Research Article Open Access
FTO Knockdown Decreases Phosphorylation of Tau in Neuronal Cells; A Potential Model Implicating the Association of FTO with Alzheimer 's Disease
Ryan T Pitman#, Jason T Fong, Amanda L Stone, Joseph T Devito and Neelu Puri*#
Department of Biomedical Sciences, University of Illinois College of Medicine, Rockford, Illinois, USA
#Both Authors have contributed equally to this work
- Corresponding Author:
- Neelu Puri
Department of Biomedical Sciences
University of Illinois College of Medicine
1601 Parkview Avenue, Rockford
Illinois 61107, USA
Tel: 815-395-5678
Fax: 815-395-5666
E-mail: neelupur@uic.edu
Received date: August 08, 2013; Accepted date: September 17, 2013; Published date: September 30, 2013
Citation: Pitman RT, Fong JT, Stone AL, Devito JT, Puri N (2013) FTO Knockdown Decreases Phosphorylation of Tau in Neuronal Cells; A Potential Model Implicating the Association of FTO with Alzheimer’s Disease. J Alzheimers Dis Parkinsonism 3:125. doi: 10.4172/2161-0460.1000125
Copyright: © 2013 Pitman RT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Recent genetic studies identify variants within the Fat Mass and Obesity Associated gene (FTO) as important contributors to the development of obesity and suggest a potential link between obesity-associated FTO variants and Alzheimer’s disease (AD). The mechanisms of association regarding FTO and AD are currently unclear; however, obesity is thought to be a well known risk factor for AD. In Alzheimer’s disease hyperphosphorylation of the Tau protein at certain epitopes causes the formation of neurofibrillary tangles due to microtubule collapse. AMP-activated protein kinase (AMPk) is known to phosphorylate Tau, and previous studies have proposed a relationship between FTO knockdown and phosphorylated AMPk (pAMPk). In this study we show that siRNA mediated knockdown of FTO expression in SH-SY5Y neuroblastoma cells decreases Tau phosphorylation. This novel finding suggests the potential for a cellular mechanism that may link FTO function with the development of AD.
Keywords
FTO; Tau phosphorylation; Alzheimer’s disease; Obesity; AMPk
Introduction
Obesity and its metabolic consequences are well-known to have wide-ranging effects in multiple body systems. Increasing evidence supports a strong link between obesity in middle-aged and elderly individuals, and the development of Alzheimer’s disease (AD) [1]. Although the pathophysiological mechanisms connecting obesity and AD are unclear, previous studies suggest a role for leptin [2], hyperglycemia [3], and mitochondrial dysfunction [4] in the pathogenesis of AD.
Variants within the FTO gene can contribute to the development of obesity-related traits and obesity worldwide [5], presumably through an alteration in energy intake [6-8]. It has been proposed that individuals who are heterozygous or homozygous for FTO variants exhibit increased expression of FTO transcript levels, which may be responsible for increased BMI and obesity related traits [9].
Recent studies suggest a genetic association between variants within the Fat Mass and Obesity Associated gene (FTO) and Alzheimer’s disease (AD) [10]. FTO is highly expressed in the human brain [11], and a loss of function mutation may lead to structural and functional brain abnormalities in humans [12]. Specifically, one study found that FTO risk allele carriers have a decreased frontal lobe brain volume, when compared to non-carriers of the FTO risk allele [13]. Furthermore, other studies have shown decreased word fluency in obese elderly men that have a FTO risk allele as well [14]. Unfortunately, there is no present cellular mechanism linking FTO and AD. However, our study presents a potential model linking FTO knockdown and the development of AD.
FTO may also be functionally coupled to the BDNF-NTRK2 signaling pathway, which is known to be involved in the pathophysiology of AD and metabolic homeostasis in humans [15,16]. Furthermore, recent studies have suggested neuropeptide Y (NPY) to have a possible role in neuroprotection from AD associated factors such as amyloid beta, through its involvement with BDNF [17]. Our previous studies investigating FTO and energy-balance related proteins, such as NPY [18], led us to look into the effect of FTO on the phosphorylation of Tau. This original idea was further developed into the major focus of this model, which attempts to link FTO knockdown to decreased phosphorylation of Tau implicating the potential role of FTO in AD.
The Tau protein stabilizes and maintains the normal morphology of microtubules in neurons of the central nervous system. However, Tau proteins are not able to stabilize microtubules when phosphorylated, which can lead to the development of neurodegenerative diseases such as AD. BDNF, AMPk, and Akt are known to associate with the phosphorylation of Tau, an important component of neurofibrillary tangles and a mediator of neuronal destruction when hyperphosphorylated [16,19,20,21]. In particular, previous studies have indicated that AMPk can directly phosphorylate Tau at Ser-396 [19,22]. Other studies have demonstrated that increased levels of Akt may correspond with a significant increase in levels of total Tau and hyperphosphorylated Tau [21]. Our lab has also shown a link between FTO knockdown and decreased levels of both pAkt and pAMPk, which serve roles in glucose metabolism and sensing metabolic intracellular energy levels, respectively [18,22,23]. In the present study, we postulate a connection between FTO expression and Tau phosphorylation, through activated AMPk.
Our findings agree with previous studies, and indicate a possible connection between FTO variants and AD through a proposed cellular mechanism [10,13]. Overall, we hypothesize that FTO knockdown may play a role in the development of Alzheimer’s disease, by reducing phospho-Tau levels.
Materials and Methods
SH-SY5Y neuroblastoma cells are a well established model for the study of Tau phosphorylation and AD pathophysiology [24]. In this study, SH-SY5Y cells were differentiated as described in one of our previous publications [18]. Prior to differentiation, knockdown of FTO expression was performed with the use of siRNA. SH-SY5Y cells were transfected with either anti-FTO-siRNA from Dharmacon (Cat no: D-004159-02; Lafayette, CO) or control siRNA (Cat No: 6568S; Cell Signaling Technology, Danvers, MA) using Dharmafect 2 transfection reagent as described previously [18].
To confirm appropriate down regulation of FTO in both undifferentiated (naive) and differentiated cells, qPCR was performed at several time points following transfection, and one week after induction of differentiation. Immunoblotting was performed as described previously [18,25], utilizing anti-FTO antibody from Novus Biologicals (Cat No: NB110-59758, Littleton, CO) and anti-β-Actin (Cat No: 5441) antibody from Sigma (St. Louis, MO). Sheep anti-mouse IgG (NA931V) and donkey anti-rabbit IgG (NA934V) secondary antibodies were obtained from GE healthcare (Piscataway, NJ). While probing for Tau phosphorylation, the anti-Tau antibody (Cat No: 4019; Cell Signaling, Danvers, MA) and anti-phospho-Tau (phospho-Ser-396) antibody (Cat No: 2934-1, Epitomics, Burlingame, CA) were used. Densitometry was performed with Image J. Experiments for each condition were repeated three times, and a representative result was shown.
Results
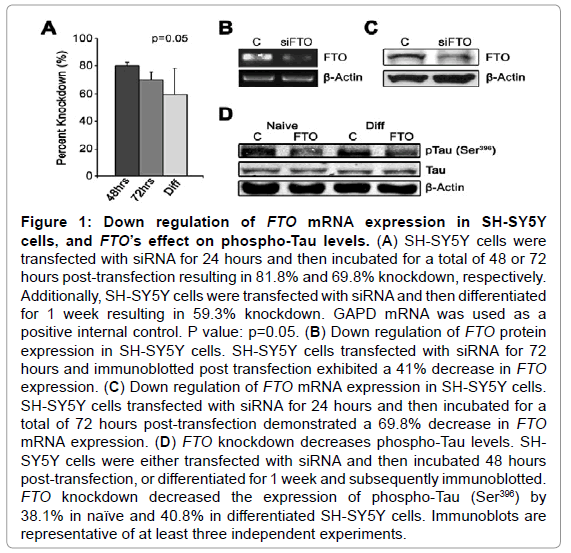
Naive SH-SY5Y cells treated with siRNA against FTO exhibited 81.8% and 69.8% reduction in FTO mRNA expression at 48 and 72 hours, respectively (Figure 1A). Sustained knockdown of FTO mRNA was validated after one week of differentiation (59.3%) (Figure 1A). FTO mRNA expression was found to be decreased by 69% after 72 hours of transfection (Figure 1B) with a similar result seen by immunoblotting, as protein expression was found to be decreased by 41% at 72 hours (Figure 1C). To determine a possible mechanism linking FTO and AD, FTO-siRNA transfected cells were immunoblotted for the presence of Tau phosphorylation. This phosphorylation could contribute to hyperphosphorylation of Tau, which is a known precursor to the development of the neurofibrillary tangles present in AD neurons [26]. In both naive and differentiated SH-SY5Y cells, FTO-siRNA transfection resulted in a significant decrease in Tau phosphorylation at Ser-396 (Figure 1D) (38% and 41%, respectively).
Figure 1: Down regulation of FTO mRNA expression in SH-SY5Y cells, and FTO’s effect on phospho-Tau levels. (A) SH-SY5Y cells were transfected with siRNA for 24 hours and then incubated for a total of 48 or 72 hours post-transfection resulting in 81.8% and 69.8% knockdown, respectively. Additionally, SH-SY5Y cells were transfected with siRNA and then differentiated for 1 week resulting in 59.3% knockdown. GAPD mRNA was used as a positive internal control. P value: p=0.05. (B) Down regulation of FTO protein expression in SH-SY5Y cells. SH-SY5Y cells transfected with siRNA for 72 hours and immunoblotted post transfection exhibited a 41% decrease in FTO expression. (C) Down regulation of FTO mRNA expression in SH-SY5Y cells. SH-SY5Y cells transfected with siRNA for 24 hours and then incubated for a total of 72 hours post-transfection demonstrated a 69.8% decrease in FTO mRNA expression. (D) FTO knockdown decreases phospho-Tau levels. SHSY5Y cells were either transfected with siRNA and then incubated 48 hours post-transfection, or differentiated for 1 week and subsequently immunoblotted. FTO knockdown decreased the expression of phospho-Tau (Ser396) by 38.1% in naïve and 40.8% in differentiated SH-SY5Y cells. Immunoblots are representative of at least three independent experiments.
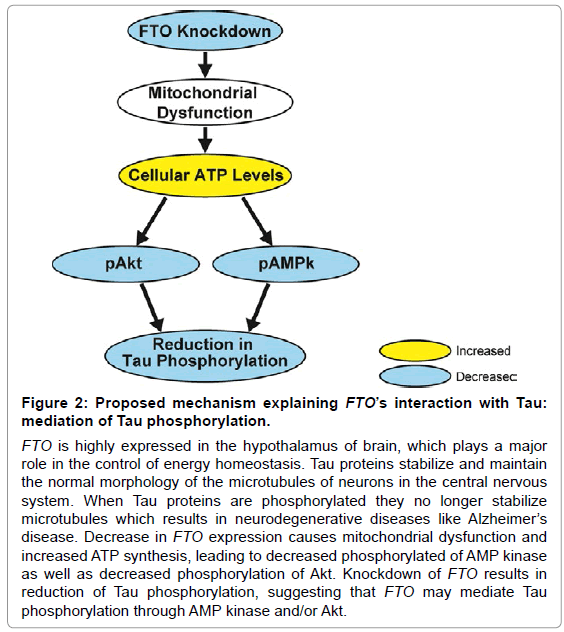
Figure 2: Down regulation of FTO mRNA expression in SH-SY5Y cells, and FTO’s effect on phospho-Tau levels. (A) SH-SY5Y cells were transfected with siRNA for 24 hours and then incubated for a total of 48 or 72 hours post-transfection resulting in 81.8% and 69.8% knockdown, respectively. Additionally, SH-SY5Y cells were transfected with siRNA and then differentiated for 1 week resulting in 59.3% knockdown. GAPD mRNA was used as a positive internal control. P value: p=0.05. (B) Down regulation of FTO protein expression in SH-SY5Y cells. SH-SY5Y cells transfected with siRNA for 72 hours and immunoblotted post transfection exhibited a 41% decrease in FTO expression. (C) Down regulation of FTO mRNA expression in SH-SY5Y cells. SH-SY5Y cells transfected with siRNA for 24 hours and then incubated for a total of 72 hours post-transfection demonstrated a 69.8% decrease in FTO mRNA expression. (D) FTO knockdown decreases phospho-Tau levels. SHSY5Y cells were either transfected with siRNA and then incubated 48 hours post-transfection, or differentiated for 1 week and subsequently immunoblotted. FTO knockdown decreased the expression of phospho-Tau (Ser396) by 38.1% in naïve and 40.8% in differentiated SH-SY5Y cells. Immunoblots are representative of at least three independent experiments.
Discussion
Interestingly, in the present study, FTO appears to regulate Tau phosphorylation. In addition, FTO knockdown only affects phospho- Tau (Ser-396) expression without a change in total Tau expression, suggesting a transcription-independent effect of FTO expression on phosphorylation of Tau. Ser-396 has been previously noted as a major epitope involved in AD [27]. Thus, when Tau is phosphorylated at this site, the protein’s capabilities in microtubule binding, assembly, and stabilization are greatly diminished [28-30]. However, the mechanistic link between FTO gene expression and Tau phosphorylation in neuronal cells is unknown. Previous studies in our lab show a connection between FTO expression and the phosphorylation of AMPk and Akt in SH-SY5Y cells [18]. Other studies demonstrate that AMPk activation may contribute to Tau phosphorylation in AD patients [19]. These previous investigations indicate a potential link between FTO and Tau phosphorylation, through AMPk activation in AD patients.
FTO is highly expressed in the hypothalamus, an area of the brain that plays a major role in the control of energy homeostasis. Thus, we hypothesize that FTO has a significant role in the control of cellular energy balance, including mitochondrial function [31] and glucose homeostasis; both of which are known to be deregulated in AD [3,4]. FTO knockdown may be able to reduce the phosphorylation of Tau, through disruption of cellular energy balance, subsequent increases in ATP synthesis, and decreases in levels of pAMPk and pAkt. Thus, AMPk and Akt may have the potential to serve as a link in this proposed pathway of interaction between FTO and phospho-Tau [18,22] (Figure 2). Overall, this proposed model may give insight into one of the many mechanisms involved in the pathophysiology of AD. However, further investigations are vital for the understanding of interactions between FTO, AMPk, Akt and Tau.
While FTO is known to be a transcriptional coactivator [32], it seems unlikely that FTO directly regulates the expression of either AMPk or Tau. FTO knockdown affects the phosphorylation of both AMPk and Akt, as shown in our previous study, without significant effects on the total levels of AMPk or Akt [18]. In the present study we found that FTO knockdown decreases the phosphorylation of Tau, however, there is no effect on total protein levels of Tau. It is unlikely that FTO co-localizes with either AMPk or Tau because FTO is primarily confined to the nucleus [33]. However, FTO codes for a 2-oxogluterate dependent nucleic acid demethylase that selectively demethylates 3-methylthymine and 3-methyluracil in single stranded DNA and RNA, respectively [34]. We speculate that FTO may be able to indirectly influence AMPk, Akt, Tau and other related proteins through demethylation reactions.
Additional investigations regarding the link between FTO variants, AD pathogenesis and other brain abnormalities, including reduced brain volume and defects in central nervous system development, will further contribute to our understanding of the involvement of FTO with AD. While our model is suggestive, we believe that it is a novel concept, and may impact future research concerning the relationship between FTO, obesity and AD. In future studies, we plan to reinforce and expand upon our proposed model, which includes investigation of other proteins that mediate down regulation of phospho-Tau through FTO. It is our hope that these observations will set the ground work and open new fields of research to further define the pathogenesis of AD.
Acknowledgements
The authors have no conflicts of interest to report.
References
- Lee EB (2011) Obesity, leptin, and Alzheimer's disease. Ann N Y Acad Sci 1243: 15-29.
- Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, et al. (2009) Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA 302: 2565-2572.
- Kim B, Backus C, Oh S, Feldman EL (2013) Hyperglycemia-induced Tau cleavage in vitro and in vivo: a possible link between diabetes and Alzheimer's disease. J Alzheimers Dis 34: 727-739.
- Zhu X, Perry G, Smith MA, Wang X (2013) Abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J Alzheimers Dis 33 Suppl 1: S253-262.
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, et al. (2007) A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316: 889-894.
- Speakman JR, Rance KA, Johnstone AM (2008) Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity (Silver Spring) 16: 1961-1965.
- Wardle J, Carnell S, Haworth CM, Farooqi IS, O'Rahilly S, et al. (2008) Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab 93: 3640-3643.
- Church C, Moir L, McMurray F, Girard C, Banks GT, et al. (2010) Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet 42: 1086-1092.
- Berulava T, Horsthemke B (2010) The obesity-associated SNPs in intron 1 of the FTO gene affect primary transcript levels. Eur J Hum Genet 18: 1054-1056.
- Keller L, Xu W, Wang HX, Winblad B, Fratiglioni L, et al. (2011) The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer's disease risk: a prospective cohort study. J Alzheimers Dis 23: 461-469.
- Dina C, Meyre D, Gallina S, Durand E, Körner A, et al. (2007) Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 39: 724-726.
- Boissel S, Reish O, Proulx K, Kawagoe-Takaki H, Sedgwick B, et al. (2009) Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am J Hum Genet 85: 106-111.
- Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, et al. (2010) A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc Natl Acad Sci U S A 107: 8404-8409.
- Benedict C, Jacobsson JA, Rönnemaa E, Sällman-Almén M, Brooks S, et al. (2011) The fat mass and obesity gene is linked to reduced verbal fluency in overweight and obese elderly men. Neurobiol Aging 32: 1159.
- Rask-Andersen M, Almén MS, Olausen HR, Olszewski PK, Eriksson J, et al. (2011) Functional coupling analysis suggests link between the obesity gene FTO and the BDNF-NTRK2 signaling pathway. BMC Neurosci 12: 117.
- Zhang F, Kang Z, Li W, Xiao Z, Zhou X (2012) Roles of brain-derived neurotrophic factor/tropomyosin-related kinase B (BDNF/TrkB) signalling in Alzheimer's disease. J Clin Neurosci 19: 946-949.
- Croce N, Gelfo F, Ciotti MT, Federici G, Caltagirone C, et al. (2013) NPY modulates miR-30a-5p and BDNF in opposite direction in an in vitro model of Alzheimer disease: a possible role in neuroprotection? Mol Cell Biochem 376: 189-195.
- Pitman RT, Fong JT, Billman P, Puri N (2012) Knockdown of the fat mass and obesity gene disrupts cellular energy balance in a cell-type specific manner. PLoS One 7: e38444.
- Vingtdeux V, Davies P, Dickson DW, Marambaud P (2011) AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer's disease and other tauopathies. Acta Neuropathol 121: 337-349.
- Ittner LM, Götz J (2011) Amyloid-β and tau--a toxic pas de deux in Alzheimer's disease. Nat Rev Neurosci 12: 65-72.
- Pei JJ, Khatoon S, An WL, Nordlinder M, Tanaka T, et al. (2003) Role of protein kinase B in Alzheimer's neurofibrillary pathology. Acta Neuropathol 105: 381-392.
- Cai Z, Yan LJ, Li K, Quazi SH, Zhao B (2012) Roles of AMP-activated protein kinase in Alzheimer's disease. Neuromolecular Med 14: 1-14.
- Bae SS, Cho H, Mu J, Birnbaum MJ (2003) Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem 278: 49530-49536.
- Mookherjee P, Johnson GV (2001) Tau phosphorylation during apoptosis of human SH-SY5Y neuroblastoma cells. Brain Res 921: 31-43.
- Puri N, Ahmed S, Janamanchi V, Tretiakova M, Zumba O, et al. (2007) c-Met is a potentially new therapeutic target for treatment of human melanoma. Clin Cancer Res 13: 2246-2253.
- Chung SH (2009) Aberrant phosphorylation in the pathogenesis of Alzheimer's disease. BMB Rep 42: 467-474.
- Hu YY, He SS, Wang X, Duan QH, Grundke-Iqbal I, et al. (2002) Levels of nonphosphorylated and phosphorylated tau in cerebrospinal fluid of Alzheimer's disease patients : an ultrasensitive bienzyme-substrate-recycle enzyme-linked immunosorbent assay. Am J Pathol 160: 1269-1278.
- Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, et al. (1993) Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron 10: 1089-1099.
- Song JS, Yang SD (1995) Tau protein kinase I/GSK-3 beta/kinase FA in heparin phosphorylates tau on Ser199, Thr231, Ser235, Ser262, Ser369, and Ser400 sites phosphorylated in Alzheimer disease brain. J Protein Chem 14: 95-105.
- Wang JZ, Wu Q, Smith A, Grundke-Iqbal I, Iqbal K (1998) Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS Lett 436: 28-34.
- Bravard A, Lefai E, Meugnier E, Pesenti S, Disse E, et al. (2011) FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes 60: 258-268.
- Wu Q, Saunders RA, Szkudlarek-Mikho M, Serna Ide L, Chin KV (2010) The obesity-associated Fto gene is a transcriptional coactivator. Biochem Biophys Res Commun 401: 390-395.
- Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, et al. (2007) The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318: 1469-1472.
- Jia G, Yang CG, Yang S, Jian X, Yi C, et al. (2008) Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett 582: 3313-3319.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 15359
- [From(publication date):
December-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10639
- PDF downloads : 4720