Editorial Open Access
Exosomes and Shedding Microvesicles are Mediators of Intercellular Communication: How do they Communicate with the Target Cells?
Suresh Mathivanan*Department of Biochemistry, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Victoria 3086, Australia
- Corresponding Author:
- Suresh Mathivanan
Department of Biochemistry
La Trobe Institute for Molecular Science
La Trobe University, Melbourne, Victoria 3086, Australia
Tel: +61 03 9479 2506
Fax: +61 03 9479 1226
E-mail: S.Mathivanan@latrobe.edu.au
Received date: June 29, 2012; Accepted date: June 30, 2012; Published date: July 11, 2012
Citation: Mathivanan S (2012) Exosomes and Shedding Microvesicles are Mediators of Intercellular Communication: How do they Communicate with the Target Cells? J Biotechnol Biomater 2:e110. doi:10.4172/2155-952X.1000e110
Copyright: ©2012 Mathivanan S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Intercellular interactions are pivotal for basic cellular activities and errors in either receiving or transmitting these signals are shown to cause pathological conditions. Whilst, such intercellular communications were once thought to be regulated by membrane surface molecules and/or soluble secreted proteins by stimulating the target cells through receptor mediated activation, increasing evidences suggest that extracellular microvesicles (EMVs) can also trigger such signaling events in the target cells. Exosomes and shedding microvesicles (SMVs) are classes of EMVs that are membrane enclosed organelles released by cells under physiological and pathological conditions [1-6]. Among the EMVs, exosomes are small (40-100 nm diameter) membraneous vesicles of endocytic origin while SMVs (also referred to as ectosomes) are large membranous vesicles (50-1000 nm diameter) that are shed directly from the plasma membrane (PM) [7]. Recent studies have shown that these EMVs mediate intercellular communication [8-10] and are shown to harbour mRNA, microRNA, proteins and lipids [8,11-14] based on the host cell.
Intercellular interactions are pivotal for basic cellular activities and errors in either receiving or transmitting these signals are shown to cause pathological conditions. Whilst, such intercellular communications were once thought to be regulated by membrane surface molecules and/or soluble secreted proteins by stimulating the target cells through receptor mediated activation, increasing evidences suggest that extracellular microvesicles (EMVs) can also trigger such signaling events in the target cells. Exosomes and shedding microvesicles (SMVs) are classes of EMVs that are membrane enclosed organelles released by cells under physiological and pathological conditions [1-6]. Among the EMVs, exosomes are small (40-100 nm diameter) membraneous vesicles of endocytic origin while SMVs (also referred to as ectosomes) are large membranous vesicles (50-1000 nm diameter) that are shed directly from the plasma membrane (PM) [7]. Recent studies have shown that these EMVs mediate intercellular communication [8-10] and are shown to harbour mRNA, microRNA, proteins and lipids [8,11-14] based on the host cell [13].
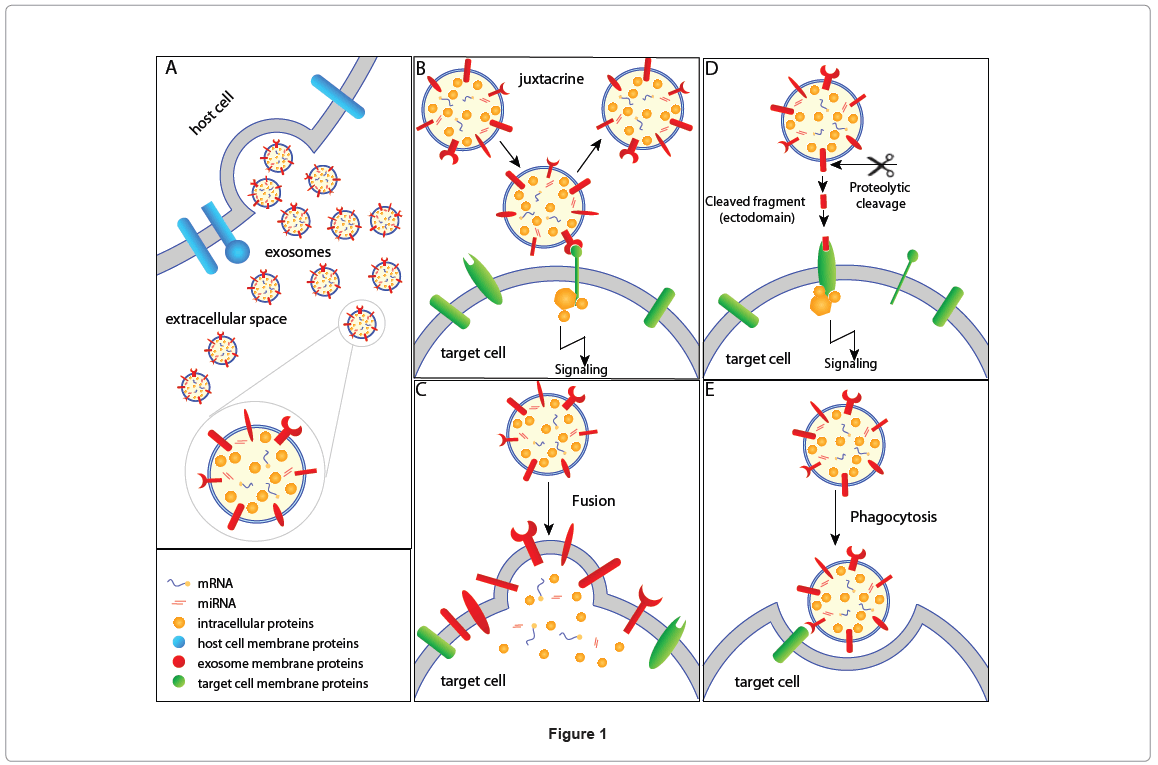
While many of such recent observations have proven the role of EMVs in cell-cell communication, the exact mechanism in which these EMVs communicate with the target cells still remains elusive. Excluding the study by Rana et al. that showed the role of tetraspanins in target selection [15], the factors that influence target cell selection are poorly understood. Possible mechanisms of EMVs communication with target cells are shown in Figure 1 (exosomes as an example). EMVs harbor membrane proteins that can interact with the target cells in a juxtacrine manner, thereby activating the target cell (Figure 1B). Alternatively, exosomes can fuse with the target cell resulting in the non-selective transfer of exosomal proteins and RNA (Figure 1C) to the target cell. In addition to proteins and lipids, exosomes also contain mRNA and microRNAs that can be transferred to the target cell, conferring new functional properties to the recipient cell after the acquisition of the exosomal genetic material. Such fusion might change the membrane features of the target cell (e.g., arachidonic acid transfer from platelets-derived shedding microvesicles to leukocytes and endothelial cells [16]) including varied lipid concentrations and the transfer of exosomal membrane proteins on the target cell surface (e.g., CD41 antigen from platelets-derived shedding microvesicles to tumor and endothelia cell surface [17,18]). Proteins that are present in the soluble secretome are not only a result of protein secretion, cell death and cell surface membrane ectodomain shedding but also due to ectodomain shedding of exosomal membrane proteins [19,20]. Exosomal membrane proteins can be cleaved by proteases and the resulting fragment may act as ligands for cell surface receptor in the target cell (Figure 1D). In addition to juxtacrine, ectodomain cleavagebased signaling and membrane fusion, exosomes can be engulfed by antigen presenting cells and phagocytosized [21] (Figure 1E). The antigen presenting cells can later process the molecular information and trigger the cellular signaling cascades [21].
Whilst these are the possible mechanisms of intercellular communication by EMVs, the precise mechanism need to be clearly understood to manipulate these bioactive vesicles as efficient drug delivery vehicles.
References
- Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2: 569-579.
- Mathivanan S, Ji H, Simpson RJ (2010) Exosomes: Extracellular organelles important in intercellular communication. J Proteomics 73: 1907-1920.
- Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G (2002) The biogenesis and functions of exosomes. Traffic 3: 321-330.
- van Niel G, Porto-Carreiro I, Simoes S, Raposo G (2006) Exosomes: a common pathway for a specialized function. J Biochem (Tokyo) 140: 13-21.
- Lotvall J, Valadi H (2007) Cell to cell signalling via exosomes through esRNA. Cell Adh Migr 1: 156-158.
- Cocucci E, Racchetti G, Meldolesi J (2009) Shedding microvesicles: artefacts no more. Trends Cell Biol 19: 43-51.
- Simpson RJ, Mathivanan S (2012) Extracellular Microvesicles: The Need for Internationally Recognised Nomenclature and Stringent Purification Criteria. J Proteomics Bioinform 5: ii-ii.
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, et al. (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654-659.
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, et al. (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med.
- Somasundaram R, Herlyn M (2012) Melanoma exosomes: messengers of metastasis. Nat Med 18: 853-854.
- Mathivanan S, Fahner CJ, Reid GE, Simpson RJ (2012) ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res 40: D1241-D1244.
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, et al. (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10: 1470-1476.
- Mathivanan S, Lim JW, Tauro BJ, Ji H, Moritz RL, et al. (2010) Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics 9: 197-208.
- Simpson RJ, Kalra H, Mathivanan S (2012) ExoCarta as a resource for exosomal research. Journal of Extracellular Vesicles 1: 18374.
- Rana S, Yue S, Stadel D, Zoller M (2012) Towards tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol.
- Barry OP, FitzGerald GA (1999) Mechanisms of cellular activation by platelet microparticles. Thromb Haemost 82: 794-800.
- Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, et al. (2005) Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer 113: 752-760.
- Barry OP, Pratico D, Savani RC, FitzGerald GA (1998) Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest 102: 136-144.
- Stoeck A, Keller S, Riedle S, Sanderson MP, Runz S, et al. (2006) A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem J 393: 609-618.
- Sanderson MP, Keller S, Alonso A, Riedle S, Dempsey PJ, et al. (2008) Generation of novel, secreted epidermal growth factor receptor (EGFR/ErbB1) isoforms via metalloprotease-dependent ectodomain shedding and exosome secretion. J Cell Biochem 103: 1783-1797.
- Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, et al. (2010) Cellular internalization of exosomes occurs through phagocytosis. Traffic 11: 675-687.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 14680
- [From(publication date):
July-2012 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10064
- PDF downloads : 4616