Special Issue Article Open Access
Functional Exhaustion of Bone Marrow Derived Endothelial Progenitor Cells in a Chronic Swine Model of Myocardial Ischemia
| Simon Maltais1,3, Nicholas A Haglund3, Jean Francois Tanguay2, Martin G Sirois2, Jean Claude Tardif2, Louis P Perrault1, Hak Joon Sung4 and Hung Q Ly2* | ||
| 1Department of Cardiac Surgery | ||
| 2Department of Medicine, Montreal Heart Institute, University of Montreal, Montreal, Quebec, Canada | ||
| 3Department of Cardiac Surgery and Cardiology, Vanderbilt University Medical Center, Nashville, Tennessee, USA | ||
| 4Department of Biomedical Engineering, Vanderbilt University Medical Center, Nashville, Tennessee, USA | ||
| *Corresponding author: | Hung Q. Ly, MD, MSc, FRCPC Department of Medicine Montreal Heart Institute University of Montreal 5000 Belanger St East, Montreal Quebec, H1T 1C8, Canada Tel: 514-376-3330 Fax: 514-376-6299 E-mail: qh.ly@umontreal.ca |
|
| Received November 24, 2011; Accepted January 09, 2012; Published January 12, 2012 | ||
| Citation: Maltais S, Haglund NA, Tanguay JF, Sirois MG, Tardif JC, et al. (2011) Functional Exhaustion of Bone Marrow Derived Endothelial Progenitor Cells in a Chronic Swine Model of Myocardial Ischemia. Autacoids S3:002. doi:10.4172/2161-0479.S3-002 | ||
| Copyright: © 2011 Maltais S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Autacoids and Hormones
Abstract
The functional impairment of bone marrow derived endothelial progenitor cells remains an important barrier for cardiac cell-based therapies. Our aim was to create a relevant swine model of chronic ischemia and document its effect on bone derived progenitor cells. We hypothesized that bone marrow derived endothelial progenitor cells would be functionally impaired in the setting of chronic cardiac dysfunction of ischemic origin. At baseline, Landrace miniswine was instrumented with a fixed occluder to the proximal left anterior descending coronary artery. We evaluated the animals over a 3-month period (0, 45 and 90 days). Focal proximal left anterior descending stenosis was angiographically confirmed in all animals (mean diameter stenosis = 96±4%, n=12). The resulting ischemic myocardium had evidence of contractile dysfunction but preserved viability. A progressive decline in circulating levels of endothelial progenitor cells was documented 3 months following instrumentation (P<0.001). Quantitative polymerase chain reaction analysis revealed that chronic myocardial ischemia produced a biphasic response in both hypoxic-inducible factor 1 and stromal-derived factor 1 mRNA expression. While initially unregulated, a gradual decline in hypoxic-inducible factor 1 and stromal-derived factor 1 mRNA expression was observed over time (from day 45 to 90). On serial assessment, endothelial progenitor cell migration in response to chemo attractant gradients of vascular endothelial growth factor (10-200 ng/mL) and stromal cell-derived factor-1 (10-100 ng/mL) was progressively impaired. Decreased circulating levels and migratory dysfunction of bone marrow derived endothelial progenitor cells were documented in a reproducible clinically relevant model of myocardial ischemia. Our model of chronic ischemic cardiac dysfunction could contribute to improved understanding of cellular mechanisms involved in the mobilization and exhaustion of endothelial progenitor cells in patients with heart failure.
Keywords |
|||
| Heart Failure; Bone Marrow; Progenitor cells | |||
| Abbreviations | |||
| BM: Bone Marrow; CHF: Congestive Heart Failure; CO: Cardiac Output; DSE: Dobutamine Stress Echocardiography; EPC: Endothelial Progenitor Cell; FS: Fractional Shortening; HIF 1: Hypoxic Inducible Factor 1; IHD: Ischemic Heart Disease; LAD: Left Anterior Descending; LV: Left Ventricle; LVEF: Left Ventricular Ejection Fraction; LVD: Left Ventricle Dimension; MNC: Mononuclear Nuclear Cell; RNA: Ribonucleic Acid; RT-PCR: Reverse Transcription Polymerase Chain Reaction; SDF-1: Stromal Derived Factor 1; TTE: Transthoracic Echocardiography; VEGF: Vascular Endothelial Growth Factor | |||
| Introduction | |||
| Ischemic heart disease (IHD) remains a leading cause of mortality worldwide [1]. Currently, there are over five million diagnosed cases of congestive heart failure (CHF) in North America [2,3]. The complex pathophysiology of CHF involves alterations at numerous levels, from altered molecular signaling pathways to direct cardiomyocyte injury, which progressively results in deleterious changes in both myocardial structure and function [4-6]. A myriad of novel pathophysiological mechanisms such as mitochondrial dysfunction underscore the growing complexities involved in CHF, which could explain some of the limited impact of current therapeutic strategies [7,8]. | |||
| Rahimtoola et al. [9] proposed a thorough description of the “hibernation syndrome”. They postulated that this condition resulted from reduced myocardial blood flow at rest whereby the heart downgrades its myocardial function to the extent that blood flow and function are maintained at equilibrium [9]. Serial studies using external coronary occluder on juvenile swine producing severe coronary stenosis demonstrated a systematic progression to myocardial hibernation/ischemia [10-12]. At the clinical level, hibernation leads to left ventricular systolic dysfunction and potentially CHF [13,14]. | |||
| A growing interest has emerged in the role of bone marrow (BM)- derived endothelial progenitor cells (EPCs) in the setting of CHF [15], as these progenitor cells act as biomarkers of extent of coronary artery disease, but also contribute to myocardial healing in the setting of ischemic injuries [16,17]. A blunted response of the “BM-cardiac axis” has been observed to lead to the progression towards CHF [18]. Insufficient levels and intrinsic functional abnormalities of EPCs such as increased apoptosis and exhaustion of BM-derived EPCs are of paramount importance in the setting of delayed vascular repair [19- 22]. There is currently poor longitudinal data on the alterations of BM-derived cells during the course of chronic myocardial ischemia in a clinically relevant in vivo model. Our study aim was to assess the underlying mechanisms driving the differences in cellular trafficking (mobilization and migration) of BM-derived EPCs. We hypothesized that BM-derived EPCs would be impaired in a chronic swine model of myocardial ischemia. | |||
| Methods by Feature Space | |||
| Chronic ischemic cardiomyopathy model creation | |||
| Juvenile Landrace miniswine (10 to 15 kg) was obtained from Primiporcs (St-Gabriel-de-Brandon, QC). All surgical procedures and postoperative care were carried out in accordance with the guidelines of the Canadian Council on Animal Care. The study protocol was approved by the Montreal Heart Institute Animal Experimentation Committee. All animals received aspirin 81mg the day before surgery. Swine were anesthetized with sodium pentobarbital (30mg/kg) and mechanically ventilated (Harvard Apparatus, South Natick, Mass.) according to our standard protocol [23]. Chronic instrumentation and experimental protocol have been previously detailed by Fallavolitta et al. [12]. Briefly, at baseline, 14 juvenile pigs were instrumented through a left anterior thoracotomy with a 1.5 to 2.25 mm rigid Delran occluder (generously provided by Dr. Canty, Buffalo, New York) on the proximal left anterior descending artery (LAD). Pigs were then weaned-off from ventilator and allowed to recover. All animals were instrumented by the same operator (S.M.) and housed under similar conditions. Blood samples for troponin measurement were drawn at baseline and after different time points to exclude myocardial infarction. Left ventriculogram and coronary angiography were performed at baseline and at the end of the study for each animal to evaluate LV function and determine the severity of coronary artery stenosis. | |||
| Echocardiographic measures | |||
| Echocardiographic (TTE) studies were performed with a M3S probe (2.0~4.3 Megahertz) and the Vivid 7 Dimension system (GE Healthcare Ultrasound, Horten, Norway). Transthoracic echocardiographic study was performed at baseline before surgery. Dobutamine stress Echocardiographic (DSE) study was performed three months following LAD instrumentation. Dobutamine (Dobutrex; Eli Lilly, Toronto, Canada) was used in incremental doses. A low DSE dosage (5-10 g/ kg/min) was used to increase the heart rate (HR) to approximately 120 beats per minute (bpm), whereas a high DSE dosage (15 40 g/kg/min) targeted a HR over 140 bpm. Parasternal short-axis views were recorded to visualize potential ischemic left ventricular (LV) wall segments. LV regional wall motion abnormalities were scored as: normal=1; hypokinesis=2; akinesis=3; dyskinesis=4 and aneurysmal=5. Wall thickness was measured at cardiac end-diastole (d) and end-systole (s) for the segments with abnormal wall motion, and wall thickening was calculated as: (wall thickness s-wall thickness d/wall thickness d × 100). Left ventricular dimensions (LVD) at both cardiac end-diastole (LVDd) and end-systole (LVDs) were measured in the short-axis view at the level of the papillary muscles (in M-mode). Left ventricular fractional shortening was calculated as: (LVDd - LVDs) / LVDd × 100. Pulsedwave Doppler measurements were used to trace the flow spectrum of the LV outflow tract in an apical 5-chamber view, and cardiac output was obtained subsequently. The average of 3 cardiac cycles was used for all measurements. | |||
| Isolation and culture of porcine endothelial progenitor cells | |||
| BM cells were aspirated from the iliac bone at 3 different time points during the course of this study (0, 45, and 90 days). For each time point, fresh BM and peripheral blood mononuclear cells (MNCs) were isolated by Ficoll Histopaque density-gradient centrifugation (Life Sciences, Baie d’Urfe, QC). After isolation of MNCs, 1.3 × 105/cm2 of MNCs were plated on fibronectin-coated culture dishes and maintained in endothelial cell basal medium-2 (EBM-2; Clonetics, San Diego, CA) supplemented with EGM-2 (Clonetics, San Diego, CA) microvascular single aliquots, 5% fetal bovine serum (FBS; Gibco, Calgary, AB), and 1% penicillin (10,000 U/ml)/streptomycin (10 mg/ml). The cells were cultured for 4 days at 37°C with 5% CO2 in a humidified atmosphere. After 4 days, non-adherent cells were discarded by washing with phosphate-buffered saline (PBS; Gibco, Calgary, AB). Thereafter, the adherent cells were cultured in complete EGM-2 medium and the medium was changed every 3 days until the first passage. Colonies of early EPCs appeared between 7 and 14days of culture, and were identified as well-circumscribed monolayers of cobblestone-appearing cells. In this study, we focused mainly on the role of early EPCs, as late EPCs represent more differentiated cells. MNCs were also preserved at 80°C at a concentration of 1.0 × 107/ml using the Bambanker serumfree medium (Wako, Richmond, VA). | |||
| Endothelial progenitor cell characterization by semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) and FACS analysis | |||
| Total RNA was extracted before the first passage from cultured EPCs (approximately 2 × 106) using the RN easy Extraction Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Any residual genomic DNA was eliminated by treatment with DNase (Ambion, Austin, TX). RNA was reverse-transcribed with Moloney Murine Leukemia Virus (MMLV) Reverse Transcriptase (RT) (Invitrogen Corporation; Carlsbad, CA). Negative controls contained RNA, but no M-MLV RT, while GAPDH was used as an internal positive control. PCR was carried out in a final volume of 50 μl with 6 μl of cDNA template, 0.75 μl of forward and reverse primers (0.5 μg/μl) (Qiagen, Valencia, CA) (Supplementary Table 1), and 1.25 units of Taq DNA Polymerase (Amersham; Piscataway, NJ) on a Genius thermo cycler (Techne Corporation; Minneapolis, MN). Initial denaturation for 4 minutes at 94°C was followed by 30 cycles of 1 minute at 94°C, 1 minute at 55°C for annealing, and 1 minute at 72°C. The final step consisted of 7 minutes of extension at 72°C. PCR products were run on 2% agarose gels and visualized with ethidium bromide against a 100 bp ladder. | |||
| The expression of the monocyte marker CD14 was evaluated by direct immune fluorescence using standard techniques [24] with a human monoclonal antibody directed against a porcine antigen (Coulter Immunology, Hialeah, FL). Immunofluorescence reactivity was determined by automated multi-parameter flow cytometry analyzing at least 104 cells (FACScan, Becton Dickinson; Mountain View, CA). | |||
| Endothelial progenitor cell in vivo fluorescent staining | |||
| Early passage (1-2) BM or peripheral-derived EPCs (~2.0 × 105 cells/well) were seeded on human fibronectin-coated 6-well plates (BD Biosciences, Mississauga, ON). The following day, attached cells were incubated with fluorescein Griffonia simplicifolia lectin I, isolectin B4 (Vector Laboratories, Burlingame, CA), acylated low-density lipoprotein labeled with 1,1-dioctadecyl-3,3,3-3-tetramethylindocarbocyanine perchlorate (DiI-ac-LDL; Biomedical Technologies, Stoughton, MA), and Topro-3 nuclear staining (Topro-3; Invitrogen, Carlsbad, CA). Using an inverted fluorescence microscope (Zeiss Axiovert, Toronto, ON), cells were examined for uptake of Dil-Ac-LDL, isolectin B4, and nucleus staining with Topro-3. | |||
| Migration assays | |||
| Chemo tactic migration assays were performed according to the original method described by Kucia et al. [16]. Briefly, cells were starved on fibronectin-coated six-well plates (BD Biosciences, Mississauga, ON) with 0.1% fetal bovine serum FBS for 24 hours. Cells were then placed in serum-free medium and equilibrated for 10 minutes at 37°C. The lower chambers of Corning Transwell 24-well plates (polycarbonate membrane 8 mm pore size; BD Biosciences, Mississauga, ON) were filled with 650ml of serum-free medium and 0.5% FBS containing either human SDF-1 (10, 100, and 200 ng/ml; R&D Systems, Minneapolis, MN), human VEGF (10, 50, and 100 ng/ml; R&D Systems, Minneapolis, MN), or medium alone (control). Subsequently, 2 × 105 cells were placed in the upper chamber and incubated for 6 hours at 37°C and 5% CO2. The migrated cells on the lower side of the filter were fixed in methanol and stained with hematoxylin/eosin. Cell counting was performed in five random high-power fields (60X) for all time points and the results are expressed relative to baseline (control conditions). Results are given as the average of 3 experiments. | |||
| Assessment of SDF-1 and HIF-1 by real-time quantitative PCR | |||
| Total BM RNA was extracted using the RNeasy Extraction Kit (Qiagen, Valencia, CA), and RNA was reverse-transcribed using the MMLV-RT as described (Invitrogen Corporation; Carlsbad, CA). Quantitative assessment of SDF-1, HIF-1, and GAPDH mRNA levels was performed by real-time RT-PCR as detailed in the supplementary method section. The relative quantitation value of target, normalized to the endogenous control GAPDH (house-keeping) gene and relative to a calibrator, is expressed as 2-ΔΔCt ( fold difference), where ΔCt=(Ct of target genes)-(Ct of endogenous control gene, GAPDH), and ΔΔCt=(ΔCt of samples for target gene)-(ΔCt of calibrator for the target gene). | |||
| Statistical analysis | |||
| Student’s t test was used for comparisons. Data are presented as mean ± standard deviation. Time-dependent changes were evaluated by ANOVA comparing data at each time point with the corresponding baseline. A level of P<0.05 was considered statistically significant and all P-values are two-sided. | |||
| Results | |||
| Chronic ischemic model | |||
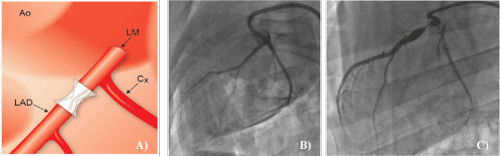
| Fourteen miniswine (average weight, 53.0 ± 5.0 kg) were followed for an average of 92 ± 5 days after the creation of LAD stenosis. The mortality rate was 7%, as one animal died from hemorrhage caused by left atrial laceration. One animal was excluded because a significant myocardial infarction was observed, leaving 12 animals that were included in this study. All animals were in good health at the end of the study with no overt clinical signs of CHF. There were no significant changes in hematocrit or arterial blood gases on serial per operative measures following the implantation of the occluder. A schematic representation of the proximal LAD occluding device is provided in Figure 1-a. Significant proximal LAD stenosis was documented by angiography for all animals. The mean diameter stenosis was 96 ± 4%. Chronic stenosis of the LAD was progressively created as observed on the coronary angiogram at 3 months (Figure 1-c). The resulting narrowing created severe coronary stenosis without total LAD occlusion, and no significant collateral circulation was noted as already documented by Fallavollita et al. [25]. As troponin release occurs early after ischemic injury in the pig and the critical time for sampling is within the first hour [26], troponin levels were measured 15 and 60 minutes as well as 24 and 48 hours following the implantation of the occluder. Troponin levels remained under 0.01mg/L in all animals. Moreover, continuous electrocardiogram recordings did not show any significant per operative ST segment elevation while no significant hemodynamic changes were observed during the per operative period. | |||
| Myocardial dysfunction assessment by dobutamine stress echocardiography | |||
| Transthoracic echocardiographic and DSE results are summarized in Table 1. Normal wall motion and wall thickening were observed at baseline studies, before surgery, and at rest of DSE tests 3 months following the intervention. Increased WMS and decreased wall thickening in the antero-septal wall were observed both at low and high dosage of dobutamine infusion. This indicated that the chronic LAD stenosis (90 days) induced a decrease in the anterior wall contractility confirming the underlying ischemic phenomenon. Normal wall motion was observed in all other visualized LV segments. Throughout all stages of DSE, dobutamine infusion produced a significant increase in FS and CO compared to rest and baseline. These results indicate well preserved compensation from non-ischemic segments. | |||
|
|||
| Endothelial progenitor cell phenotype and numbers | |||
| EPCs were expanded in vitro from the isolated MNCs fraction as previously described. Adherent EPCs were identified 7 to 14 days following culture in complete EGM-2 medium as well-circumscribed cobblestone-appearing cells. These MNCs-derived adherent cells were positive for isolectin B4 staining and dil-ac-LDL uptake (Figure 3). Semi-quantitative RT-PCR showed that EPCs were positive for stem cell markers CD34 and CD133, expressed hematopoietic markers CD31 (PECAM-1), CD144 (VE-cadherin), von willebrand factor (vWF), and KDR (VEGFR-2), but were negative for the monocyte marker CD14 (data not shown). Fluorescence-activated cell sorting (FACS) analysis further confirmed that the monocyte marker CD14 was not detectable at the cell surface before proceeding with experiments. | |||
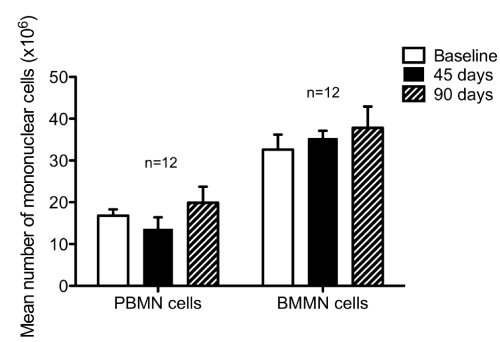
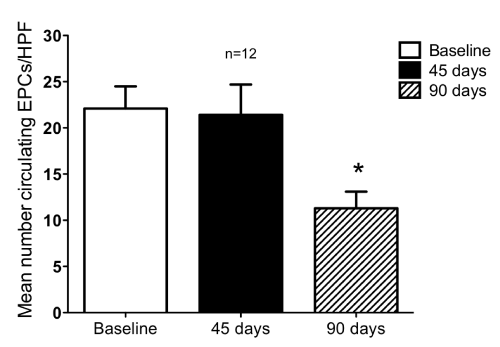
| There were no differences in total peripheral MNCs isolated at each time point observed (Figure 2). As depicted in Figure 4, there was a decrease in the number of circulating EPCs at three months compared with baseline (P<0.001). | |||
| Endothelial progenitor cell functional assessment | |||
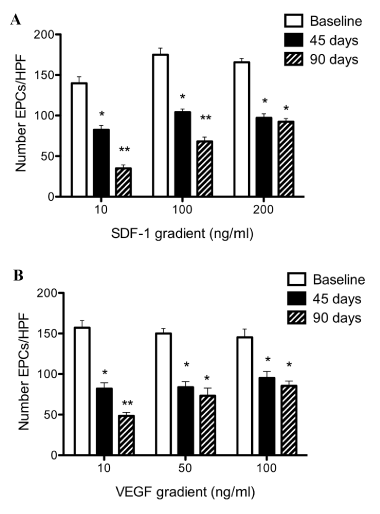
| Evaluation of the BM-derived EPC functional capacity was done by migration assays (Corning Transwell 8mm polycarbonate membrane plates). The latter assays documented a reproducible and progressive decrease in EPCs chemo taxis along SDF-1 and VEGF gradients following LAD instrumentation (Figures 5-a, 5-b). | |||
| Alterations of BM physiology by cardiac dysfunction | |||
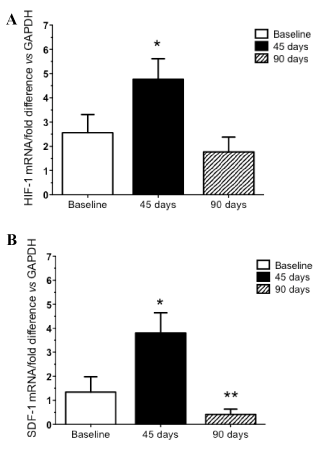
| The effect of a chronic state of myocardial ischemia on the BM was evaluated by studying the expression levels of HIF-1 and SDF-1 in BM MNCs. Using fresh BM aspirates; whole BM MNCs were isolated using a Ficoll gradient. We evaluated BM-MNCs mRNA expression of HIF-1 and SDF-1 for all animals at every time points. The expression of these motomorphogens was maximally unregulated 45 days following LAD instrumentation (P=0.008). As demonstrated in Figure 6, chronic myocardial ischemia produced a biphasic response in mRNA expression with HIF-1 (Figure 6-a) and SDF-1 (Figure 6-b) initially unregulated 45 days following the intervention. Conversely, a relative decrease in mRNA expression at the end of the study (at 90 days) was found for both HIF-1 and SDF-1. Compared to baseline, only SDF-1 showed a significant decrease in mRNA expression at 3 months (P=0.03). | |||
| Discussion | |||
| Mounting evidence from preclinical and clinical studies now support the concept that BM-derived cells are capable of inducing repair and, possibly, regeneration in various tissues and organs including the myocardium [27-30]. EPCs are a subtype of circulating BM-derived cells that have the ability to proliferate and differentiate into mature endothelial cells [31]. Previous studies have shown that this process is involved in the prevention of endothelial dysfunction and cardiovascular disease progression [17]. BM-derived progenitor cells from patients with CHF have reduced homing ability to sites of ischemia and poorer neovascularisation potential in models of hind limb ischemia [18]. Kissel et al. [21] recently reported that a functional exhaustion of BM-derived EPCs could account for the unfavorable LV remodeling process in patients with ischemic heart failure [21]. However, several questions remain concerning the kinetics of BMderived EPCs mobilization at various clinical stages of CHF. Therefore, the objective of this study was to explore the functional capacity of BMderived EPC in a reproducible swine model of myocardial ischemia. Our aims were also to investigate key underlying mechanisms involved in the mobilization of BM-derived EPCs in patients with progressive CHF. | |||
| The major findings of the present study are: (1) chronic severe stenosis of the proximal LAD using the Delran occluder creates a reproducible chronic ischemic myocardium swine model, in accordance with prior evidence [32,33]; (2) progression towards myocardial ischemia was associated with an environment of active ischemia resulting in a decrease in the number of circulating EPCs at 3 months compared to baseline; (3) functional exhaustion of BM-derived EPCs was observed as attested by a decreased chemo tactic capacity of EPCs to SDF-1 and VEGF; (4) quantitative mRNA expression of HIF-1 and SFD-1 measured in the BM was maximally unregulated 45 days following LAD instrumentation, with a subsequent decrease of both genes at 90 days. | |||
| EPC mobilization is a complex and multifaceted phenomenon with numerous elicited pathways according to injurious conditions. Among others, the stromal-derived factor 1 (SDF-1)/CXCR4 axis regulates the chemo taxis of BM progenitor cells through activation and regulation of integrin molecules [34]. New hormonal hypothesis have recently emerged and proposed evidence showing that mineral corticoids could induce accelerated senescence of progenitor cells leading to their reduced survival in HF [35]. Standard mechanisms hypothesized also that the up regulation of SDF-1 during hypoxia can be facilitated by the transcription factor hypoxic-inducible factor 1 (HIF-1) [36]. SDF-1 activation is promoted by HIF-1 expression, resulting in an increased expression of SDF-1 in ischemic tissues [36]. Upon entering the BM environment, SDF-1 activates several proteinases expressed by BM stromal cells such as matrix metalloproteinase 9 (MMP-9) [37]. MMP-9 is closely involved in the egress of BM-derived EPCs into the circulation through BM sinusoids [38]. In the present study, there was a biphasic response in mRNA expression of both HIF-1 and SDF-1 genes. These genes were initially up regulated 45 days following the intervention, while we noted a subsequent relative decrease in mRNA expression of SDF-1 at the end of the study. This differential mRNA expression could be explained by a modification in BM-cell biology leading to a functional migratory exhaustion of BM-derived EPCs mobilized in the setting of chronic ischemic myocardial injury. Hence, the local microenvironment, also termed the «stem cell niche», provides essential cues to maintain stem and progenitor cell functions and direct cell fate decisions in the bone marrow. A disturbed niche might lead to cell exhaustion [39]. | |||
| Clinical correlation | |||
| To our knowledge, this is the first study to reproducibly document in a large animal model a potential link for the «BM-heart axis». We proposed that chronic myocardial ischemia could alter the BM physiology through an adverse alteration of BM-derived EPC number and migratory capacity in the setting of myocardial hibernation/ ischemia. Our aim was to study the kinetics of BM-derived EPCs mobilization according to various stages of progressive ischemia in a clinically relevant, large animal model of myocardial ischemia. We postulate that, in the setting of chronic ischemia, there is a state of BM functional exhaustion (as evidenced by decreased EPC numbers and chemo tactic properties) that could partially be mediated through a relative decrease in HIF-1 and SDF-1 expression over time. The concept of interdependence of the BM and the heart, supported by our findings, is consistent with previous reports on BM-derived cardiac progenitors in chronic ischemic cardio myopathies [40,41]. The present observations have important conceptual implications in the understanding of BMdependent cardiac repair and regeneration, and provide a rationale for further studies aimed at optimizing therapeutic cardiac cellbased therapy by modulation of BM-derived progenitors or BM microenvironment to promote progenitor cell mobilization. This large model provides a reproducible translational platform to investigate the role of more established EPCs-modulating agents such as granulocyte colony stimulating factor [42], satins [43] or novel pharmacological strategies to mobilize BM-derived cells. Finally, our findings highlight that the BM functional response (through quantitative and qualitative assessment of its progenitor cells i.e. EPCs in this study) may constitute a relevant clinical target, potentially amenable to pharmacological manipulation in order to promote cardiac healing after ischemic injury. | |||
| Limitations | |||
| This model has obvious limitations. It is an attempt to reproduce a chronic ischemic model of viable myocardium. Designed to isolate a phenomenon influenced by several natural variables (age, atherosclerosis), this translational large animal model is only still a partial reproduction of complex human pathologies. In this study, each animal is used as its own control overtime. However, the absence of a formal control group should be mentioned. We further used DES in order to evaluate the ischemic myocardium 3 months after the occlusion. Magnetic resonance imaging could also be considered in order to characterize this chronic ischemic model. | |||
| Conclusion | |||
| This study of altered BM-derived progenitor cell biology in the setting of chronic myocardial ischemia has shown that mechanisms driving the differences in BM-derived EPC mobilization can be investigated with a minis wine model of chronic myocardial ischemia. Progressive functional exhaustion of BM-derived EPCs occurs with time and is associated with a decreased migratory capacity related to a relative decrease in SDF-1 and HIF-1 mRNA expression. Thus, an interaction between the heart and the BM likely occurs. Understanding the basic cellular changes and potential functional impairment occurring in patients during the sequence of events leading to progressive CHF development will be critical when establishing the clinical relevance of the crosstalk in the BM-cardiac axis. | |||
| Acknowledgements | |||
| We thank M.P. Mathieu, S. Blanchet, J. Lebel, S. Gilligan, P. Geoffroy and D. Lauzier for their precious help in this study, and Dr. Yan Fen Shi for the echocardiographic analysis. Drs. Maltais, Ly and Perrault are scholars of the Fonds de la recherche en santé du Québec (FRSQ). Dr. Perrault is supported by the National Institute of Health (NIH), the Canadian Institutes of Health Research (CIHR), and the Department of Surgery, University of Montreal. Dr Ly is supported by the Heart and Stroke Foundation of Canada (HSFC), the Cell Network of the FRSQ and the Stem Cell Network of Canada. Dr. Tardif holds the University of Montreal research chair in atherosclerosis and is supported by CIHR. | |||
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13768
- [From(publication date):
January-2011 - Apr 14, 2025] - Breakdown by view type
- HTML page views : 9122
- PDF downloads : 4646