wagner R, Deml L, Schirmbeck R, Niedrig M, Reimann J, et al. (1996) Construction, expression and immunogenicity of chimeric HIV-1 virus-like particles. Virology 220: 128-140.
- Peacey M, Wilson S, Baird MA, Ward VK (2007) Versatile RHDV virus-like particles: incorporation of antigens by genetic modification and chemical conjugation. Biotechnol Bioeng 98: 968-977.
- Paz De la Rosa G, Monroy-García A, Mora-GarcíaMde L, Peña CG, Hernández-Montes J, et al. (2009) An HPV 16 L1-based chimeric human papillomavirus-like particles containing a string of epitopes produced in plants in able to elicit humoral and cytotoxic T-cell activity in mice. Virol J 6: 2.
- Buonaguro L, Racioppi L, Tornesello ML, Arra C, Visciano ML, et al. (2002) Induction of neutralizing antibodies and cytotoxic T lymphocytes in Balb/c mice immunized with virus-like particles presenting a gp120 molecules from a HIV-1 isolate of clade A. Antiviral Res 54: 189-201.
- Buonaguro L, Tornesello ML, Tagliamonte M, Gallo RC, Wang LX, et al. (2006) Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T-cell responses. J Virol 80: 9134-9143.
- Deml L, Kratochwil G, Osterrieder N, Knüchel R, Wolf H, et al. (1997) Increased incorporation of chimeric human immunodeficiency virus type 1 gp120 protein Pr55 gag virus-like particles by Epstein-barr virus gp220/350-derivrd transmembrane domain. Virol 235: 10-25.
- Wang BZ, Liu W, Kang SM, Alam M, Huang C, Ye L, et al. (2007) Incorporation of high levels of chimeric human immunodeficiency virus envelope glycoproteins into virus-like particles. J Virol 81: 10669-10878.
- Zhang R, Zhang S, Li M, Chen C, Yao Q (2010) Incorporation of CD40 ligand into SHIV virus-like particles (VLP) enhanced SHIV-VLP-induced dendritic cell activation and boosts immune response against HIV. Vaccine 28: 5114-5127.
- Yildiz I, Shukla S, Steinmetz NF (2011) Applications of viral nanoparticles in medicine. Curr Opin Biotechnol 22: 901-908
- Shishido T, Mieda H, Hwang SY, Nishimura Y, Tanaka T, et al. (2010) Affibody-displaying bionanocapsules for specific drug delivery to HER2-expressing cancer cells. Bioorg Med Chem Lett 20: 5726-5731.
- Takahashi R, Kanesashi S, Inoue T, Enomoto T, Kawano M, et al. (2008) Presentation of functional foreign peptides on the surface of SV40 virus-like particles. J Biotechnol 135: 385-392.
- Nassal M, Skamel C, Kratz PA, Wallich R, Stehle T, et al. (2005) A fusion product of the complete Borreliaburgdorfei outer surface protein A (OspA) and the hepatitis B virus capsid protein is highly immunogenic and induced protective immunity similar to that seen with an effective lapidated OspA vaccine formula. Eur J immunol 35: 655-665.
- Ako-Adjei D, Johnson MC, Vogt VM (2005) The retroviral capsid domain dictates virion size, morphology, and coassembly of gag into virus-like particles. J Virol 79: 13463-13472.
- Campbell S, Vogt VM (1995) Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J Virol 69: 6487-6497.
- Haynes JR, Dokken L, Wiley JA, Cawthon AG, Bigger J, et al. (2009) Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine 27: 530-541.
- Keller PW, Johnson MC, Vogt VM (2008) Mutations in the spacer peptide and adjoining sequences in Rous sarcoma virus Gag lead to tubular budding. J Virol 82: 6788-6797.
- Jorgenson RL, Vogt VM, Johnson MC (2009) Foreign glycoproteins can be actively recruited to virus assembly sites during pseudotyping. J Virol 83: 4060-4070.
- Scheifele LZ, Kenny SP, Cairns TM, Craven RC, Parent LJ (2007) Overlapping roles of the Rous sarcoma virus Gag p10 domain in nuclear export and virion core morphology. J Virol 81: 10718-10728.
- Kuate S, Stahl-Hennig C, Stoiber H, Nchinda G, Floto A, et al. (2006) Immunogenicity and efficacy of immunodeficiency virus-like particles pseudotyped with the G protein of vesicular stomatitis virus. Virology 351: 133-144.
- Morrison TG (2010) Newcastle disease virus-like particles as a platform for the development of vaccines for human and agricultural pathogens. Future Virol 5: 545-554.
- Liew MW, Rajendran A, Middelberg AP (2010) Microbial production of virus-like particle vaccine protein at gram-per-liter levels. J Biotechnol 150: 224-231.
- Yin S, Sun S, Yang S, Shang Y, Cai X, et al. (2010) Self-assembly of virus-like particles of porcine circovirus type 2 capsid protein expressed from Escherichia coli. Virol J 7: 166.
- Marcekova Z, Psikal I, Kosinova E, Benada O, Sebo P, et al. (2009) Heterologous expression of full-length capsid protein porcine circovirus 2 in Escherichia coli and its potential use for detection of antibodies. J Virol Methods 162: 133-141.
- Schädlich L, Senger T, Kirsching CJ, Muller M, Gissmann L (2009) Refining HPV16 L1 purification from Escherichia coli: reducing endotoxin contaminations and their impact on immunogenicity. Vaccine 27: 1511-1522.
- Roldão A, Mellado MCM, Castilho LR, Carrondo MJT, Alves PM (2010) Virus-like particles in vaccine development. Expert Rev Vaccines 9: 1149-1176.
- Cho HJ, Shin HJ, Han IK, Jung WW, Kim YB, et al. (2007) Induction of mucosal and systemic immune responses following oral immunization of mice with Lactococcuslactis expressing human papillomavirus 16 L1. Vaccine 25: 8049-8057.
- Aires KA, Cianciarullo AM, Camiero SM, Villa LL, Boccardo E, et al. (2006) Production of human papillomavirus type 16 L1 Virus-like particles by recombinant Lactobacillus casei cells. Appl Environ Microbiol 72: 745-752.
- Bermúdez-Humarán LG, Kharrat P, Chatel JM, Langella P (2011) Lactococci and lactobacilli as mucosal delivery vectors for the therapeutic proteins and DNA vaccines. Microb Cell Fact.
- Bryan JT (2007) Developing an HPV vaccine to prevent cervical cancer and genital warts. Vaccine 25: 3001-3006.
- Freivalds J, Dislers A, Ose V, Pumpens P, Tars K, et al. (2011) Highly efficient production of phosphorylated hepatitis B core particles in yeast. Protein Expr Purif 75: 218-224.
- Gurramkonda C, Adnan A, Gäbel T, Lünsdorf H, Ross A, et al. (2009) Simple-high-cell density fed-batch technique for high-level recombinant protein production with Pichia pastoris. Application to intracellular production of Hepatitis B surface antigen. Microb Cell Fact 8: 13.
- Lunsdorf H, Gurramkonda C, Adnan A, Khanna N, Rinas U (2011) Virus-like particle production with ultrastructural and immunocytochemical insights into Pichia pastoris producing high levels of the Hepatitis B surface antigen. Microb Cell Fact 10: 48.
- Roth JF (2000) The yeast Ty virus-like particles. Yeast 16: 785-795.
- Mandell RB, Koukuntla R, Mogler LJK, Carzoli AK, Holbrook MR, et al. (2010) Novel suspension cell-based vaccine production systems for Rift valley fever virus-like particles. J Virol Methods 169: 259-268.
- Sakuragi S, Goto T, Sano K, Morikawa Y (2002) HIV type 1 Gag virus-like particle budding from spheroplasts of Saccharomyce cerevisiae. Proc Natl Acad Sci U S A 99: 7956-7961.
- Morikawa Y, Goto T, Yasuoka D, Momose F, Matano T (2007) Defect of human immunodeficiency virus type 2 Gag assembly in Saccharomyces cerevisiae. J Virol 81: 9911-9921.
- Tagliamonte M, Visciano ML, Tornesell ML, De Stradis A, Buonaguro FM, et al. (2011) HIV-Gag VLPs presenting trimeric HIV-1 gp140 spikes constitutively expressed in stable double transfected insect cell line. Vaccine 29: 4913-4922.
- Krammer F, Schinko T, Palmberger D, Tauer C, Messner P, et al. (2010) Trichoplusia ni cells (High Five) are highly efficient for the population of influenza A virus-like particles: a comparison of two insect cell lines as producing platforms for influenza vaccines. Mol Biotechnol 45: 226-234.
- Senger T, Schädlich L, Gissmann L, Müller M (2009) Enhanced papilloma virus-like particle production in insect cells. Virology 388: 344-353.
- Vieira HLA, Estêvão C, Roldão A, Peixoto CC, Sousa MF, et al. (2005) Triple layered rotavirus VLP production: Kinetics of vector replication, mRNA stability and recombinant protein production. J Biotechnol 120: 72-82.
- Lee JM, Chung HY, Kim KI, Yoo KH, Hwang-Bo J, et al. (2011) Synthesis of double-layered rotavirus-like particles using internal ribosomal entry site vector system in stably-transformed Drosophila meranogaster. Biotechnol Lett 33: 41-46.
- Chung YC, Chen CY, Lin SY, Chung YC, Chiu HY, et al. (2010) Enterovirus 71 virus-like particle vaccine: Improved production conditions for enhanced yield. Vaccine 28: 6951-6957.
- Drugmand JC, Schneider YJ, Agathos SN (2011) Insect cells as factories for biomanufacturing. Biotechnol Adv.
- Rueda P, Fominaya J, Langeveld JP, Bruschke C, Vela C, et al. (2000) Effect of different baculovirus inactivation procedures on the integrity and immunogenicity of porcine parvovirus-like particles. Vaccine 19: 726-734.
- Giorgi C, Franconi R, Rybicki EP (2010) Human papilloma virus vaccines in plants Expert Rev Vaccines 9: 913-924.
- Santi L, Huang Z, Mason H (2006) Virus-like particles production in green plants. Methods 40: 66-76.
- Biemelt S, Sonnewald U, Galmbacher P, Willmitzer L, Müller M (2003) Production of human papillomavirus type 16 virus-like particles in transgenic plants. J Virol 77: 9211-9220.
- Guetard D, Greco R, Cervantes Gonzalez M, Celli S, Kostrzak A, et al. (2008) Immunogenicity and tolerance of following HIV-1/HBV plant-based oral vaccine administration. Vaccine 26: 4477-4485.
- Scotti N, Alagna F, Ferraiolo E, Formisano G, Sannino L, et al. (2009) High-level expression of the HIV-Pr55gag polyprotein in transgenic tobacco chloroplasts. Planta 229: 1109-1122.
- Walsh G (2006) Biopharmaceutical benchmarks 2006. Nat Biotechnol 24: 769-776
- Hammonds J, Chen X, Zhang X, Lee F, Spearman P (2007) Advances in methods for the production, purification, and characterization of HIV-Envpsuedovirion vaccines. Vaccine 25: 8036-8048.
- Wu CY, Yeh YC, Yang YC, Chou C, Liu MT, et al. (2010) Mammalian expression of virus-like particles for advanced mimicry of authentic influenza virus. PLoS One 5: e9784.
- Lai JC, Chan WW, Kien F, Nicholls JM, Peiris JS, et al. (2010) Formation of virus-like particles from human cell lines exclusively expressing influenza neuraminidase. J Gen Virol 91: 2322-2330.
- Gomez-Casado E, Gomez-Sebastian S, Núñez MC, Lasa-Covarrubias R, Martínez-Pulgarín S, et al. (2011) Insect larvae biofactories as a platform for influenza vaccine production. Protein Expr Purif 79: 35-43.
- Millan AF, Gomez-Sebastian S, Nunez MC, Veramendi J, Escribano JM (2010) Human papillomavirus-like particles vaccine efficiently produced in a non-fermentative system based on insect larva. Protein Expr Purif 74: 1-8.
- Molinari P, Peralta A, Taboga O (2008) Production of rotavirus-like particles in Spodoptera frugiperda larvae. J Virol Methods 147: 364-367.
- Motohashi R, Shimojima T, Fukagawa T, Maenaka K, Park EY (2005) Efficient large-scale protein production of larvae and pupae of silkworm by Bombyx mori nuclear polyhedrosis virus bacmid system. Biochem Biophys Res Commun 326: 564-569.
- Kato T, Kajikawa M, Maenaka K, Park EY (2010) Silkworm expression system as a platform technology in life science. Appl Microbiol Biotechnol 85: 459-470.
- Higashihashi N, Arai N, Enjo T, Horiuchi T, Saeki Y, et al. (1991) High-level expression and characterization of hepatitis B virus surface antigen in silkworm using a baculovirus vector. J Virol Methods 35: 159-167.
- Tian T, Medina V, Mayhew DE, Maeda S, Falk BW (1995) Beet western yellows luteovirus capsid proteins produced by recombinant baculoviruses assemble into virion-like particles in cells and larvae of Bombyx mori. Virology 213: 204-212.
- Feng H, Liang M, Wang H, Zhang T, Zhao P, et al. (2011) Recombinant canine parvovirus-like particles express foreign epitopes in silkworm pupae. Vet Microbiol 154: 49-57.
- Deo VK, Tsuji Y, Yasuda T, Kato T, Sakamoto N, et al. (2011) Expression of an RSV-gag virus-like particle in insect cell lines and silkworm larvae. J Virol Methods 177: 147-152.
- Tsuji Y, Deo VK, Kato T, Park EY (2011) Production of Rous sarcoma virus-like particles displaying human trans-membrane protein in silkworm larvae and its application to ligand-binding assay. J Biotechnol 155: 185-192.
- Li Z, Yi Y, Yin X, Zhang Z, Liu J (2008) Expression of foot-and-mouth disease virus capsid proteins in silkworm-baculovirus expression system and its utilization as a subunit vaccine. PLoS One 3: e2273.
- Li Z, Yin X, Yi Y, Li X, Li B, et al. (2011) FMD subunit vaccine produced using a silkworm-baculovirus expression system: Protective efficacy against two type Asia1 isolates in cattle. Vet Microbiol 149: 99-103.
- Lee KS, Sohn MR, Kim BY, Choo YM, Woo SD, et al. (2012) Production of classical Swine Fever virus envelope glycoprotein e2 as recombinant polyhedral in baculovirus-infected silkworm larvae. Mol Biotechnol 50: 211-220.
- Gong Z, Jin Y, Zhang Y (2005) Oral administration of a cholera toxin B subunit-insulin fusion protein produced in silkworm protects against autoimmune diabetes. J Biotechnol 119: 93-105.
- Jin R, Lv Z, Chen Q, Quan Y, Zhang H, et al. (2008) Safety and immunogenicity of H5N1 influenza vaccine based on baculovirus surface display system of Bombyx mori. PLoS ONE 3: e3933.
- Roy P, Noad R (2008) Virus-like particles as a vaccine delivery system: myths and facts. Hum Vaccin 4: 5-12.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 17168
- [From(publication date):
specialissue-2012 - Nov 27, 2025] - Breakdown by view type
- HTML page views : 12266
- PDF downloads : 4902