Research Article Open Access
Extraction, Determination and Bioremediation of Heavy Metal Ions and Pesticide Residues from Lake Water
| Sandip R Sabale1*, Bhaskar V Tamhankar1, Meena M Dongare3 and B S Mohite2 | |
| 1P.G. Department of Chemistry, Jaysinpur College, Jaysingpur-416101, M.S., India | |
| 2Department of Chemistry, Shivaji University, Kolhapur-416004, M.S., India | |
| 3Department of Botany, Shivaji University, Kolhapur-416004, M.S., India | |
| Corresponding Author : | Sandip R Sabale P.G. Department of Chemistry Jaysinpur College, Jaysingpur-416101, M.S., India E-mail: sandip_ana@rediffmail.com |
| Received: March 01, 2012; Accepted: April 10, 2012; Published: April 12, 2012 | |
| Citation: Sabale SR, Tamhankar BV, Dongare MM, Mohite BS (2012) Extraction, Determination and Bioremediation of Heavy Metal Ions and Pesticide Residues from Lake Water. J Bioremed Biodegrad 3:143. doi:10.4172/2155-6199.1000143 | |
| Copyright: © 2012 Sabale SR, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The natural or manmade water bodies in and near the settlements play very important role in improvement and conservation of local environment but many of them are on the way of eutrophication. This problem needs an urgent and sincere attention of environmentalists. The pollution due to heavy metal ions, pesticides and fertilizers has been investigated and analyzed by using atomic absorption spectrometer and Liquid Chromatography Tandem Mass Spectrometry. The present study focuses on the determination of heavy metal ions from aquatic plants so as to evaluate its curative power as well as extraction method of pesticides from water and its determination using liquid chromatography tandem mass spectrometry. Nearly 11 pesticides have been found in ppb level. The curative power and remediation of pesticide residues and heavy metal ions using microbial culture has also been tried and reported.
| Keywords |
| Heavy metal ions; Pesticides; Bioremediation; AAS; LCMS/ MS |
| Introduction |
| Pollution due to heavy metal ions and pesticides is dangerous as they tend to bioaccumulate and therefore toxic to the plant, human and animal health. Metal ions are accumulated in living beings when they are taken up and stored faster than they are metabolized or excreted. Heavy metal ions can enter a water supply through industrial and consumer waste. Lead, cadmium, chromium and mercury are the most potential heavy metal ions cause water pollution. Aquatic plants are well known for accumulating and in concentrating heavy metal ions [1,2]. Several workers have shown that constructed wetlands are very effective in removing heavy metal ions from polluted waste-waters [3]. Different wetland species differ, however, in their abilities to take-up and accumulate various trace elements in their tissues [4]. Laboratory studies of water hyacinth have demonstrated the potential use of this species in removing metals from polluted water [5,6]. |
| Pesticides are the chemicals used in agriculture, in order to protect the crops from the attacks of pests, diseases and rodents. They are toxic and cause environmental contamination as well as generate public health problems. The analysis of pesticide residue in water is difficult, since these compounds occur at very low concentration level [7]. High Performance Liquid Chromatography (HPLC) is the method often used when the compounds to be analyzed are polar, nonvolatile and thermally labile [8]. The use of biodegradable pesticides in making HPLC is the favorite analytical technique, allowing large volumes of injection of aqueous samples [9]. Although, many authors reported the multiresidue pesticide analysis in water by HPLC [10] and Gas Chromatographic (GC) techniques [11], the use of hyphenated techniques like LC/MS and GC/MS are more easy, suitable and precise, and give accurate and confirmatory results [12,13]. |
| Kolhapur, a prominent city of Southwestern Maharashtra (India) is rapidly emerging as a leading industrial and commercial centre. The development of the city has caused directly or indirectly, a number of water quality problems. In this city, there is a Wetland in the form of a beautiful Lake called as Rankala, which is man-made reservoir, situated in southwestern region of the city. The total catchment area is of 5.21 sq km. and is of 94.33 million cubic feet storage capacity. The maximum water depth is up to 88 feet [14]. In this lake, the Eichhornia crassipes plant species were growing enormously up to 2008 [15], but in the year 2009 Eichhornia seems to be replaced by Salvinia occupying whole lake (Figure 1), which looks like a big foot ball ground. Because of rapid urbanization, increasing use of fertilizers and pesticides in the agricultural land around the lake subjected to great amount of ecological stress. The mixing of sewage and agricultural waste, results into the increase of organic matter, silt and nutrients like nitrogen, potassium and phosphorus in the lake. The lake is also suffering from abuse due to which soluble metal ions, detergents, fertilizers and solid wastes are accumulating. The large area and good sunlight, supports rapid growth of aquatic/eutrophic plants like Salvinia, Eichhornia, hydrilla and other macrophytes in the lake. |
| The microorganisms are the agents which consume many organic as well as inorganic compounds and degrade them. They are widely used for the degradation of organic compound like dyes, pesticides and other. The microorganism, Proteus spp. SUK 7 has been used by Patil et al. [16] for the decolorization of Reactive Blue 59. Several bacteria capable of dye decolourization have been reported [17-20]. The Bacillus Thurigiensis MOS-5 has been used for the biodegradation of malathion by Zeinat et al. [21]. Many other reports were also found for the biodegradation of pesticides by microorganisms or bacteria [22-25]. Microorganisms are also the agents used for the biosorption of the metals [26]. The mechanism of biosorption, bacterial biosorbants and the biosorption by the various microorganisms were described by Vijayraghavan et al. [27]. The Bacteria isolated from the various sources which were used for the biosorption of heavy metals are reported [28-30]. But there are no any attempts were found on the use of Bacillus thuringensis NCIM 2159 and Proteus spp SUK 7 for the biosorption of the metals and the removal of pesticide residue. |
| The investigation about the presence of heavy metal ions and the qualitative as well as quantitative assessment of pesticide residues present in water body (Rankala) is presented in this paper. |
| Experimental |
| Reagents and materials |
| Double distilled, deionized water has been used for washing the plant materials as well as for the determination of trace metals. All the reagents used are of analytical grade and are used without further purification. |
| Analytical reference standards of pesticides were obtained from Dr. Ehrenstorfer (Augsburg, Germany) and Accustandard (New Haren, USA) always with the purity certified >98%. All the solvents namely, dichloromethane, methanol and water were all HPLC grade. The anhydrous sodium sulphate was of analytical reagent grade and was purchased from the RFCL limited (New Delhi, India). It was activated by heating at 300°C for 4 hours before use and kept in oven at 60°C. HPLC grade water was collected from ultra pure water purification system (SG Water, Germany). |
| Instruments |
| The pH and conductance measurements were carried out using digital pH meter (LI-127, Elico, India) and digital conductivity meter (EQ-660 A, Equip-Tronics, India). The metal ion detection was carried out using Atomic Absorption Spectrometer, Perkin-Elmer, AAnalyst 300 series. A Perkin-Elmer 200 series HPLC system (Massachusetts, USA) hyphenated to an API 2000 mass spectrometer (Applied Biosystems, MDS Sciex, Canada) was used. A high speed homogenizer (Heidolph, Germany), low volume concentrator (Telemachanic, Magelies), high volume refrigerated centrifuge (Z 36 HK, HARMLE Labortechnik, GmbH), low volume refrigerated centrifuge (Z 233M K2, HARMLE Labortechnik, GmbH), were used for residue analysis. |
| Sampling |
| The plant samples of Eichhornia, Salvinia and hydrilla were collected at random from different areas of lake covering all directions. The plant material was washed to remove periphyton, dust and sediment particles. The material was stored in polythene bags, at the same time the water samples around the plants were collected randomly and brought to laboratory. The temperature of water at the time of sampling was recorded. All individual plant samples were again washed with distilled deionized water in laboratory. These materials were sun dried for 10 days in separate containers and were heated in oven at 110°C for 12 hours. The dried samples were grounded using mortar till dry powder was formed. All the physical and chemical parameters of water were determined and the water was stored in refrigerator. |
| Sample preparation for AAS analysis of trace metals |
| The dried samples of Eichhornia, Salvinia and hydrilla were weighed accurately and dissolved in HNO3 and HClO4 (in the ratio 3:1). The resulting mixtures were evaporated to dryness and extracted with distilled, deionized water. The solutions were heated to boiling and filtered. The volumes of the diluted sample were made to 100 mL each. 1.0 L water sample was heated to reduce the volume, acidified and total 100 mL volume was made. The metal ion concentrations in all the samples were analyzed by Atomic Absorption Spectrometer. For determination of unknown concentrations of all metals, the calibration charts for each element were used. All analyses were done in triplicate and one blank sample. |
| Extraction of pesticide residue from water sample |
| One liter water sample was filtered using vacuum and 100 g of sodium chloride was added. The salt was dissolved and the pesticides were extracted with 60 mL dichloromethane in a separating funnel. The organic layer was passed through sodium sulphate and collected in a 200 mL evaporation tube. The aqueous layer was then again extracted twice with two 60 mL fractions of dichloromethane in same manner. All the dichloromethane layers were then evaporated under a gentle stream of nitrogen in large volume concentrator up to 2 mL and the solution was transferred into 10 mL test tube. The mixture was evaporated to near dryness under a gentle stream of nitrogen in a low volume concentrator at 35°C. The residues were dissolved in a mixture of 1.0 mL 0.1% acetic acid (in water) and 1.0 mL methanol followed by vortexing. This solution was centrifuged at 10000 rpm for 5 min. The supernatant extract was filtered through 0.2 μm polyvinylidine fluoride (PVDF) membrane filter and then analyzed by LC-MS/MS. |
| Microorganism treatment for the removal of trace metals and pesticide residue |
| The microorganism Bacillus thuringiensis NCIM 2159 was obtained from MTTC Chandigarh (India) while the Proteus spp. SUK 7 was isolated by the known method [16]. These two bacteria were used in consortium. |
| Medium for the growth of microorganisms: 6.0 g/L Na2HPO4, 3.0 g/L K2HPO4, 2.0 g/L NH4Cl, 5.0 g/L NaCl, 8.0 g/L Glucose, 0.1 g/L of MgSO4 and 0.1 g/L of MnSO4 were used as a nutritional medium for the growth of the microorganisms. |
| Microorganisms were grown in the minimal media composition as described above and 5% of them were transferred to water sample. Flask was kept on rotary shaker at 120 rpm at 30°C for 7 days. After 7 days, microbial cell mass was removed by centrifugation at 7000 rpm for 15 minutes. The supernatant obtained was used for further studies. |
| Liquid chromatography mass spectrometric method |
| The high performance liquid chromatography was performed using Perkin-Elmer 200 series liquid chromatography system equipped with series 200 Vaccum degasser, series 200 auto sampler, series 200 pump, and series 200 column oven. PrincetonSPHER C18 (150mm × 4.6 mm × 5 μm) column (Princeton, NJ, USA) was maintained at 30°C at the flow rate of 1.2 mL/min. The mobile phase was composed of (A) methanol/water (20:80 v/v) with 5.0 mM ammonium formate and (B) methanol/water (90:10 v/v) with 5.0 mM ammonium formate; gradient 0-1.0 min/90% A, 1-2 min/90%-0% A, 2-16 min/0%A, 16-17 min/0%- 90% A, 17-23 min/90% A. Prior to use, the solvents were filtered through 0.22 μm filter with applied vacuum. 20 μL of the sample extract was injected. |
| The triple quadrupole mass spectrometer was equipped with Turbo Ion Spray Interface (ESI). The instrument was operated in positive ion electrospray mode with 118 transitions monitored during LC separation in the multiple reaction monitoring (MRM) modes. The MS parameters included ion spray voltage of 5500 V; nebulizer gas 30 psi; curtain gas 20 psi; heater gas 60 psi and the ion source temperature of 500°C. |
| Selection and tuning of the transitions as well as analyte dependent parameters, DP (Declustering Potential), CE (Collision Energy) and CXP (Cell Exit Potential) were performed by direct infusion of individual pesticide solution in 10 mM ammonium formate in methanol/water (1:1, v/v) at a concentration of 1.0 mg/L. Analyte MS/MS transitions, retention times and instrument conditions are presented in table 1. |
| Stock solution of individual pesticides at 500 mg/kg was prepared by exact weight and solution in methanol for LC-MS/MS (gravimetric control was followed). The stock solution was stored at -10°C in a freezer. The intermediate stock standard mixture of 5 mg/L was prepared by mixing the appropriate quantities of the individual stock solutions followed by requisite volume makeup by methanol as required. A working standard mixture of 0.5 mg/L was prepared by diluting the intermediate stock solution, from which the calibration standards within the range 0.01-0.250 mg/L were prepared by serial dilution with methanol-water (1:1, v/v) for LC-MS/MS to plot the calibration curve, from which unknown concentration of pesticide residues from water sample was determined. |
| Results and Discussion |
| Determination of heavy metal ions from water and plant samples by AAS |
| The pollution in stagnant water bodies is mainly due to accumulation of plant nutrients, heavy metal ions and pesticide residues. Apart from actual chemical analysis the pollution level can be judged by observation of vegetative and animate life in the water. Both the plants and the animals in a polluted ecosystem show different modifications, indicating the types and extent of the pollution. Among these indicators plants perform better. The monstrous growth of aquatic plants indicates the eutrophication of water body. This indicates the excessive presence of plant nutrients in water. The heavy metal ion pollution is amongst most severe problems of water pollution as the toxic metals like lead, mercury, copper and cadmium directly heat the vital organs of the human beings. |
| The aquatic plants are bioaccumulators of heavy metal ions, so they are both indicator and corrective systems. The capacity and preference of accumulation of particular metal ion in plant body is a finger print i.e. for different aquatic plants preference of accumulation and the extent varies. For the present work the plants Salvinia, Eichhornia and hydrilla were studied. |
| The analysis carried out with the help of AAS technique revealed that, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn ions are present in these plant samples. The data of concentrations in ppm with standard deviation are presented in Table 2. Similar analysis carried out for water samples around plants reveals that, there is relatively less concentration of toxic metal ions in water. Thus the accumulation of these ions by the plants is quite evident. |
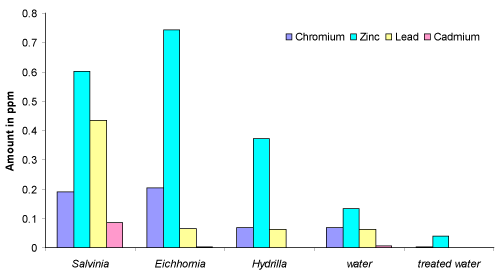
| The results show that the accumulation of Pb, Cd, Ni and Co is highest in Salvinia than Eichhornia and hydrilla, while the accumulation of Cu, Mn, Zn, Cr and Fe is found to be higher in Eichhornia. The extent of concentration of metal ions in Salvinia, Eichhornia and hydrilla are compared and the order is |
| Salvinia: Mg > Ca > Fe > Cu > Zn > Pb > Mn > Ni > Cr > Cd > Co |
| Eichhornia: Ca > Cu > Fe > Mg > Mn > Zn > Cr > Ni > Pb > Co > Cd |
| hydrilla: Ca > Cu > Mg >Fe > Zn > Ni > Mn > Cr > Pb > Co > Cd |
| The present investigation shows that the plants, Salvinia, Eichhornia and hydrilla are effective and inexpensive absorbents for many toxic metal ions. The plants can be used to purify water from industrial effluents. In the Rankala Lake, the sources of toxic metal ions like Pb, Cr must be dumping of batteries, effluents from the electroplating industries, the submersion of Ganesh idols, sewage, leather industry effluent and leaching of the residue due to rain water and effluent to the lake water. The enormous growth of these plants (Figure 1) indicates the nature and volume of pollution of Rankala Lake which shows the initiation of eutrophication. Figure 2 reveals that the Salvinia plant was found to be efficient for the absorption of Pb, Cd, Zn and Cr while Eichhornia was found efficient towards Zn and Cr ions. |
| Determination of heavy metal ions from water sample treated with microorganism |
| The water sample was treated with the microorganisms as per the described procedure. The treated water sample was subjected to analyze the metal concentration by AAS. From the results it is found that there is a decrease in the concentration of metal ion which shows that these microorganisms are consuming the metals and playing very important role in the removal of the metal ions. It has been found that the concentration of metal ions in the microbial treated water is decreased, while the concentration of magnesium and manganese ions seems to be slightly increased after the treatment. This may be due to the metal ions entering from cultural media. The microorganisms, Bacillus thuringiensis NCIM 2159 and Proteus spp. SUK 7 were found to be effective for removal of toxic metal ions from water sample under study. It has been found that, at a given concentration of the metal ions the cobalt, nickel, lead and cadmium were removed completely while zinc, copper and chromium were partially removed (Table 3). Remediation of copper is about 45% while that of chromium is about 90%. |
| Pesticide residue analysis from water by liquid chromatography tandem mass spectrometer |
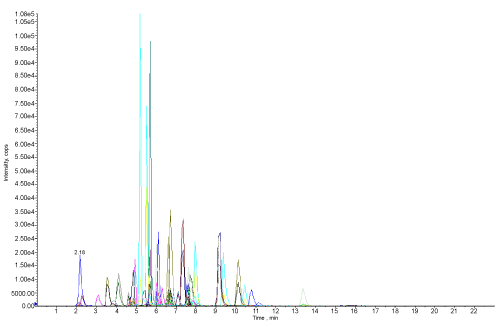
| Figure 3 shows the chromatogram of standard 59 pesticides of 0.250 mg/L. The water sample was extracted as per the procedure and 20 μL of sample was run in LCMS/MS. The water sample after treatment with microorganisms was extracted by the same procedure and sample was run in LCMS/MS. |
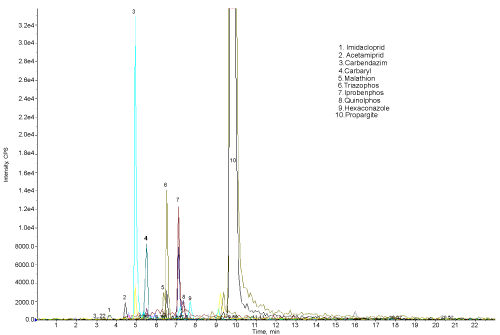
| The results show that 11 pesticide residues are found to be present in the studied water sample. The amount of propargite found was very high. The results show that the Rankala Lake is contaminated with pesticides. Most of the pesticides enter the lake through water streams coming from the agricultural land. The pesticide residues are found in the order |
| Propargite > Propenophos > Quinolphos > Hexaconazole > Carbendazim > Imidacloprid > Triazophos > Iprobenphos > Malathion > Carbaryl > Acetamiprid |
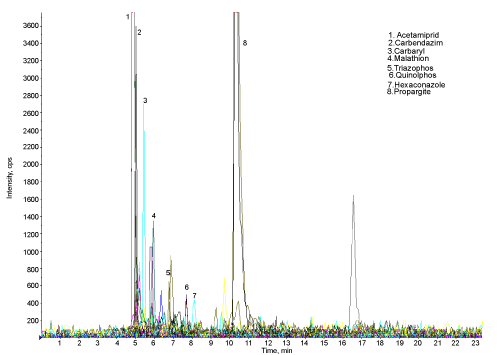
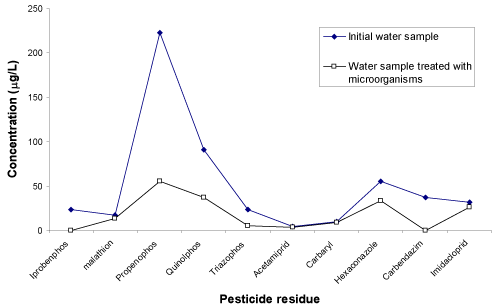
| The Figure 4 shows the chromatogram of water sample indicating the 10 peaks of different pesticides (except propenophos, low response hence there is no peak). Figure 5 shows the chromatogram of water sample treated with microorganisms. The results show that, these organisms are efficient for the degradation of all the 11 pesticides. The amount and percentage of degradation of pesticides by the micro organisms are as shown in Table 2. The graph of concentration of pesticide residue in water sample and microbial treated water sample (Figure 6) shows the efficiency of microorganisms for the removal of pesticides residue from water, except malathion, carbaryl and imidacloprid. |
| Conclusions |
| The study reveals that, the pollution levels of stagnant water bodies in small cities have also reached to a severe stage. From the study it is concluded that, the lake water is polluted by pesticide residues entering through the irrigation of agricultural lands in vicinity. The residues of eleven pesticides have been found in the water and the level of propargite is very high. The lake water is also found to be contaminated by heavy metal ions like Pb, Cr, Zn and Cd probably entering from industrial waste, through drainage and battery industries in the vicinity. At the same time, the aquatic plants like Eichhornia and Salvinia appears to play a vital role in removing the heavy metal ions and correcting the system. But the plants should be carefully controlled and harvested to remove the toxic metal residues. The microorganisms like Bacillus thuringiensis NCIM 2159 and Proteus spp. SUK 7 are found effective in assimilation and degradation of many of the pesticide residues. However, further investigation regarding scaling up and commercial operation is necessary. The natural or manmade water bodies in and near the settlements play very important role in improvement and conservation of local environment, but many of them are on the way of eutrophication. This problem needs an urgent and sincere attention of environmentalists. |
| Acknowledgements |
| Author is thankful to Shanghai University for post doctoral fellowship and also thankful to Prof. Dr. Genxi Li, Dean, School of Life Sciences, Shanghai University, Shanghai, China, for valuable discussion and support during the work. Author is also thankful to Prof. K. J. Patil and Dr. R. R. Kumbhar for their valuable suggestions for this work. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16450
- [From(publication date):
April-2012 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 11527
- PDF downloads : 4923
