Research Article Open Access
Fabricating Functional Ti-Alloy Biomedical Implants by Additive Manufacturing Using Electron Beam Melting
Lawrence E Murr1,2*, Sara M Gaytan1,2, Edwin Martinez1,2, Frank R Medina2 and Ryan B Wicker21Department of Metallurgical and Materials Engineering, The University of Texas at El Paso, El Paso, TX 79968, USA
2W. M. Keck Center for 3D Innovation, The University of Texas at El Paso, El Paso, TX 79968, USA
- Corresponding Author:
- Dr. Lawrence E Murr
Department of Metallurgical and Materials Engineering
The University of Texas at El Paso
El Paso, TX 79968, USA
Tel: +915-747-6929
Fax: +915-747-8036
E-mail: lemurr@utep.edu
Received date: March 07, 2012; Accepted date: March 24, 2012; Published date: March 26, 2012
Citation: Murr LE, Gaytan SM, Martinez E, Medina FR, Wicker RB (2012) Fabricating Functional Ti-Alloy Biomedical Implants by Additive Manufacturing Using Electron Beam Melting. J Biotechnol Biomaterial 2:131. doi:10.4172/2155-952X.1000131
Copyright: © 2012 Murr LE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
A wide range of biocompatible and biofunctional implant devices from dental screw posts to porous hip stems designed for stress compatibility, cementless fixation by bone cell ingrowth, and long-term infection defense employing antibacterial, nanoparticulate silver are illustrated in this review of contemporary biomaterial appliances. Emphasis is placed on Ti-6Al-4V, but new alloys providing low Young’s modulus for stress-shielding reduction as well as Co-base alloys are highlighted. Open-cellular structure monoliths fabricated by additive manufacturing using electron beam melting are illustrated along with observations of their microstructures observed by light optical and electron microscopies and associated mechanical properties; including hardness, tensile and fatigue strength.
Keywords
Open-cellular structures; Ti and Co-base alloys; Electron beam melting; Biomedical implants; Light and electron microscopy; Structure-property relationships
Introduction
Metal prostheses or implants in the broadest sense have been utilized in the form of screws, plates, rods and combined, complex configurations manufactured from cast or wrought precursors. Functionality can involve a wide range of structural replacement issues as well as issues relating to biocompatibility in its broadest sense: toxicity, mechanics, corrosion, wear, fatigue, etc. All involve skeletal remediation or replacement: skull plates, dental replacements and augmentation, joint replacements, bone replacements and strengthening augmentation, etc. Simultaneous and continual improvement in both implant materials in device or appliance design and clinical implantation techniques have led to generally wellaccepted and predictable dental and medical procedures [1-3]. These have also involved the optimization of osseointegration whereby living tissue, especially bone tissue, and the implant become structurally and functionally connected.
Unlike any metal or alloy implant appliance, bone is a complex viscoelastic composite of living material capable of revision or remodeling of mature tissue by new bone tissue: self-repair. In a biomechanical context, a distinction can be made between the mechanical behavior of bone tissue as a material, and the whole bone structure as it relates to mechanical behavior. As a dynamic, living tissue regime, bone microstructure, macrostructure and shape (geometry) are continuously adjusting to optimize this structural framework. This process is compromised by disease or metabolic disorders which alter or compromise mineral components of bone microstructure, especially calcium hydroxyapatite (Ca10 (PO4)6 (OH)2) which composes the bone composite microarchitecture [4-9].
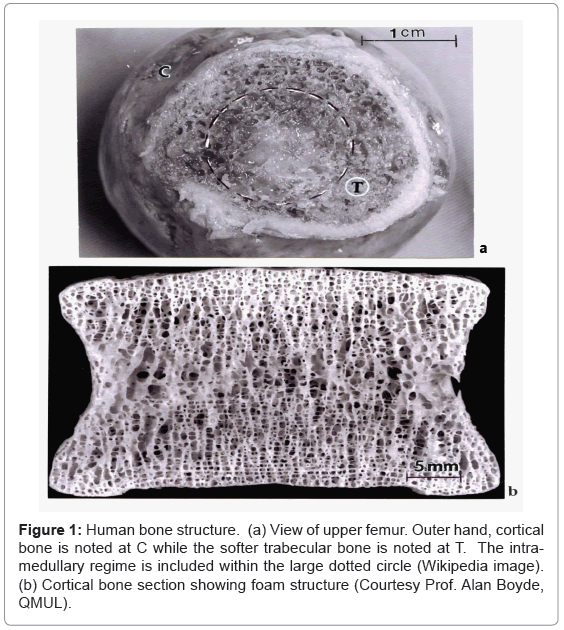
The complexity of bone is shown generally in the illustrations provided in figure 1. Figure 1a shows an upper view of the human femur bone composed of central cancellous (soft) or spongy bone structure which is functionally graded into the outer cortical or hard bone whose porous, foam structure is shown in the cortical section in figure 1b. The soft femoral bone center or intramedullary region shown by the dashed circle in figure 1a also contains a propensity of vascular (blood) cell structures. In its natural state, the femur carries its external loads primarily in the cortical (C) (outer bone) regime where, in contrast to the softer trabecular (T) bone, the stiffness or elastic (Young’s) modulus (E) can be at least on order of magnitude greater: < 2 GPa for trabecular (T) bone in contrast to ~20 GPa for cortical (C) bone. Bone density (and corresponding porosity) apparent in figure 1b normally varies from 1.7 to 2.1 g/cm3. The associated bone compressive strength (or compressive yield stress) varies from 0.13 to 0.18 GPa, and the bending strength is generally represented by E/100 [1,6].
Figure 1: Human bone structure. (a) View of upper femur. Outer hand, cortical bone is noted at C while the softer trabecular bone is noted at T. The intramedullary regime is included within the large dotted circle (Wikipedia image). (b) Cortical bone section showing foam structure (Courtesy Prof. Alan Boyde, QMUL).
As evident in figure 1b, the foam structure of bone is characterized by a ligament network whose length and thickness (or cross-section) can change geometrically. The actual microstructure of the ligaments, composed of lamellar collagen fibrils ~200 nm in diameter intermixed with calcium hydroxyapatite crystals ~20 nm in size; often arranged into mineral rods ~100 nm across [10,11]. These intercalated lamellar composites allow for complex ligament stresses: bending, torsion, compression and stretching- to accommodate complex skeletal loading. In addition, the open-cellular, foam structure of bone (Figure 1) optimizes impact energy absorption as well as thermal conduction (or skeletal heat flow) and fluid flow and cell migration [9,12-14]. Of course the structural and microstructural features implicit in figure 1 will change to accommodate the skeletal functionality throughout the body: skull, spine, long and short bone sections, etc.
The use of metals and alloys to augment or replace bone and bone function in more modern times (the past 2 or 3 decades) has been dominated by Ti and its alloys (especially Ti-6Al-4V), stainless steels (especially low carbon 316 (316L)), and Co-base alloys (especially Co- 29Cr-6Mo-0.22C) [15]. These alloys exhibit low or negligible toxicity, low corrosion and correspondingly high corrosion resistance and high strength [16,17]. The Co-base alloy of choice noted also has exceptional wear resistance since its stiffness or elastic (Young’s) modulus (210 GPa) is nearly twice that for Ti-alloys.
It can be noted in figure 1a that in the case of the femur, which supports a significant portion of the body’s load, that the load is distributed over a wide range of load-bearing structure with a modulus ranging from < 0.2 at the bone center to ~20 GPa in the outer cortex. Inserting a metal rod or stem into the dashed circled area of the femur in Figure 1(a) would shift a significant part of the load-carrying capacity to the intramedullary stem where, for Ti-6Al-4V for example, the lowest stiffness of ~0.2 GPa would be replaced with a core stiffness of ~110 GPa; a factor of 550. As a consequence, the bone is subjected to much reduced stresses, a phenomenon referred to as stress shielding. In addition, the vascular system composing the intramedullary regime is eliminated and the stress reduction relative to the natural load-bearing regime will cause the bone to adapt by reducing its mass by internal remodeling (becoming more porous (Figure 1b), or by becoming thinner (external remodeling or resorption) [14,18]. However this practice of introducing an intramedullary stem to correct severe femur fracture is much more reliable than adding plates to the outer cortex using screws, which have been especially prone to fatigue failure as a consequence of cyclic loading resulting from severe walking gates which shift the load from side-to-side.
In many cases, metal implant fixation strategies have involved the use of polymeric (acrylic) cements, such as polymethylmethacrylate (PMMA), but these cements are also prone to fatigue and loosening of the implant in response to cyclic loading [19,20]. A more effective strategy since about 1975 has involved the application of porous surface coating features (including sintered powders or wire sponge) to metal implants in order to allow bone cell ingrowth to provide biological fixation as a part of bone remodeling [21-23]. However natural oxides such as TiO2 on the surface of Ti and Ti-alloys also provide a highly biocompatible surface which permits effective osseointegration especially for endosseous dental implants in the form of screws, rods, posts or blades [24].
It seems to be apparent from figure 1 that effective implant strategies which might account for the multi-functional features of bone should focus on fabricating novel foam or open-cellular metal structures. These would address the range of osseointegration phenomena implicit in the previous discussions.
Ideal open cellular structures: foam model
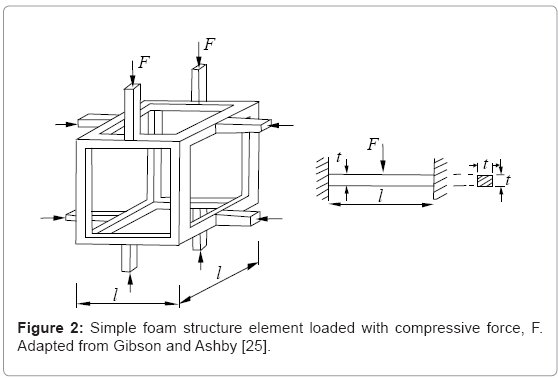
Since the basic deformation mechanism of foams in general is bending dominated, Gibson and Ashby [25] considered that foam such as the bone structure shown in figure 1b consists of a network of short beams or struts. These are often referred to as Timoshenko-type beams [26] whose behavior is described by shear deformation and rotational inertia effects. In this simple, classical mechanics representation shown reproduced in figure 2, relative density (ρ/ρs; where ρs is the fully solid density) for the cubic cell shown as short, connected struts, and the second moment of area of a rectangular strut member, I, are related to the beam member (or strut) dimensions by [25]:
ρ/ρs∝ (t/l)2, (1)
I ∝t4 ; (2)
where t and l are the strut dimensions shown in figure 2. The corresponding elastic (Young’s) modulus or stiffness for the opencellular element shown in figure 2 is given by [25,26]:
E= σ/E= C1EsI/l4= C1Est4/l4, (3)
where from Equation (1):
E/Es= C1 (ρ/ρs)2; (4)
where Es is the fully dense (solid) modulus, and C1 is a constant. This ideal foam or open-cellular structure model has been demonstrated to characterize a wide range of polymeric foams, aluminum and aluminum alloy foams, as well as other metallic, open-cellular structures [25,27,28].
The model in Figure 2 represents a unit cell which can be the basis for a periodic structure such as a lattice block [29] or reticulated mesh structure with high stiffness and strength, with only a fraction of the solid material weight [30]. However, the irregular, stochastic networks representing bone (Figure 1b) are generated either in a regular manner with the help of Kelvin cells, (or regular-faced convex polyhedra), or irregularly by irregular-faced polyhedra with connecting edges (ligaments) having triangular or distorted triangular cross-sections rather than the more regular circular or rectangular cross-sections for struts (Figure 2) [31,32].
Fabrication of open-cellular structures by electron beam melting (ebm)
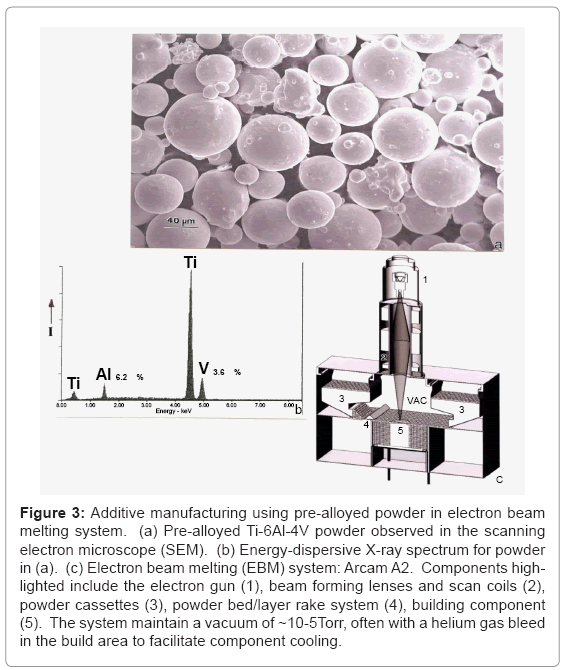
The EBM System: Figure 3a shows a typical Ti-6Al-4V (nominal) atomized (in argon) powder having an average diameter of ~40 μm, while figure 3b illustrates a typical energy-dispersive (X-ray) spectrum for this powder. Figure 3c illustrates a general schematic for the Arcam A2 electron beam melting system where the powder represented in figure 3a is loaded into cassettes marked (3) and gravity fed to the build area (5) and raked (4) into a uniform layer roughly 3 particle diameters thick (~100 μm). The electron gun shown at (1) in Figure 3(c) generates an electron beam accelerated at 60 kV potential which is focused and scanned by electromagnetic lenses and scan coils in the system portion marked (2) in figure 3c. Each raked (4) layer is preheated by the beam in multiple x-y passes prior to the final melt scan. The preheat scan rate is roughly 104 mm/s while the melt scan is ~102 mm/s. By altering the scan strategies controlled by the beam focus, scan rate, beam current (or energy density) along with the scan width as shown by Thijs et al. [33] for laser beam melting, the microstructure and associated mechanical properties of fabricated components can be somewhat selectively adjusted. In addition, since Ti-6Al-4V represents a multiphase system (α, α+β, β, α’, etc.) cooling variances from the beta transus (994°C) can produce associated phases or phase mixtures [34] to be illustrated later.
Figure 3: Additive manufacturing using pre-alloyed powder in electron beam melting system. (a) Pre-alloyed Ti-6Al-4V powder observed in the scanning electron microscope (SEM). (b) Energy-dispersive X-ray spectrum for powder in (a). (c) Electron beam melting (EBM) system: Arcam A2. Components highlighted include the electron gun (1), beam forming lenses and scan coils (2), powder cassettes (3), powder bed/layer rake system (4), building component (5). The system maintain a vacuum of ~10-5Torr, often with a helium gas bleed in the build area to facilitate component cooling.
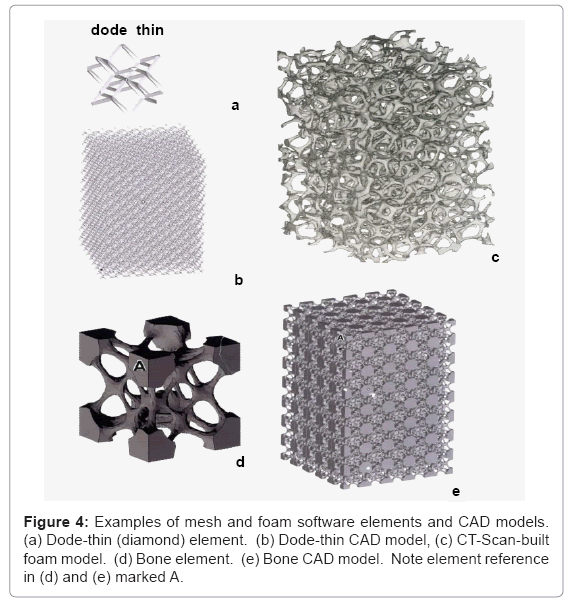
Cad models for open-cellular structure fabrication: There are numerous design and design software strategies available for creating CAD models for fabricating open-cellular structures-lattice, mesh, or foam-by EBM. These can involve structure generators or a lattice structure unit (cell) such as the simple cell represented in figure 2. By changing the cell size or strut dimensions (l or t in Figure 2) using appropriate instruction sets, the physical building of threedimensional solids is accomplished by the selective melting of each raked layer (Figure 3c) as directed in the CAD model. In this research, software packages included MATERIALISE/MAGICS (http://www. materialise.com) lattice structure units and SELECTIVE SPACE STRUCTURES (3s) (www.pro-fit.de) bone structure unit cell software for fabricating lattice structures. Foam structures were fabricated from CT-scans of aluminum alloy foams to create build element volumes which were embedded in CAD models allowing for cell size (or pore size) variations along with ligament cross-section variations which are essentially the same as the strut dimension variances used for mesh or lattice fabrication as implicit in figure 2 [27]. These model features are illustrated in figure 4. While CT-scan foam models (Figure 4c) represent a convenient approach to CAD software development, foam models can also be constructed from mathematical computer models as well [32,35].
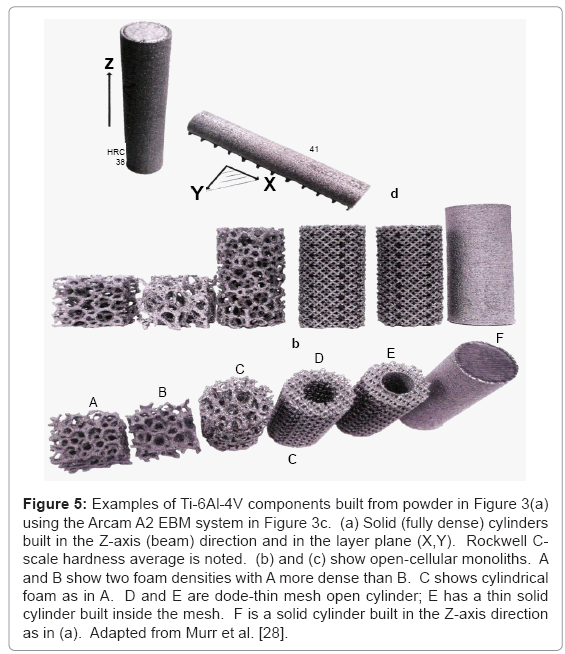
Figure 5 shows a wide range of components utilizing the dodethin lattice element shown in figure 4a and 4b, and the foam model illustrated in figure 4c. Figure 5a illustrates solid (fully dense) Ti- 6Al-4V cylinders built in the Z direction parallel to the electron beam in figure 3c, and in the plane of the powder bed (X-Y) in figure 3c. Corresponding Rockwell C-scale hardness (HRC) values (HRC 38 and HRC 41) are indicative of microstructure variations which result by different cooling rates associated with these build geometries. Figure 5b and 5c show complex, open-cellular monoliths fabricated by EBM.
Figure 5: Examples of Ti-6Al-4V components built from powder in Figure 3(a) using the Arcam A2 EBM system in Figure 3c. (a) Solid (fully dense) cylinders built in the Z-axis (beam) direction and in the layer plane (X,Y). Rockwell Cscale hardness average is noted. (b) and (c) show open-cellular monoliths. A and B show two foam densities with A more dense than B. C shows cylindrical foam as in A. D and E are dode-thin mesh open cylinder; E has a thin solid cylinder built inside the mesh. F is a solid cylinder built in the Z-axis direction as in (a). Adapted from Murr et al. [28].
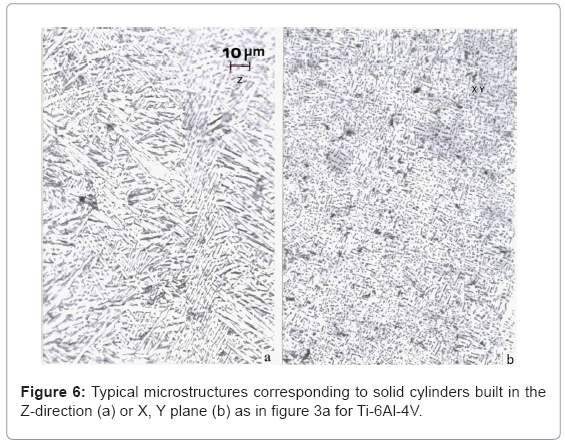
Ti-6Al-4V component microstructures: Figure 6 compares the microstructures typical for Ti-6Al-4V solid cylinders fabricated by EBM in the Z-axis or X, Y-axis configurations represented in figure 5a. The Z-axis microstructure in the vertical reference plane parallel to the build direction (Z) has shown in figure 6a represents the classical, acicular, Widmanstatten α-plate (grain) structure. The hcpα (a= 0.295 nm, c= 0.466 nm) is separated by a thin interfacial region (dark contrast) composed of bcc β (a= 0.33 nm). The typical hardness corresponding to this microstructure is shown for comparison with other grades and fabrication processing in table 1. In contrast, the X, Y-axis microstructures represented by figure 6b show a much
| Solid Material (r= 4.43 g/cm3) |
HV (GPa) |
HRC | YS (ksi) |
UTS (ksi) |
YS (GPa) |
UTS (GPa) |
Elongation (%) |
| Wrought (1) (acicular a) |
3.8 | 48 | 170 | 178 | 1.17 | 1.23 | 12 |
| Wrought (2) (equiaxeda/b) |
4.3 | 52 | 177 | 187 | 1.22 | 1.29 | 14 |
| ASTM Grade 5 (nominal) |
--- | 37 | 131 | 145 | 0.90 | 1.00 | 15 |
| EBM Vertical * Cylinder (Z) |
3.6 | 38 | 149 | 161 | 1.03 | 1.11 | 13 |
| EBM Horizontal * Cylinder (X) |
4.1 | 41 | 158 | 166 | 1.09 | 1.14 | 9 |
| EBM Horizontal * Cylinder (Y) |
--- | --- | 161 | 169 | 1.11 | 1.17 | 12 |
Table 1: Comparative mechanical properties for Ti-6Al-4V
refined distribution of β fragments in the α matrix which is roughly 10 percent harder and stronger (Table 1). These comparative cylindrical component geometries are characterized by variations in heating and cooling, where the X, Y-built components cool more rapidly than the Z-axis built components.
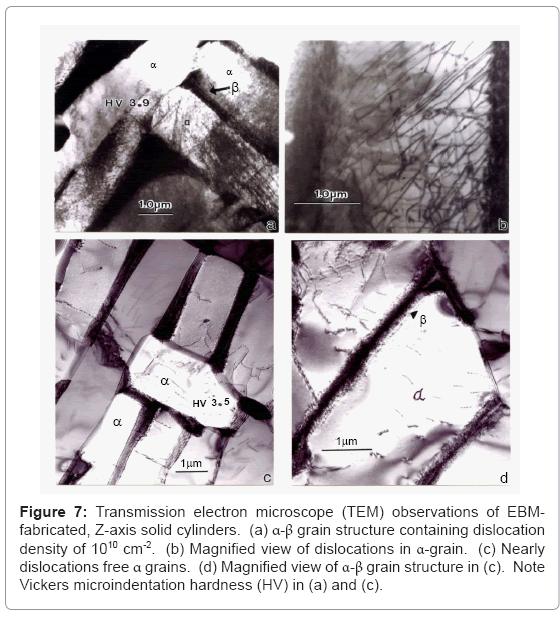
Figure 7 compares dislocation substructures which can also be varied by altering the scan strategies, especially utilizing sub-melt scans following layer melting to affect annealing, or using multiple melt scans to promote acicular α-phase grain growth and annealing of dislocations. The dislocation density in figure 7a and 7b was measured to be ~1010 cm-2 in contrast to figure 7c and 7d where it was measured to be ~108 cm-2. This two-order of magnitude density variation is reflected in a microindentation hardness variation of 3.9 GPa (Figure 7a) versus 3.5 GPa (Figure 7c).
Figure 7: Transmission electron microscope (TEM) observations of EBMfabricated, Z-axis solid cylinders. (a) α-β grain structure containing dislocation density of 1010 cm-2. (b) Magnified view of dislocations in α-grain. (c) Nearly dislocations free α grains. (d) Magnified view of α-β grain structure in (c). Note Vickers microindentation hardness (HV) in (a) and (c).
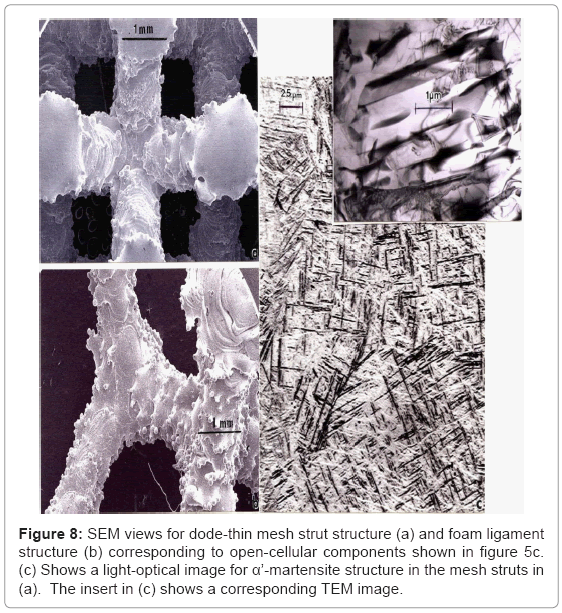
Figure 8a and 8b illustrate the mesh strut and foam ligament structures, respectively for the corresponding open-cellular structure components shown in figure 5b and 5c. Strut microstructure is illustrated in figure 8c which shows primarily α’-martensite platelets (hcp: a= 0.294 nm; c= 0.468 nm [36,37]) as a consequence of rapid solidification and cooling of the thin strut cross-sections. Ahmed and Rack [34] have shown that cooling rates above 410°C s-1 result in a fully martensitic microstructure, and a massive transformation occurring between 410 and 20°C s-1. The corresponding microindentation hardness (HV) measured for varying strut and ligament dimensions varied between ~3.7 GPa and 4.2 GPa. Consequently, martensite in Tialloys strengthens to a much lesser extent than in steels, and equivalent strengthening and hardening can be achieved by either refining the α-phase platelets or by α’-martensite formation (compare Figures 6b and 8c).
Cranio-maxillofacial implant examples
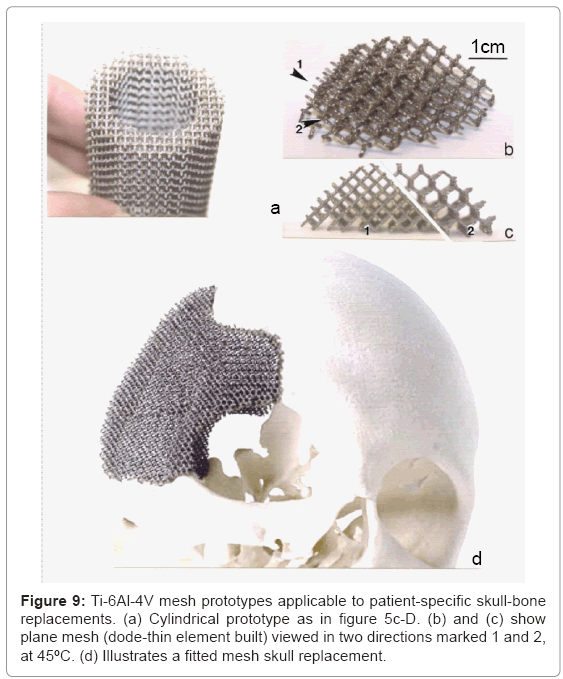
Skull replacement mesh structures : Solid skull replacements, although historically useful, are lacking in tissue ingrowth, especially at the bone margin. Prospects for fabricating mesh arrays as illustrated in figure 9 (specifically Figure 9d) are particularly encouraging since the porosity can be tailored to optimize both the strength/weight ratio and bone cell ingrowth. The mesh orientation, implicit in figure 9a to 9c can also allow for stress optimization. Studies of bone cell ingrowth in relation to pore size have demonstrated that ingrowth will not occur for cell sizes <100 μm [38,39], while recent work by Yang et al. [40] and Ryan et al. [41] has suggested that pore sizes ranging from 0.5 to 1 mm promote fibrous tissue formation which enhances the component biocompatibility. Mesh arrays as shown in Figure 9d can be patientspecific fabrications implanted without cement fixation.
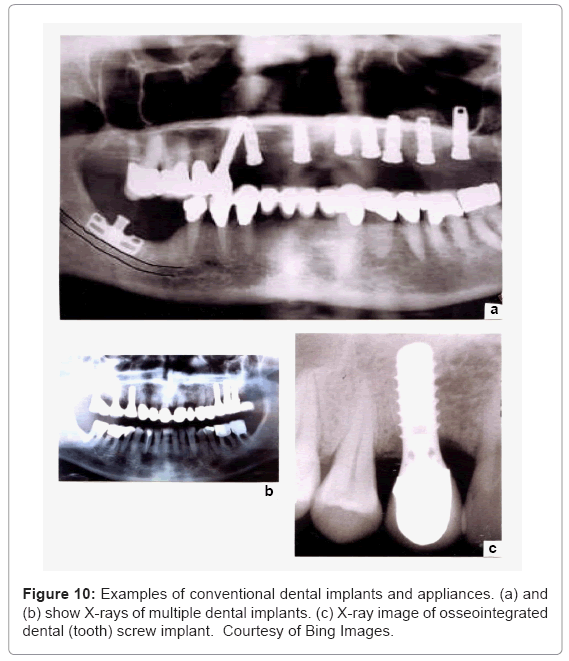
Dental protheses and implants: Ti-6Al-4V restorations pose attractive alternatives to more traditional metals, including crown and bridge protheses. It has become well-accepted and even the material of choice in endosseous implant devices. These include a variety of implant screws and dental porcelain-to-titanium or Ti-alloy crowns illustrated in the X-ray images shown in figure 10. Figure 10c in particular illustrates the close apposition of hard and soft tissue to the metal screw surface as a consequence of the passivating oxide (TiO2) on the implant surface. Although EBM fabrication can produce these appliances and appliance components, most are cast. However, because of their small size, EBM fabrication produces stronger prototypes whose α’-martensitic microstructure (Figure 8c) is well suited to the dental environment. Koike et al. [42] have recently shown that EBM-fabricated dental prototypes wear measurably better than either wrought and especially cast products, and also exhibit corrosion resistance comparable to either wrought or cast products. Because of the relatively small size of dental screw implants (Figure 10), there is no significant stress shielding and corresponding bone revision.
Hip implants and femoral stems
Hip implants, especially the femoral, intramedullary stem component, pose the greatest concern for bone stress shielding since the bone, especially the trabecular (soft) bone regime is often completely filled by the metal or alloy implant. Consequently, the edges of the implant can extend well beyond the dotted circle region or figure 1a and coincident with the inner extent of the outer cortical bone. Consequently, the implant stem will carry most of the femoral load, and for Co-base stems; for example, the modulus (E) or stiffness difference will be roughly an order of magnitude larger for the alloy stem than the cortical bone. Using Ti-6Al-4V would correspondingly reduce this difference to a factor of 5. In either case, stiffness matching strategies using open-cellular structure prototypes based upon CAD models and components represented in figures 4 and 5, respectively, will have significant benefits in reducing bone revision and in providing effective fixation of the implant to the existing bone without cement. This can also apply to the acetabular shell attached to the pelvic bone.
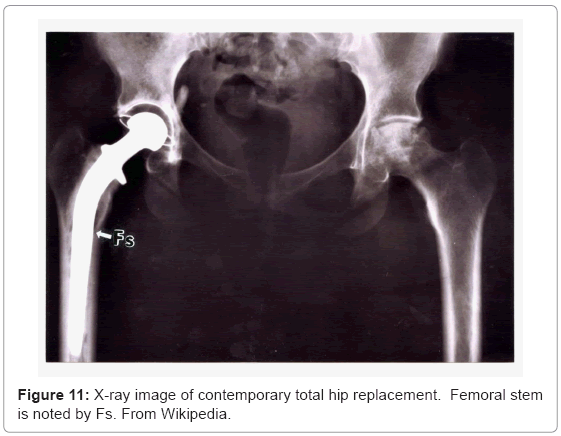
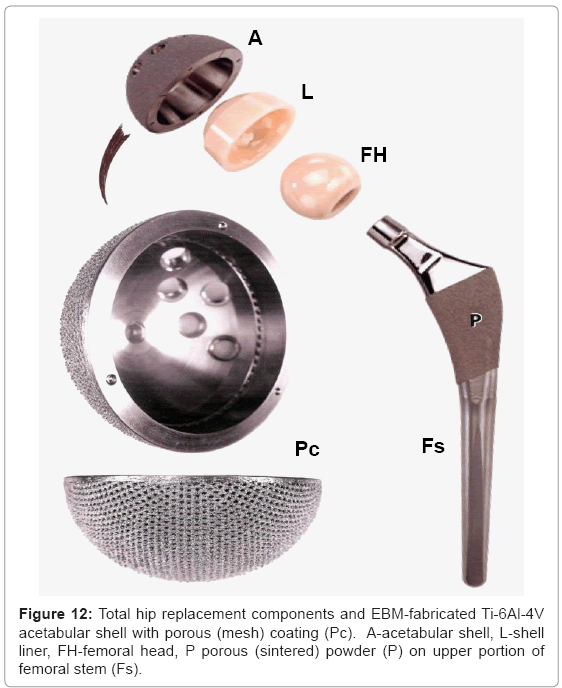
Figure 11 illustrates these appliance features for a total (right) hip replacement. Contemporary, commercial appliances contain a porous-coated stem (Fs in Figure 11) from the stem collar to the tip, or only in the upper stem section from the collar which has been a popular fixation strategy for the past two decades [18,23]. This feature is illustrated in the hip implant component view shown in figure 12 at P. The femoral stem (Fs) in figure 12 is attached to a highly crosslinked polyethylene head (FH) which fits into a corresponding or matching liner (L). The liner fits into the metal (Ti-6Al-4V) acetabular shell or cup (A in Figure 12) which is often screwed into the pelvic bone. This cup has been manufactured using EBM by Ala Ortho in Italy, and used in thousands of hip replacement appliances for several years. The porous mesh outer structure shown in figure 12(at Pe) is fabricated in the monolithic acetabular shell component, and provides effective stress shielding reduction and fixation by bone in growth.
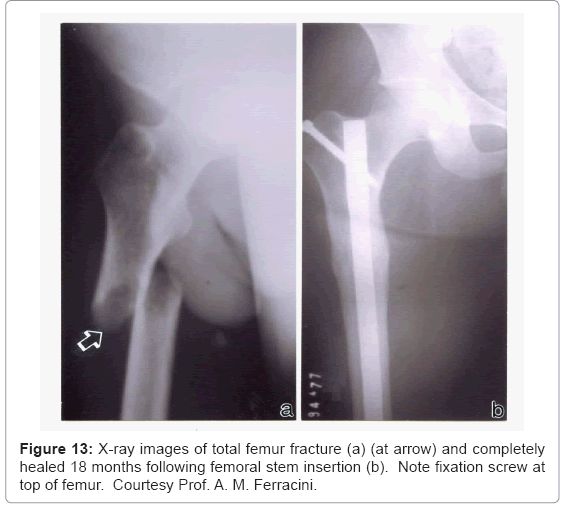
In contrast to the total hip arthroplasty illustrated in the X-ray of figure 11, figure 13 shows a completely broken and displaced rightside femur which was repaired using a solid Ti-6Al-4V rod inserted from the top of the femur and anchored by a screw. In contrast to the femoral hip stem (Fs) shown in figure 11, the intramedullary rod (or stem) in figure 13 occupies a smaller area of the bone cross-section as illustrated in figure 1a. However this rod surface meets the relatively soft trabecular bone regime, and the stiffness variation between bone and alloy implant is probably greater than a factor of 50, presenting large stress shielding. However, novel porous monolithic rods could be fabricated by EBM which could dramatically reduce the stress shielding and also allow for effective implant fixation, which can be a particular problem for intramedullary rod implants.
Strategies for stress shielding reduction and biocompatibility optimization of implants: The ability to tailor stiffness of implant stems in particular is implicit in the consideration of open-cellular structures in Equation (4), and examples provided in figure 5b and 5c for EBM-fabricated Ti-6Al-4V components. While graded porosity from the stem exterior to a solid, interior alloy rod may be effective, the development of metal or alloy stems with a porous core could provide an efficient scaffold for the development of vascular structures which could eventually render the implant even more biofunctional or biocompatible than reducing stress shielding and providing for efficient fixation by bone cell ingrowth [38-40].
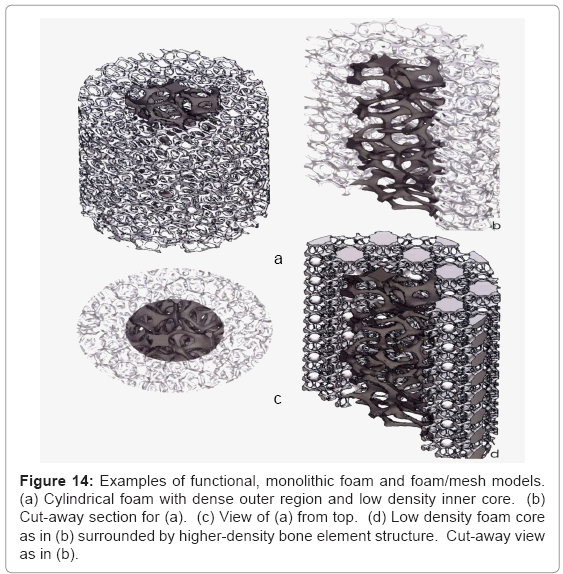
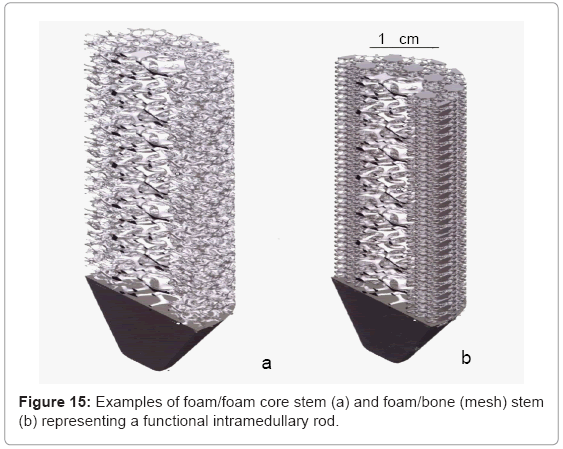
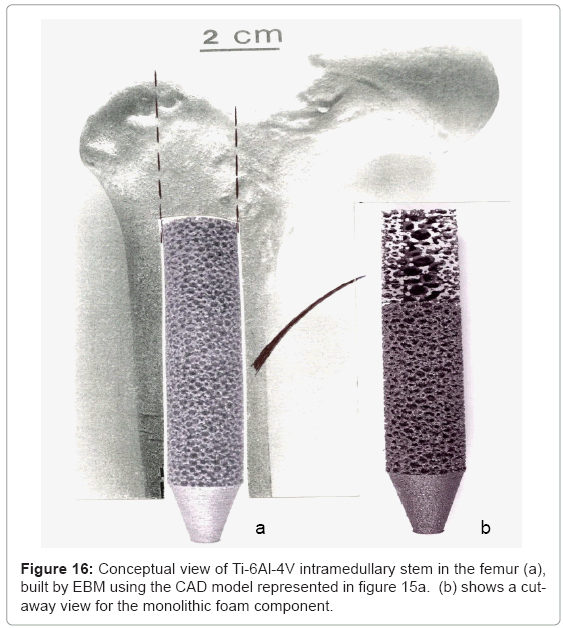
Figure 14 illustrates some examples of functional bone stem models which might be considered to replace the solid intramedullary (femoral) rod shown in figure 13, or even the femoral stem of figures 11 and 12. The implications of providing for a more biocompatible or biofunctional implant appliance using a variety of open-cellular structure models or functional models are perhaps more tractable in the more extended models of figure 15, and the view of a fabricated Ti- 6Al-4V component in figure 16, built using the CAD model of figure 15a; which is an extension of figure 14a.
Figure 14: Examples of functional, monolithic foam and foam/mesh models. (a) Cylindrical foam with dense outer region and low density inner core. (b) Cut-away section for (a). (c) View of (a) from top. (d) Low density foam core as in (b) surrounded by higher-density bone element structure. Cut-away view as in (b).
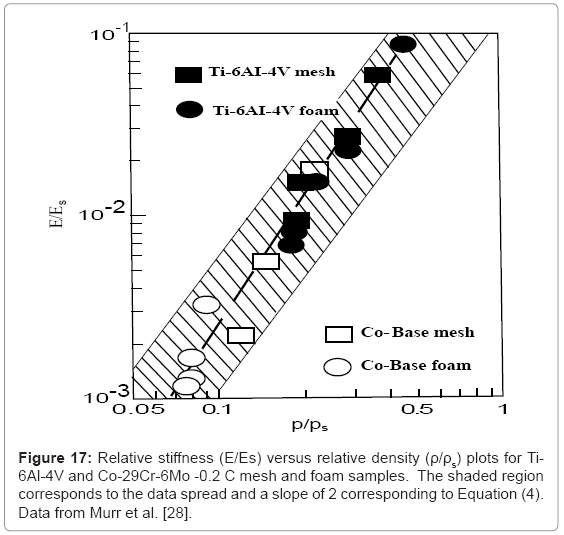
Murr et al. [28] have recently shown that stiffness and density measurements for Ti-6Al-4V mesh and foam structures (represented in Figure 5b and 5c) along with Co-29Cr-6Mo superalloy mesh and foam structures fabricated by EBM, essentially follow the Gibson- Ashby model represented in figure 2 and Equation (4), when plotted (log-log) as relative stiffness (E/Es) versus relative density (ρ/ρs). This data is shown reproduced in Figure 17 where the slope fitted to the data corresponds to n≅2.1 while the shaded area representing the spread of the data corresponds to a slope of n=2 (as in Equation (4)). Correspondingly, chosing an appropriate range of stiffness values (E) for either Ti-6Al-4V or Co-29Cr-6Mo porous implants would identify a range of densities as a design parameter for building requisite opencellular structures: mesh, foam, or intermixed arrays illustrated in figure 14d and figure 15b. This strategy can also be used for other implant components, such as the acetabular shell shown in Figure 12.
It can be observed from figure 17 that in order to match an intermedullary bone regime having a stiffness of ~10 GPa would require E/Es of ~0.09 for Ti-6Al-4V (with Es=110 GPa in Equation (4)). The corresponding relative density (ρ/ρs) would be ~0.6 (for ρs=4.43 g/cm3); or ρ=2.7 g/cm3. This density would require a mesh or foam pore or cell dimension (l in Figure 2) close to the cell ingrowth size limit (~ 100 μm), and the optimum biocompatible open-cellular structure would be more efficiently achieved using the Co-base alloy. However for smaller implant stem dimensions which would require matching much lower stiffness values (<2 GPa), Ti-6Al-4V foam or mesh prototypes would perform adequately for a porous structure with ρ ≅1 g/cm3.
Figure 17 illustrates that in the fabrication of open-cellular implant monoliths with low density and higher porosity, high modulus (stiffness) materials such as Co-base alloys may be preferred while in cases where fully dense alloys must be employed, such as fixation screws and the like, biomechanically derived properties would favor lower modulus and stronger materials.
Recent development of β-Ti alloys with lower stiffness’s ranging from 50 GPa to 90 GPa have been developed over the past 5 years [43]. Of particular interest has been Ti-24Nb-4Zr-8Sn (designated Ti 2448) [44]. The large Nb content acts as a β-stabilizer while the Zr and Sn additions prevent martensitic transformations upon quenching from the β field [44]. Stiffness values as low as 42 GPa have been achieved in this β-phase alloy [44].
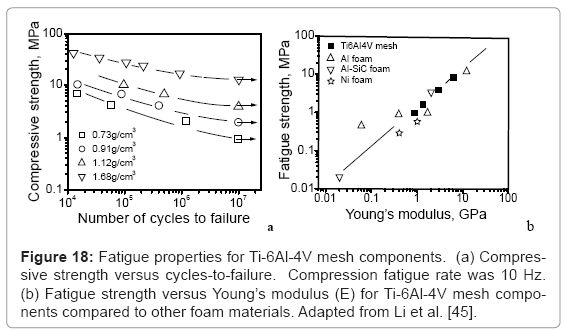
Considering only the biomechanical issues, low modulus alloys are certainly preferred, but in the larger biomedical context where fixation and implant strength (including yield stress and fatigue strength) are a concern, the design strategy becomes more complex. Fatigue, particularly low-cycle fatigue (LCF) is a particular concern since walking and attendant bone loading and unloading is characterized by roughly 1 cycle s-1 (1 Hz). This is particularly important for fatigue of open-cellular structures which has only recently been reported for Ti-6Al-4V mesh structures fabricated by EBM [45], as shown in figure 18. Figure 18a shows that as the open-cellular (mesh) structure density decreases, the corresponding compressive strength decreases proportionately with fatigue cycles to failure. Figure 18b illustrates that as the mesh density increases, the corresponding Young’s modulus (E) and the fatigue strength also increase consistent with other foam materials.
Fatigue resistance is a crucial factor for the long term applications of implants subjected to cyclic loading, especially hip and knee arthroplasties. Fatigue has been an important consideration even for acrylic cements in more conventional implant fixation where bone cement fracture has been a major factor in aseptic loosening and implant failure [46-48].
Knee arthroplasty examples
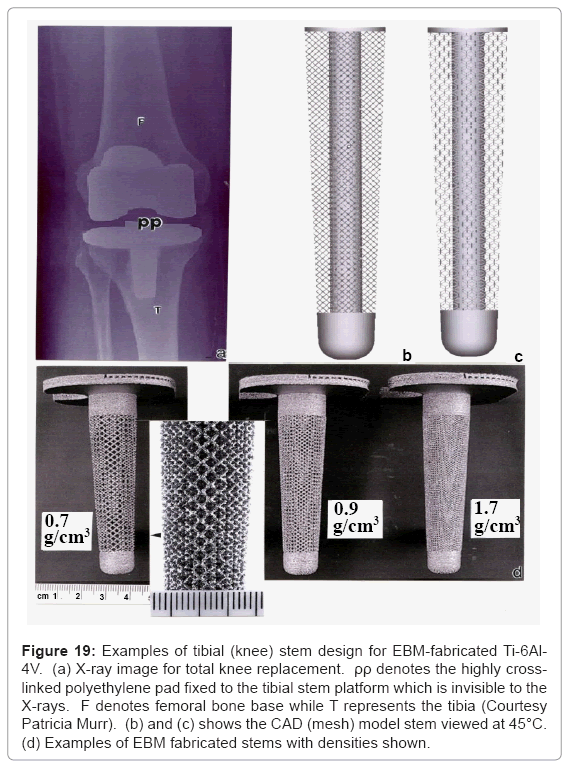
Totalknee replacements illustrated in the X-ray image of Figure 19a often utilize a Co-base alloy for the femoral (F) component while Ti- 6Al-4V is used to fabricate the tibial (T) stem appliance which holds the highly cross-linked polyethylene pad (PP) which bears the connecting load from the femoral appliance. Figure 19b and 19c illustrates the corresponding 45°C views for a dode-thin mesh element (Figure 4a) model having a solid, central stem, while Figure 19d shows several examples of EBM-fabricated Ti-6Al-4V tibial stem appliances using this model. The most dense mesh appliance (mesh density = 1.7 g/cm3) has an average pore size of ~400 μm, and corresponds to a stiffness, E4 GPa from figure 17 (E/Es = 0.036; Es= 110 GPa).
Figure 19: Examples of tibial (knee) stem design for EBM-fabricated Ti-6Al- 4V. (a) X-ray image for total knee replacement. ρρ denotes the highly crosslinked polyethylene pad fixed to the tibial stem platform which is invisible to the X-rays. F denotes femoral bone base while T represents the tibia (Courtesy Patricia Murr). (b) and (c) shows the CAD (mesh) model stem viewed at 45°C. (d) Examples of EBM fabricated stems with densities shown.
Novel antibacterial strategies for open-cellular implants
Aside from implant failure through fixation cement fatigue and fracture, fatigue of appliances or appliance components (such as screws), the wear and fracture of polymer components such as the polyethylene pad in knee arthroplasty (Figure 19a), bone revision, and the like, device related infections pose one of the most significant issues in implant surgery. Despite antiseptic precautions in fabricating and sterilizing the implant, and in surgical procedures, bacterial attachment and proliferation on biomedical device surfaces pose a substantial health risk. Most polymers, including acrylic cements, are readily colorized by bacteria. Bacterial attachment and growing bacterial colonies can produce an exocellular polysaccharide matrix which protects them against antibiotics and normal body defenses [49,50]. In the case of hip and knee implants it is especially important to prevent bacterial infection while bone cell ingrowth occurs, especially with open-cellular prototypes illustrated in figures 16 and 19.
Contemporary implant fixation acrylics are often mixed with antibiotic drugs such as gentamicin and norfloxacin [51,52] to serve as a local release antibacterial strategy. Silver nanoparticles have also been incorporated into polymeric components as well [52]. Silver, including silver compounds, have been known in antiquity to have antibacterial properties and colloidal silver in more recent times has been identified as an antifungal and antiviral agent as well as an antibacterial agent [53,54]. Many commercial products, including refrigerator linings, wound dressings and burn coverings, hospital bedding, socks, and silver nano-particle-coated medical devices such as catheters, etc. are currently available world-wide.
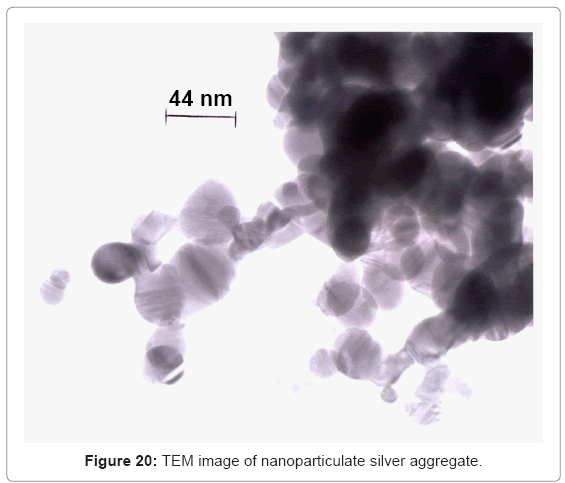
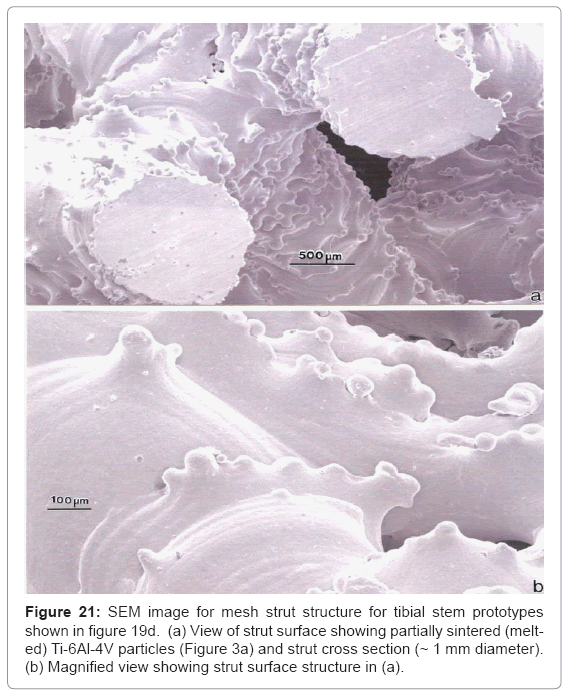
Figure 20 shows some commercially manufactured silver nanoparticle/ nano-particle aggregates. The primary (single) silver nanoparticles are single crystalline or often contain twins or twin faults observed as bands within the particles which occur during growth. The single silver particles in figure 20 vary in size from ~5 nm to 50 nm; with aggregate sizes ranging from ~0.1 μm to >1 μm. Consequently open-cellular implant components such as hip (femoral) and knee (tibial) stems (Figure 16 and 19, respectively) could be dipped into sterilized silver nanoparticles to fill the pores with requisite amounts of nanoparticle aggregates. These would stick to the open-cellular ligament or strut microstructures illustrated typically in figure 8, and in more detail in figure 21 which shows a mesh structure corresponding to the tibial stems shown in figure 19. Conceptually similar processes
Summary and Conclusions
The ability to fabricate complex, open-cellular monolithic structures by additive manufacturing using electron beam melting from prealloyed precursor powders provides a new and more comprehensive range of functionality for implant prototypes. These functional features include mesh and foam design strategies for reducing or eliminating biomechanical stress shielding and the ability to provide intrinsic implant fixation by allowing bone cell growth into these opencellular structures. Additionally, open-cellular implant prototypes can provide greater fatigue resistance than customary acrylic fixation cements, and the open cellular structures are ideal for loading and retention of antibacterials such as nanoparticulate silver. Functionally graded, open-cellular structures can also be fabricated as monolithic arthroscopic appliances which may allow for vascularization in stem cores and encourage more natural skeletal bone function
The wide range of Young’s moduli or stiffnesses for biocompatible alloys-42 GPa to 110 GPa for Ti-alloys and 210 GPa for Co-base alloyspresents novel design strategies for implants, ranging from dental screws to femoral stems, by fabricating open-cellular monoliths using EBM. In addition, these can be fabricated from micro - CT scans to produce patient-specific appliances as originally proposed by Harryson and Cormier [56]. Appliances incorporating monolithic designs are impossible to fabricate using more conventional manufacturing or processing technologies, and because of the elimination of process waste in producing appliances from cast or wrought alloy stock, novel appliances can be produced cheaper and more efficiently because excess powder is almost completely recycled in the EBM process
Acknowledgements
This research was supported in part by Mr. and Mrs. MacIntosh Murchison Endowments at the University of Texas at El Paso.
References
- Roesler H (1987) The history of some fundamental concepts in bone biomechanics. J Biomech 20: 1025-1034.
- Wiesel SW, Delahay JN (1997) Essentials of Orthopedic Surgery. (3rdedn), Springer, NY.
- Cascarini L, Schilling C, Gurney B, Brennan P (2012) Oxford Handbook of Oral and Maxillofacial Surgery (Oxford Medical Hanbooks) Oxford University Press, England.
- Monolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for pathogenesis and treatment of osteoporosis. Endocr Rev 21: 115-137.
- Boskey A (2003) Bone mineral crystal size. Osteoporosis Int 14: S16-S21.
- Currey JD (1999) What determines the bending strength of compact bone. J Exp Biol 202: 2495-2503.
- Augat P, Schorlemmer S (2006) The role of cortical bone and its microstructure in bone strength. Age Ageing 35: ii27-ii31.
- Yaszemski MJ, Trantolo DJ, Lewandrowski, K-U, Hasirci V, Altobelli DE,Wise DC (Eds.) (2004) Biomaterials in Orthopedics, Marcel Dekker, Inc. NY.
- Wrek GE, Bowlin GL (2008) Encyclopedia of Biomaterials and BiomedicalEngineering Vol. 1, Informa Healthcare USA, New York.
- Bocciarelli DS (1970) Morphology of crystallites in bone. Calcified Tissue Res 5: 261-269.
- Mackie IG, Green M, Clarke H, Isaac DH (1989) Osteoporatic bone microstructure by collagenase etching. Ann Rheum Dis 48: 464-469.
- Moses WM, Witthaus FW, Hogan HA, Laster WR (1995) Measurement of the thermal conductivity of cortical bone by an inverse technique. Exp Thermal Fluid Sci 11: 34-39.
- Panagiotspoulos E, Kostopoulos V, Tsantzalis S, Fortio AP, Douglas A (2005) Impact energy absorption by specimens from the upper and of the human femur. Injury 36: 613-617.
- Currey JD (2002) Bones: Structure and Mechanics, Princeton University Press, NJ.
- Park JB in Bronzino J D (2000) The Biomedical Engineering Handbook, Vol. 1,CRC Press, LLC, Boca Raton, FC.
- Niinomi M (2001) Recent metallic materials for biomedical applications. Metall Mater Trans A 33: 477-486.
- Niinomi M (2008) Mechanical compatibilities of titanium alloys for biomedical applications. J Mech Behav Biomed Mater 1: 30-42.
- Huiskes R, Weinans H, Van Rietbergen B (1992) The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop Relat Res 274: 124-134.
- Krause W R, Mathis R S (1988) Fatigue properties of acrylic bone cements: review of literature. J Biomed Mater Res Appl Biomater 22: 37-53.
- Dunne NJ, Orr JF, Mushipe MT, Eveleigh RJ (2003) The relationship between porosity and fatigue characteristics of bone cements. Biomater 24: 239-245.
- Pilliar RM, Cameron HU, Macnab I (1975) Porous surfaced layered prosthetic devices. Biomed Eng 10: 126-131.
- Pilliar RM (1983) Powder metal-made orthopedic implants with porous surface for fixation by tissue ingrowth. Clin Orthop Relat Res 176: 42-51.
- Engh CA, Bobyn JD, Glassman AH (1987) Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br 69: 45-55.
- McCracken M (1999) Dental implant materials: Commercially pure titanium and titanium alloys. J Prosthodont 8: 40-43.
- Gibson LJ, Ashby MF (1982) The mechanics of three-dimensional cellular materials. Proc R Soc Lond 382: 43-59.
- Timoshenko S, Gene JM (1972) Mechanics of Materials, Van Nostrand, Reinhold Co., New York.
- Murr LE, Gaytan SM, Medina F, Lopez H, Martinez E, et al. (2010) Next generation biomedical implants using additive manufacturing of complex, cellular and functional mesh arrays. Philos Transact A Math Phys Eng Sci 368: 1999-2032.
- Murr LE, Amato KN, Li SJ, Tian YX. Cheng XY, et al. (2011) Microstructure and mechanical properties of open-cellular biomaterials prototypes for total knee replacement implants fabricated by electron beam melting. J Mech Behav Biomed Mater 4: 1396-1411.
- Aboudi J, Gilat R (2005) Micromechanical analysis of lattice blocks. Int J Solids Struct. 42: 4372-4392.
- Zhou J, Shrotriya P, Soboyejo WO (2004) On the deformation of aluminum, lattice block structures: from struts to structures. Mech Mater 36: 723-737.
- u J, Shrotriya P, Soboyejo WO (2004) On the deformation of aluminum, lattice block structures: from struts to structures. Mech Mater 36: 723-737.
- Gibson LJ, Ashby MF (1997) Cellular Solids: Structures and Properties. (2ndedn), Cambridge University Press, London.
- Jang WY, Kraynik AM, Kyriakides S (2008) On the microstructure of open-cell foams and its effect on elastic properties. Int J Solids Struct 45: 1845-1875.
- Thijs L,Verhaeghe F, Craeghs T, Humbeeck JV, Kruth JP (2010) A study of the microstructure evolution during selective laser melting of Ti-6Al-4V. Acta Mater 58: 3303-3312.
- Ahmed T, Rock HJ (1998) Phase transformations during cooling in a+b titanium alloys. Mater Sci Eng 243: 206-211.
- Brakke KA (1992) The surface evolver. Exp Math 1: 141-165.
- Singh AK, Ramachandra C, Tavafoghi M, Singh V (1993) Structure of martensite in titanium alloy Ti-6Al-1.6Zr-3.3Mo-0.3Si. J Mater Sci Lett 12: 697-699.
- Lin C, Yin G, Zhao Y, Ge P, Liu Z (2011) Analysis of the effect of alloy elements on martensitic transformation in titanium alloy with the use of valance electron structure parameters. Mater Chem Phys 125: 411-417.
- Holy CE, Shoichet MS, Davies JE (2000) Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds. J Biomed Mater Res 51: 376-382.
- Freyman TM, Yannas IV, Gibson LJ (2001) Cellular materials as porous scaffolds for tissue engineering. Prog Mater Sci 46: 273-282.
- Yang S, Leong, KF, Du Z, Chua CK (2001) The design of scaffolds for use in tissue engineering. Part 1.Traditional factors. Tissue Eng 7: 679-689.
- Ryan G, Pandit A, Apatsidis DP (2006) Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 27: 2651-2670.
- Koike M, Greer P, Owen K, Lilly G, Murr LE, et al. (2011) Evaluation of titanium alloys fabricated using rapid prototyping technologies - Electron beam melting and laser beam melting. Materials 4: 1776-1792.
- Niinomi M (2007) Recent research and development in metallic materials for biomedical, dental, and healthcare products. Mater Sci Forum 539-543: 193-200.
- Hao YL, Li SJ, Sun SY, Zheng CY, Yang R (2007) Elastic deformation behavior of Ti-24Nb-4Zr-7.9Sn for biomedical applications. Acta Biomater3: 277-286.
- Li SJ, Murr LE, Cheng XY, Zhang ZB, Hao YL, et al. (2012) Compression fatigue behavior of Ti-6Al-4V mesh arrays fabricated by electron beam melting. Acta Mater 60: 793-802.
- Hertzberg RW, Monson JA (1980) Fatigue of Engineering Plastics. Academic Press, London.
- Krause W, Mathis RS (1988) Fatigue properties of acrylic bone cements: review of the literature. J Biomed Mater Res 22: 37-53.
- Dunne NJ, Orr JF, Mushipe MT, Eveleigh RJ (2003) The relationship between porosity and fatigue characteristics of bone cements. Biomaterials 24: 239-245.
- Hoyle BD, Costerton JW (1991) Bacterial resistance to antibiotics: the role of biofilms. Prog Drug Res 37: 91-105.
- Darouiche RO (2004) Current concepts-treatment of infections associated with surgical implants. N England J Med 350: 1422-1429.
- Baleari M, Cristofolini L, Mineri C, Toni A (2003) Fatigue strength of PMMA bone cement mixed with gentamicin and barium sulfate versus pure PMMA. Proc Inst Mech Engng H 217: 9-12.
- O?Raye T (1998) The Wonder Drug Time Forgot: Colloidal Silver, Revised Edition, AWIECA Inc., London.
- Vasilev K, Cook J, Griesser HJ (2009) Antibacterial surfaces for biomedical devices. Expert Rev Med Devices 6: 553-567.
- Rai M, Yadar A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27: 76-83.
- Harryson O, Cormier DR (2005) Direct fabrication of custom orthopaedic implants using electron beam melting technology. John Wiley & Sons Ltd., London 9: 191-206.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 18846
- [From(publication date):
March-2012 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 13672
- PDF downloads : 5174