Research Article Open Access
Exploring the Viability of Modified Cellulosic Polymer and its Use as Reinforcement to Obtain Green Composites by Advanced Analytical and Evaluation Techniques
Ashish Chauhan* and Balbir Kaith
Department of Chemistry, Dr. B. R. A. National Institute of Technology, Jalandhar, India
- *Corresponding Author:
- Ashish Chauhan
Department of Chemistry
Dr. B. R. A. National Institute of Technology
Jalandhar-144011(Punjab) India
E-mail: aashishchauhan26@gmail.com
Received date: November 21, 2012; Accepted date: November 27, 2012; Published date: December 05, 2012
Citation: Chauhan A, Kaith B (2012) Exploring the Viability of Modified Cellulosic Polymer and its Use as Reinforcement to Obtain Green Composites by Advanced Analytical and Evaluation Techniques. J Anal Bioanal Tech 3:156. doi: 10.4172/2155-9872.1000156
Copyright: © 2012 Chauhan A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The cellulose rich Roselle stem fiber found in abundance throughout the world was graft copolymerized by vinyl monomeric mixtures. The graft copolymer were characterized for the change in chemical structure by modern analytical techniques and evaluated for change in physical, chemical, thermal properties. These graft copolymers were then used as reinforcement in polymer matrix to form green composites. These composites had advanced physico-chemico-thermo-mechanical properties and viability that can have multi-disciplinary utilization for scientific, industrial applications that could serve as pioneer for the development of science and technology.
Keywords
Graft copolymerization; Monomer; Chemical reactivity; Crystallinity
Introduction
The term ‘composite’ in material science refers to a material made up of a matrix containing reinforcing agents. The beginning of composite materials may have been the bricks, fashioned by the ancient Egyptians from mud and straw. Considering the high performance standard of composite materials in terms of durability, maintenance and cost effectiveness, applications of natural fiber reinforced composites as construction material, have done wonders and are environment friendly material for the future use.
Natural fibers and polymer are essential for the survival of mankind as it fulfills the primary and the secondary needs. Major sections of the scientist throughout the world have focused their attention towards utilization of renewable and sustainable resources because of environment and health concerns. In order to improve its properties various techniques like graft copolymerization, mercerization, acetylation, benzoylation and treatment with various coupling agent can be used. They have been widely studied and gave many fruitful results. Graft copolymerization has transformed the properties of the biomass and given some remarkable results for the development of technology [1-4].
The virgin Roselle (Hibiscus sabdariffa in short Hs) stem fiber has not been studied moreover graft copolymerization by Ethyl acrylate as primary monomer while in binary vinyl monomeric mixture with Methyl acrylate (MA) and Acrylonitrile (AN) as secondary monomer. Roselle fiber has high cellulose content of 73.9% and high tensile strength and low weight that promotes its use as backbone for grafting. The graft copolymers thus obtained were subjected to characterization and evaluation for use as reinforcement in green composite. Most of these studies remain unnoticed even after the well known fact that Roselle is a substitute to jute.
Material and Methods
Monomers (Merck) and chemicals (s.d. fine-Chem, Pvt. Ltd, Mumbai, India) were used as received. Composites were prepared in Compression Molding Machine (SANTEC India Ltd.) and the samples were tested on the Universal Testing Machine (HOUNSFIELD, H25KS). Monomers (Merck) and chemicals (s. d. fine-Chem, Pvt. Ltd,Mumbai, India) were used as received. Roselle fiber was obtained from the Department of Agronomy, Chaudhary Sarwan Kumar Himachal Krishi Vishwavidyalaya, Palampur (H.P), India and refluxed in acetone prior to use.
Characterization
IR spectra of the H. sabdariffa and its graft copolymers were recorded on Perkin Elmer Fourier Transform Infrared (FTIR) spectrophotometer while using anhydrous KBr (Sigma Aldrich) and hydraulic punching machine. Scanning Electron Micrographs (SEM) of H. sabdariffa and its graft copolymers were obtained by using Electron Microscopy Machine (LEO 435-25-20), under ambient conditions. Thermo gravimetric–Differential Thermo gravimetric Analysis (TGDTA) was performed on thermal analyzer (LINSEIS, L-81 11). TGA of raw and grafted fiber has been studied as a function of percentage wt. loss vs. temperature. X-ray powder diffraction (XRD) studies were performed on X-ray diffractometer (Bruker D8 Advance) under ambient conditions using Cu Kα (1.5418 Å) radiation, Ni-filter and scintillation counter as detector at 40 KV and 40 mA on rotation between 13° (2θ) to 25° (2θ) at 1s accumulation time and step size of 0.01 degree with 0.5° or 1.0 mm of divergent and anti-scattering slit. Corundum and quartz were used as the reference to verify and calibrate the instrument. The counter reading at the peak intensity at 22.68° (2θ) represents the crystalline material and the peak intensity at 15° (2θ scale) corresponds to the amorphous material in the cellulose Percentage Crystallinity (% Cr) and Crystallinity index (C.I.) were calculated by Eq. 1 and 2 [5]:
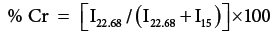 (1)
(1)
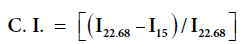 (2)
(2)
Graft copolymerization
Graft copolymerization of the monomer (EA) onto H. sabdariffa was carried-out for the optimization of different reaction conditions (reaction time, reaction temperature, monomer concentration, concentration of initiator system and pH) in order to obtain the maximum graft yield, under ambient conditions. The raw fiber was activated by swelling (0.5 g fiber in 100 ml) in distilled water for 24 h prior to use. Mixture of ceric ammonium nitrate (initiator) and conc. nitric acid (oxidizer) was slowly added to the reaction medium under continuous stirring followed by addition of the monomer. On completion of the reaction, poly (EA), poly (MA) and poly (AN) were removed on extraction with acetone, methanol, chloroform, di-methyl formamide and water. The graft co-polymers were dried at 50°C, till a constant weight. The percent grafting (Pg) was calculated by Eq. 3 [5]:
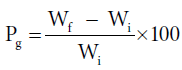 (3)
(3)
Where, Wf = final weight of the fiber, Wi = initial weight of the fiber.
Preparation of the polymer matrix resin and green composite
Different ratios of P:F was optimized with respect to their mechanical behavior so as to achieve better mechanical properties. P:F ratio of 0.75:1.0 was found to be the optimum ratio exhibiting best mechanical properties. Green composites were prepared by mixing ratio of phenol:formaldehyde (0.75:1.0) and fiber to resin (12.7:87.3), respectively. The mixture was then placed in the mold of a particular dimension: 40-80 mm (length), 5×5 mm (cross section). Degasification of sample was performed in Compression Molding Machine and the samples were kept for curing at 120°C for 10 minutes under 400 kg/ cm2 pressure. Composites thus prepared by reinforcing the raw fiber and its graft copolymers in each case, the number of specimen used for the determination of mechanical properties were three. Tests were conducted under ambient laboratory conditions [6,7].
Physico-chemical evaluation
The important properties of the fiber that have industrial significance like moisture absorbance, acid base resistance, dye uptake and swelling were taken up for study.
Moisture absorption study
Moisture absorbance studies were carried-out at various relative humidity levels from 30-90 % at ambient temperature as studied before and given in the Eq. 4 [1-3,6]:
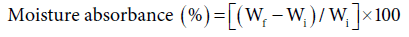 (4)
(4)
Acid and base resistance: Acid and base resistance studies were studied for 72 hours as studied before and as shown in Eq. 5 [1-3,6], under ambient conditions.
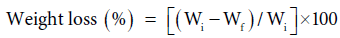 (5)
(5)
Swelling behavior in different solvents: 250 mg of grafted and raw sample was immersed in 100 ml of solvents under ambient conditions for a period of 24 hours and excess solvent were removed with filter paper as discussed before and given in Eq. 6 [1-3,6]:
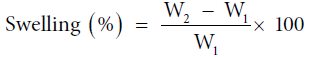 (6)
(6)
Where, W1 and W2 are the initial and final weights of samples, respectively.
Dye uptake behavior: 0.1% Gentian violet solution was prepared in distilled water as 10% NaCl solution and a few drops of acetic acid were added to this solution. Optical densities of the test solutions were observed using Digital Photo Colorimeter for seven consecutive hours and the concentrations of test solution were calculated as studied before [1-3,6]:
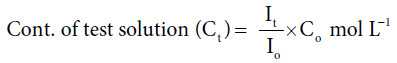 (7)
(7)
Where, Io, It, and Co is optical density of standard solution, optical density of test solution and concentration of standard solution, respectively.
Mechanical evaluation
Modulus of rupture (MOR)/Flexural strength: MOR was determined according to ASTM D 790 standard and was calculated as discussed before using the Eq. 8 [6,7]:
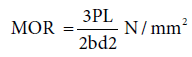 (8)
(8)
Where, P=peak load, L=length of the sample, b=width of the sample and d=thickness of the sample.
Modulus of elasticity (MOE)/Young’s modulus: MOE was determined according to ASTM D 790 standard and was calculated as discussed before using the following equation 9 [6,7]:
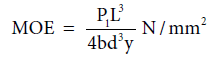 (9)
(9)
Where, P1=load at the limit of proportionality and y=rate of bending.
Stress at the limit of proportionality (SP): Stress at the limit of proportionality was calculated as discussed before by using the equation 10 [6,7]:
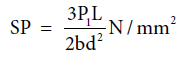 (10)
(10)
Hardness: Hardness is an important property of composites. This property of the composites was tested using Rockwell Hardness tester (Balancing Instrument and Equipments Miraj Pvt. Ltd., Model-TSM) following ASTM D785 standard, using B-scale, 100 Kg as a major load and 10 Kg as a minor load with 1/16 inch indenter steel ball and was measured in HRB [6,7].
Results and Discussion
Mechanism
The graft copolymerization of poly (EA) as principal monomer onto H. sabdariffa fiber was effective (Pg: 117.30). Use of ethyl acrylate as principal monomer in binary vinyl monomeric mixture with secondary monomers like MA and AN resulted in Hs-g-poly (EA-co- MA) and Hs-g-poly (EA-co-AN), respectively (Table 1). C2, C3, and C6 hydroxyls and C-H groups were the active sites for the incorporation of polymeric chains through grafting onto cellulosics. Ceric ammonium nitrate (initiator) was used as a source of ceric ion and the presence of concentrated nitric acid (oxidizer) played an important role during graft copolymerization. In aqueous medium, ceric ion existed as [Ce- O-Ce]6+. Since, the large size [Ce-O-Ce]6+ ions were unable to form complex with the fiber so, due to the presence of nitric acid, more Ce4+ and [Ce(OH)3]3+ ions were formed. Then these ions easily underwent complex formation with the fiber. Ceric ion formed the chelate complex with the cellulose through C-2 and C-3 hydroxyl groups of the anhydro glucose unit. Transfer of the electron from the cellulose molecule to Ce (IV) followed, leading to its reduction to Ce (III), breakage of the bonds at C-2 and C-3 that resulted in the formation of the free radical sites. Grafting of vinyl monomer onto polymeric backbone occurred as follows [5,6].
| Sample | Binary mixture(×10-3 mole L-1) | Mean Pg | ± SD | ± SE |
|---|---|---|---|---|
| Hs-g-poly (EA ± MA) | 2.26 ± 0.92 | 99.90 | ± 4.41 | ± 2.54 |
| 2.26 ± 1.84 | 141.06 | ± 2.60 | ± 1.50 | |
| 2.26 ± 2.76 | 179.96* | ± 7.15 | ± 4.13 | |
| 2.26 ± 3.68 | 112.10 | ± 2.57 | ±1.48 | |
| 2.26 ± 4.60 | 81.80 | ± 2.60 | ± 1.50 | |
| Hs-g-poly (EA ± AN) | 2.26 ± 1.25 | 175.10* | ± 4.40 | ± 2.54 |
| 2.26 ± 2.51 | 84.90 | ± 3.54 | ± 2.05 | |
| 2.26 ± 3.76 | 48.70 | ± 7.16 | ± 4.13 | |
| 2.26 ± 5.03 | 35.60 | ± 3.58 | ± 2.06 | |
| 2.26 ± 6.28 | 28.50 | ± 1.75 | ± 1.01 |
Where,* refers to the effective Pg
Table 1: Effect of the binary mixtures on Pg using EA as a principal monomer.
The optimized reaction conditions using EA as principal monomer onto the fiber were: monomer conc.: 2.26×10-3 mol L-1; CAN: 2.41×10-4 mol L-1; HNO3 conc.: 1.46×10-3 mol L-1; pH of the medium: 7; time: 150 mins. ; Temperature: 35°C that yielded Pg of 117.30 (± SD: 7.13; ± SE: 4.12). The use of ethyl acrylate as principal monomer for graft copolymerization onto H. sabdariffa fiber yielded a high Pg. It was due to high rate of propagation (Kp), low rate of termination (Kt), higher transfer rate constant (Cm) and higher reactivity of the monomer. MA is an efficient monomer for grafting onto H. sabdariffa backbone, its combination with ethyl acrylate as comonomer results in high Pg (179.96). Acrylonitrile because of high reactivity, high solubility in the reaction medium, suitable reactivity ratio strikes a perfect hydrophobichydrophilic balance that stimulates grafting (Pg: 175.10) in binary mixture with EA (Table 1). However, many other factors also determine the graft yield like the type of fiber, swelling, number of active sites; the nature and amount of the solvent and temperature of polymerization strongly influence the reactivity ratios. Elevated temperature favors the degradation, reduces the initiator half life, modifies the rate or specificity of the reaction, and influences the solubility and rheological parameters [8-16].
Characterization
FT-IR: IR spectrum of the H. sabdariffa showed a broad peak at 3424.0 cm-1 (-OH group) and peaks at 2924.7 cm-1, 1246.9 cm-1 and 1032.0 cm-1 were observed due to–CH2, C-C and C-O stretching, respectively. However, in case of Hs-g-poly (EA) an additional peak due to >C=O groups of the EA was witnessed at 1734.0 cm-1 whereas a peak at 2235.0 cm-1 (–CN group) in Hs-g-poly(EA-co-AN) and 1734.0 cm-1 (>C=O stretch) in Hs-g-poly (EA-co-MA) confirms the incorporation of the secondary monomers in these graft copolymers [6].
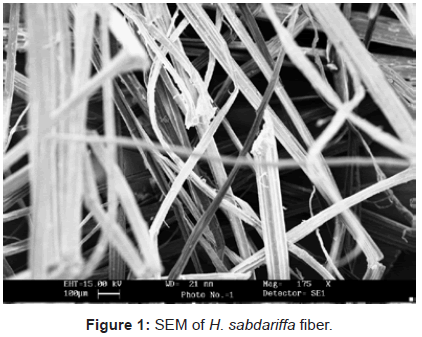
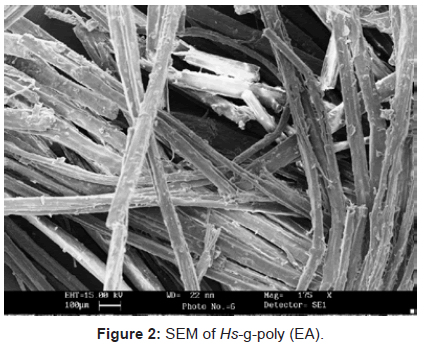
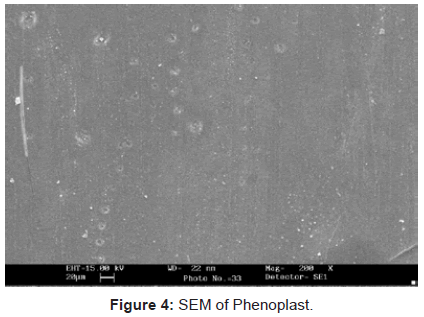
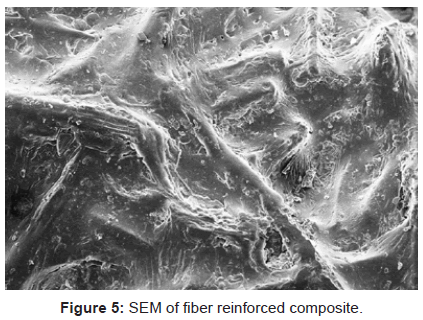
Scanning electron microscopy: Since, cellulose and P-F matrix had non-conducting behavior so they were gold plated to have an impact.It is quite evident from the figures 1-3, that there has been a sufficient deposition of polyvinyl monomers onto fiber. Comparison of the scanning electron micrographs of raw H. sabdariffa fiber with the graft copolymers reveals the distinction between the isolated and randomly distributed ungrafted fiber and the grafted samples that started forming the bundles in close packing, depending upon the Pg. Similarly, the phenoplast when embedded with fiber and graft copolymers completely changed the surface (Figures 4 and 5) [6,16].
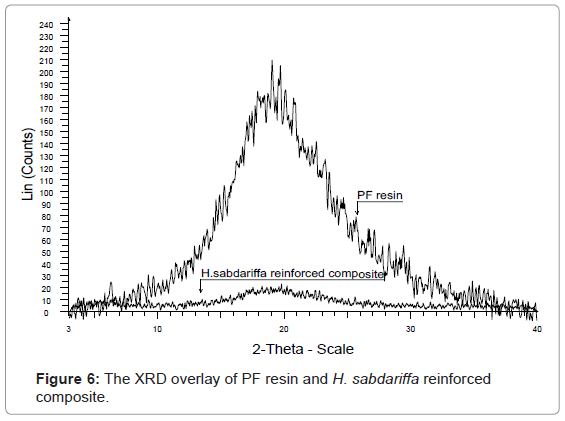
X-RD studies: It is evident from the table 2 that the percentage crystallinity and crystallinity index were found to decrease with increase in percentage of grafting. Since, the incorporation of monomer in the backbone impairs the natural crystalinity of the fiber; therefore, the graft copolymerization of EA onto H. sabdariffa fiber resulted in impaired crystallinity and increased the amorphous content in the fiber. Thus, with increase in percentage grafting, the percentage crystallinity and crystallinity index decreased along-with reduction in stiffness and hardness. However, the cellulose form I remained unchanged. This clearly indicates that the cellulose crystals are better oriented and have high crystallinity in raw H. sabdariffa fiber rather than in Hs-g-poly (EA) and other graft copolymers. The PF resin gave a halo pattern while there was further increase in amorphous content with increase in fiber and grafted fiber content in the composite as seen in figure 6 [5,6,16].
| Sample | Pg | 2θ Scale | %Cr | C.I. | |
|---|---|---|---|---|---|
| I15 | I22.68 | ||||
| H. sabdariffa | - | 40 | 136 | 77.20 | 0.70 |
| Hs-g-poly(EA) | 117.30 | 10 | 29 | 74.34 | 0.65 |
| Hs-g-poly(EA-co-MA) | 179.96 | 10 | 16 | 62.50 | 0.37 |
| Hs-g-poly(EA-co-AN) | 175.10 | 10 | 19 | 65.51 | 0.47 |
Where, % Cr=Percentage of crystallinity, C. I.=Crystallinity Index
Table 2: The % Cr and C. I. of the H. sabdariffa fiber and its graft copolymers.
Thermal analysis
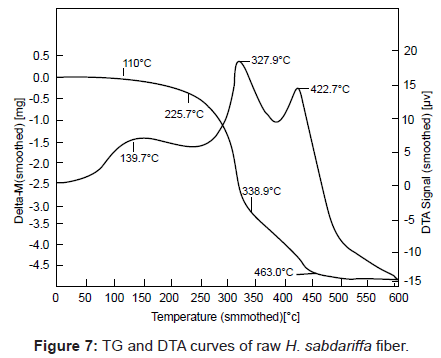
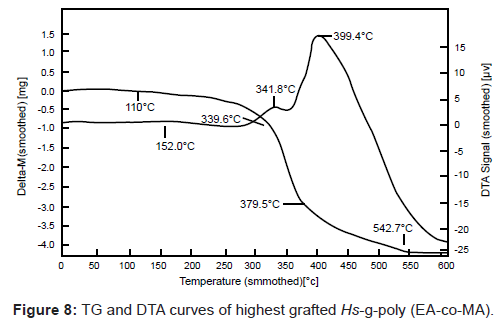
TG-DTA studies of graft copolymers: Cellulosic in H. sabdariffa degrades by dehydration, glycogen formation and depolymerization. In case of H. sabdariffa, two-stage thermal degradation has been found in the temperature range of 225.7-338.9°C with 54.0 % weight loss and between 338.9-463.0°C with 26.00% weight loss (Table 3 and Figure 7). The former stage is attributed to the loss by dehydration, volatilization processes whereas the later stage is referred to loss by depolymerization, delignification and oxidation of the char. Graft copolymers i.e. Hs-gpoly (EA) and Hs-g-poly (EA-co-vinyl monomer) showed two major stages of decomposition (Table 3 and Figure 8). The first stage pertains to loss of moisture and decarboxylation and the second stage indicates the breaking up of crystallites and covalent bonds in vinyl monomer. Thus, it is evident from the TGA data that grafted fibers are thermally more stable than the raw fibers due to the incorporation of poly (EA) and poly (vinyl) monomer chains on backbone polymer through strong covalent bonding or mechanically due to surface grafting, confirming the additional strength and thermal stability to the fiber [6,9].
| Sample Hs-g-poly- |
Pg | TGA | DTA | ||
| IDT | FDT | % Residue | Peaks in °C(μV) | ||
| H. sabdariffa | - | 225.7 | 463.0 | 20.00 | 139.7 (6), 327.9 (18.0), 422.7 (14) |
| (EA) | 117.3 | 312.4 | 500.0 | 06.70 | 125.0 (2), 361.2 (1.2), 432.5 (29) |
| (EA-co-AN) | 175.1 | 268.0 | 520.0 | 03.30 | 176.4 (17.1), 340.0(21), 425 (22.7) |
| (EA-co-MA) | 179.9 | 339.6 | 542.7 | 28.33 | 152.0 (1.2), 341.8(3.3), 399.4 (17) |
| Phenoplast | - | 500.0 | 600.0 | - | 551.0 (150) |
| Hs-r-PF-C | - | 540.0 | 680.0 | 10.0 | 610.0 (164) |
where, C= composite, IDT = initial decomposition temperature, FDT= final decomposition temperature.
Table 3: TG-DTA of H. sabdariffa, graft copolymers and composite.
In DTA results, H. sabdariffa has been found to exhibit two exothermic peaks at 327.9°C (18 μV) and 422.7°C (14 μV). Exothermic peak at 327.9°C corresponds to decomposition stage between 225.7- 338.9°C while the exothermic peak at 422.7°C corresponds to second decomposition stage (338.9-463.0°C) in TGA (Table 3 and Figure 7). In case of graft copolymers, the first and second transition peaks revealed the dehydration, adsorption and oxidation of the semi-crystalline host and the major peak signifies the fusion and irreversible dissociation of the crystallites (Table 3 and Figure 8) [6,16].
TG-DTA studies of phenoplast and composite: The TGA results of the P-F composite, showed 500°C and 600°C as the initial decomposition temperature and final decomposition temperature, respectively. The percentage weight loss, during this decomposition was found to be 26.61% in the temperature range of 50-500°C while it was 73.39% during the final decomposition in the temperature range of 500-600°C. The higher initial and final decomposition temperature could be due to three dimensional network structure of the thermoset. The DTA showed prominent exothermic peaks at 551°C (150 μV) that corresponds to the major decomposition due to irreversible decomposition of the crystallites between 500-600°C as a function of temperature, respectively. The fiber reinforced composite has higher initial and final decomposition temperature due to strong network of covalent bonds that is further supported by DTA results (Table 3) [6].
Physico-chemico evaluation
Moisture absorbance study: It has been observed that with increase in graft yield, there was significant decrease in the percentage of moisture absorbed. It was due to blocking of the sites vulnerable for moisture absorbance with hydrophobic poly (EA), poly (vinyl) monomer chains, thereby, transforming the fiber less sensitive to moisture. Similarly, the graft fiber reinforced composite complex showed a marked decrease in moisture absorbance due to intricate three dimension bonds in the resin that formed strong fiber matrix complex moreover, the monomer acted as a coupling agent to reinforce the strength in comparison to the phenoplast as seen in table 4 [1-3,6,16].
| Graftcopolymer | Pg | % Chemical resistance% wt. loss after 72 hour | % Moisture absorbanceat different RH after 12 hours | ||||
|---|---|---|---|---|---|---|---|
| Hs-g-poly- | 1N HCl | 1N NaOH | 30-35% | 50-55% | 60-65% | 85-90% | |
| H. sabdariffa | - | 55.0 | 43.0 | 0.5 | 0.8 | 1.8 | 2.5 |
| (EA) | 117.30 | 04.0 | - | - | - | - | 0.2 |
| (EA-co-AN) | 175.10 | - | - | - | - | - | - |
| (EA-co-MA) | 179.96 | - | - | - | - | - | - |
| Phenoplast | - | 04.0 | 02.0 | - | - | 3.0 | 5.0 |
| Hs-r-PF-C | - | 01.0 | - | - | - | - | 3.0 |
Where C= composite.
Table 4: Chemical resistance and moisture absorbance studies of composite, graft copolymers vis-a-vis back bone.
Acid–base resistance study: It has been observed that acid- base resistance of the fiber increased with the increase in percent grafting. It could be accounted by the fact that poly (EA) and poly (vinyl) monomer chains grafted and covalently bound onto H. sabdariffa fiber had lesser reactivity for 1 N HCl and 1 N NaOH as compared to the free hydroxyl groups present in the ungrafted fiber (Table 4). Similarly, the graft fiber reinforced composite showed a marked increase in chemical resistance due to strong fiber matrix complex formation in comparison to the Phenoplast [1-3,6,16].
Swelling behavior study: The swelling behavior studies were carried-out in different solvents like Water, MeOH, BuOH and DMF. It has been observed that H. sabdariffa fiber showed maximum swelling in Water (59%) followed by MeOH (46%), BuOH (38%) and DMF (30%). However, the swelling behavior of the graft copolymers followed the pattern: DMF>BuOH>Water>MeOH and the trend obtained had a direct correlation with the solubility parameters like solvent basicity, the molar ratio, hydrogen bond formation and the percentage grafting. The differential swelling behavior of the fibers could be justified depending upon the chemical nature and the property of the pendent groups (-COCH3,-COC2H5and-CN) that had different interactions with the solvent. Higher swelling in DMF and BuOH in grafted copolymers could be due to better interaction with the pendent groups that increases with increase in Pg. However, a reverse trend was found in the case of raw H. sabdariffa. Since, the cellulose is semi crystalline, polar polymer and miscible with in polar solvent due to hydrogen bond formation and imbibitions therefore, the raw fiber had higher swelling in water and MeOH followed by DMF and BuOH. Moreover, other factors like the fiber size, steric hindrance and temperature also influence the percentage of swelling (Table 5) [1-3,6,16].
| Sample |
Pg |
% Swelling | |||
|---|---|---|---|---|---|
| Water | MeOH | BuOH | DMF | ||
| H. sabdariffa | raw | 59.00 | 46.00 | 38.00 | 40.00 |
| Hs-g-poly(EA) | 117.30 | 14.00 | 10.00 | 62.00 | 78.00 |
| Hs-g-poly(EA-co-AN) | 175.10 | 10.00 | 10.00 | 76.00 | 94.00 |
| Hs-g-poly(EA-co-MA) | 179.96 | 12.00 | 08.00 | 80.00 | 95.00 |
Table 5: Effect of solvents on swelling behavior of graft copolymers vis-a-vis backbone.
Dye-uptake behavior: The graft copolymers exhibited less dye uptake as compared to the raw fiber as it was found to be a function of Pg. It was observed that dye uptake decreases with increase in Pg. Cellulose is semi crystalline polymer that easily swells due to competitive processes of adsorption through hydrogen bonding and the scission of the internal hydrogen bonds between the amorphous molecules. Presence of free reactive sites like–OH and–CH2OH in raw fiber helped in the absorption of the dye. But, these sites get occupied with poly (EA) chains and poly (EA-co-MA) and poly (EA-co-AN) chains in the backbone that restrain dye uptake depending upon the Pg (Table 6) [1-3,6,16].
Sample |
Pg | Dye concentration of the test solution at different time intervals (×10-4 mol L-1) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | 7 h | ||
| H. sabdariffa | raw | 4.96 | 4.38 | 4.08 | 4.08 | 3.79 | 3.21 | 3.21 |
| Hs-g-poly(EA) | 117.3 | 5.84 | 5.84 | 5.54 | 5.54 | 5.54 | 5.54 | 5.54 |
| H-g-ply(EA-co-AN) | 175.10 | 5.84 | 5.54 | 5.54 | 5.54 | 5.54 | 5.54 | 5.54 |
| Hs-g-ply(EA-co-MA) | 179.96 | 5.54 | 5.54 | 5.54 | 5.54 | 5.54 | 5.54 | 5.54 |
Table 6: Dye uptake studies of the graft copolymers vis-a-vis back bone.
Mechanical accreditation
Hs-reinforced-PF composites with phenol: formaldehyde of 0.75:1.0 and fiber to matrix ratio of 12.7:87.3 depicted better mechanical behaviors as compared to the P-F resin (Table 7). Inadequate amount of fiber within the polymer matrix did not support the composite under continuous stress and strain whereas, the excess of the fiber led to debonding. Loading of the matrix beyond optimum ratio decreased the stiffness and impaired the needed fiber matrix interaction. The better mechanical behavior could be accounted due to monomer incorporated onto the fiber that acts as coupling agent, compatible fiber-matrix interaction and orientation of the fiber. However, some deviation in the results could be justified by other governing factors for overall mechanical performance like nature and amount of matrix and fiber, orientation, distribution of the fiber with respect to the matrix axis, form of reinforcement used (woven or non-woven, grafted or ungrafted), strength of the interfacial bond between the fiber and matrix, length of the fiber (continuous or discontinuous), aspect ratio that on mere imbalance may lead to debonding and cracking [6,13,15].
| Composite | Strength Test | |||
|---|---|---|---|---|
| MOR(N/mm2) | MOE(N/mm2) | SP(N/mm2) | Hardness(HRB) | |
| Phenol-formaldehyde | 43.20 | 496.12 | 38.40 | Brittle |
| H. sabdariffa-r-PF | 69.99 | 614.40 | 57.60 | 67.00 |
| Hs-g-poly(EA)-r-PF | 82.04 | 898.56 | 74.24 | 75.00 |
| Hs-g-poly(EA-co-AN)-r-PF | 116.40 | 1096.96 | 02.84 | 79.00 |
| Hs-g-poly(EA-co-MA)-r-PF | 80.80 | 690.99 | 74.78 | 78.00 |
where, -r-PF refers to reinforced Phenol-formaldehyde
Table 7: MOR, MOE, SP and Hardness studies of graft copolymers reinforced P-F composite vis-a-vis P-F resin.
Conclusions
Transformation of waste renewable biomass to graft copolymers i.e. Hs-g-poly (EA) Pg: 117.30, Hs-g-poly (EA-co-AN) Pg: 175.1 and Hs-g- poly (EA-co-MA) Pg: 179.9 had many improved properties. With increase in grafting, undesired features like dye uptake, hydrophylicity, percentage of crystallinity and crystallinity index decreased, but increased the promising features like physico-chemico-thermal resistance and miscibility in organic solvents, hydrophobicity, that was needed for industrial use. The synthesis of such novel graft copolymers is judicious, economic, fruitful means to utilize the waste renewable biomass for scientific applications and the development of science and technology. The use of graft copolymers as reinforcement in phenoplast matrix based green composite improved the physico-chemico-thermomechanical properties, eased the degradation of the composite, helped in its sustained availability and serve an alternate to glass fiber for reinforcement. These novel graft copolymers and green composites could be used in insulator, transportation, packaging and aerospace and various other applications.
References
- Kaith BS, Singha AS, Sharma SK (2004) Synthesis of Graft Copolymers of Binary Vinyl Monomer Mixtures and Flax Fiber using FAS-KPS Redox System. Int J Chem Sci 2: 37-43.
- Kaith BS, Singha AS, Sharma SK (2003) Graft Copolymerization of Flax Fibers with Binary Vinyl Monomer Mixtures and Evaluation of Swelling, Moisture absorbance and Thermal Behavior of the Grafted fibers. J Polym Mater 20: 195.
- Singha AS, Kaith BS, Kumar S (2004) Evaluation of Physical and Chemical Properties of FAS –KPS induced Graft Co-polymerization of Binary Vinyl monomer mixtures onto mercerized Flax. Int J Chem Sci 2: 472.
- Kaith BS, Singha AS, Dwivedi DK, Kumar S, Kumar D, Dhemeniya A (2003) Preparation of Polystyrene Matrix Based Composites using Flax-g-copolymers as Reinforcing agent and Evaluation of the Mechanical Behavior. Int J Plast Technol 7: 119-125.
- Ashish Chauhan, Balbir Singh (2011) X-Ray Powder Diffraction Studies to Accredit the Morphological Transformations in Hibiscus sabdariffa Graft- copolymers. International Journal of Polymer Analysis and Characterization 16: 319-328.
- Chauhan A (2009) Synthesis and Evaluation of Physico-Chemico-Mechanical properties of polymer matrix based Composites using Graft copolymers of Hibiscus sabdariffa as reinforcing agents. Ph. D. Thesis, Punjab Technical University.
- Kaith BS, Chauhan A (2008) Synthesis, Characterization and Mechanical Evaluation of Phenol formaldehyde composites. E-Journal of Chemistry 5: 1015-1020.
- Singha AS, Rana RK (2012) Chemically induced graft copolymerization of acrylonitrile onto lignocellulosic fibers. J Appl Polym Sci 124: 1891–1898.
- Mithu M,Rajeev J,Balbir Singh K,Asim Kumar J (2012) Induction of physico-chemical and thermal resistance on Saccharum spontaneum L by grafting under microwave irradiation. International Journal of Polymeric Materials 61: 1-16.
- Taghizadeh MT, Khosravy M (2003) Kinetics and mechanism of graft copolymerization of vinyl monomers (Acrylamide, Acrylic acid and Methacrylate) onto starch by potassium Dichromate as redox initiator. Iran Polym J 12: 497-505.
- Kaith BS, Kalia S (2007) Synthesis and Characterization of Graft co-Polymers of Flax Fiber with Binary Vinyl Monomers. International Journal of Polymer Analysis and Characterization 12: 401-412.
- Fried J (2003) Conformation, solution, and molecular weight. In: Polymer Science and Technol. (2ndedn), Prentice Hall, NJ.
- Brandrup J, Immergut EH (1975) Polymer Handbook, Wiley Interscience, John Wiley and Sons, New York 2 II-105.
- Ham G (1964) High Polymer, Copolymerization, Interscience Publishers 18: 698.
- Brandrup J, Immergut EH (1975) Polymer Handbook, Wiley Interscience, John Wiley and Sons, New York 2: II-387.
- Chauhan A, Kaith B (2011) Synthesis, Characterization and Chemical studies of Hibiscus sabdariffa-g-copolymers. Fibers and polymers 12: 1-7.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14161
- [From(publication date):
December-2012 - Apr 06, 2025] - Breakdown by view type
- HTML page views : 9593
- PDF downloads : 4568