Research Article Open Access
Examination of Protein-Damaging Activity of Phosphorus(V) Porphyrin Photosensitizer for Photodynamic Therapy
Kazutaka Hirakawa1*, Taiki Yamanaka1 , Jin Matsumoto2 and Masahide Yasuda2
1Department of Applied Chemistry and Biochemical Engineering, Graduate School of Engineering, Shizuoka University, Japan
2Department of Applied Chemistry, Faculty of Engineering, University of Miyazaki, Japan
- *Corresponding Author:
- Kazutaka Hirakawa
Department of Applied Chemistry and Biochemical Engineering
Graduate School of Engineering
Shizuoka University, Johoku 3-5-1
Naka-ku, Hamamatsu, Shizuoka 432-8561, Japan
Tel: +81-53-478-1287
Fax: +81-53-478-1287
E-mail: tkhirak@ipc.shizuoka.ac.jp
Received date: April 27, 2013; Accepted date: May 29, 2013; Published date: May 31, 2013
Citation: Hirakawa K, Yamanaka T, Matsumoto J, Yasuda M (2013) Examination of Protein-Damaging Activity of Phosphorus (V) Porphyrin Photosensitizer for Photodynamic Therapy. J Anal Bioanal Tech S1:003. doi: 10.4172/2155-9872.S1-003
Copyright: © 2013 Menon D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
For the fundamental study of photodynamic therapy, protein-damaging activity of the water-soluble phosphorus(V) porphyrin (DDP(V)TPP) was examined. Absorption spectrum measurement demonstrated the binding interaction between DDP(V)TPP and human serum albumin, a water-soluble protein. Photo-irradiated DDP(V)TPP damaged the amino acid residue of human serum albumin, resulting in the decrease of fluorescence intensity from the tryptophan residue of human serum albumin. Protein damage photosensitized by DDP(V)TPP was inhibited by the addition of sodium azide and enhanced in deuterium oxide, indicating the contribution of singlet oxygen. However, sodium azide could not completely inhibit the damage of human serum albumin, suggesting that the electron transfer mechanism contributes to protein damage as does singlet oxygen generation. Isolated tryptophan was also damaged by a photosensitization of DDP(V)TPP in solution, whereas DDP(V)TPP did not induce photodamage to tyrosine and phenylalanine. Using the application of a fluorometry of hydrogen peroxide, the deactivation of catalase photosensitized by DDP(V)TPP was demonstrated.
Keywords
P(V) porphyrin; Human serum albumin; Amino acid; Catalase; Protein damage; Photosensitizer; Singlet oxygen; Electron transfer; Folic acid
Abbreviations
1O2: Singlet Oxygen; CT: Charge Transfer State; DDP (V) TPP: di (3,6-dioxadecyloxo) Tetraphenyl Porphyrinato- Phosphorus(V) Chloride; HPLC: High Performance Liquid Chromatography; HSA: Human Serum Albumin; LED: Light-Emitting Diode; NaN3: Sodium Azide
Introduction
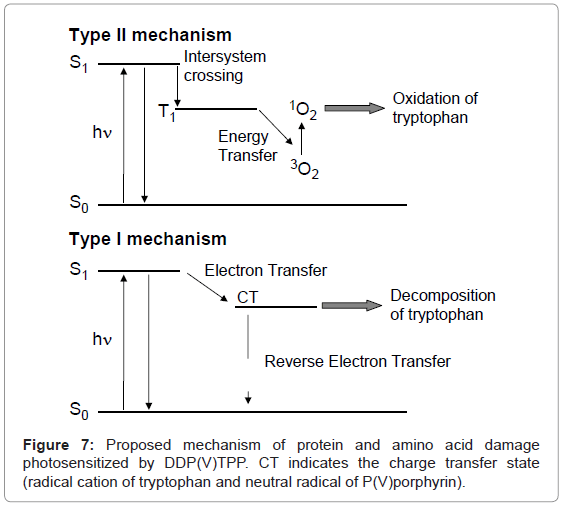
The purpose of this study is the examination of protein-damaging activity of photosensitizers for photodynamic therapy (PDT) by simple analysis method. PDT is a less invasive treatment of cancer and some non-malignant conditions [1-3]. In general, porphyrins are used as the photosensitizer for PDT. Administered photosensitizers damage cancer cells by the oxidation of biomacromolecules including protein and DNA through the following two mechanisms during visible-light irradiation [4-6]. One mechanism is the generation of singlet oxygen (1O2) through energy transfer to molecular oxygen from the photoexcited photosensitizer (Type II mechanism). Another mechanism is the electron abstraction from biomacromolecules to the photoexcited photosensitizer (Type I mechanism). The Type II mechanism is easily induced by visible-light excitation; however, the oxygen concentration in a cancer cell is relatively low [7], resulting in the restriction of activity of the PDT photosensitizer. To avoid this low oxygen concentration problem, the Type I photosensitizer may play a key role in the molecular design for PDT. The Type I mechanism requires highly oxidative activity (a lower reduction potential) in the photoexcited state of the photosensitizer. Ultraviolet photosensitizers mainly induce biomolecule photodamage through the electron transfer mechanism, whereas a visible-light photosensitizer is not appropriate for this mechanism. Therefore, it is important to select the appropriate molecular design to achieve electron transfer-mediated biomolecule damage using a visible-light photosensitizer. Since high-valent porphyrin complexes demonstrate a lower reduction potential in their photoexcited state than free-base or low-valent metal complexes, these porphyrins are advantageous for the oxidative electron transfer reaction [8-14]. Indeed, derivatives of high-valent porphyrin complexes, such as P(V) [10] and Sb(V) [14] complexes, photosensitize DNA damage through two mechanisms: 1O2 generation and the electron transfer reaction. In this study, photosensitized damage of protein and amino acid by a porphyrin P(V) complex, di(3,6-dioxadecyloxo) tetraphenylporphyrinato-phosphorus (V) chloride (DDP (V) TPP, Figure 1), [15] was investigated. As a target protein model, human serum albumin (HSA), a water-soluble protein, was used because its structure and property were well elucidated [16]. Further, the deactivation of catalase as enzyme model photosensitized by DDP (V) TPP was also examined.
Materials and Methods
Materials
Synthesis of DDP (V) TPP was previously reported [15]. Human serum albumin and catalase were purchased from Sigma-Aldrich Co. LLC. (St. Louis, MO, USA). Tryptophan, tyrosine, phenylalanine, hydrogen peroxide (H2O2), and sodium azide (NaN3) were from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Distilled water (H2O) was from Kanto Chemical Co., Inc. (Tokyo, Japan). Deuterium oxide (D2O) was from Acros Organics (Geel, Belgium). Sodium phosphate buffer (pH 7.6) was from Nakalai Tesque Inc. (Kyoto, Japan).
Spectroscopic measurements
The absorption spectrum of DDP (V) TPP and HSA was measured with the UV-Vis spectrophotometer UV-1650PC (Shimadzu, Kyoto, Japan). The fluorescence spectra of DDP (V) TPP and HSA were measured with an F-4500 fluorescence spectrophotometer (Hitachi, Tokyo, Japan). Fluorescence decay was measured using a timecorrelated single-photon counting method with a Fluorescence Lifetime System TemPro (HORIBA, Kyoto, Japan). Laser excitation at 390 nm was achieved by using a diode laser (NanoLED-390, HORIBA) in at a repetition rate of 1.0 MHz.
Determination of damage of human serum albumin photosensitized by DDP (V) TPP
The sample solution containing 10 μM DDP (V) TPP and 10 μM HSA in a sodium phosphate buffer (pH 7.6) was irradiated with a lightemitting diode (LED) (λmax=519 nm, 1 mW cm-2, CCS Inc., Kyoto, Japan). The intensity of the LED light source was measured with a photo-power meter (8230E, ADCMT, Tokyo, Japan). Protein damage by DDP (V) TPP was evaluated by measurement of the fluorescence intensity from the amino acid residues as previously reported (Ex=298 nm, Em=350 nm) [17,18].
Determination of damage of amino acid photosensitized by DDP (V) TPP
The sample solution containing 10 μM DDP (V) TPP and 100 μM amino acid (tryptophan, tyrosine, or phenylalanine) in a sodium phosphate buffer (pH 7.6) was irradiated with LED (λmax=519 nm, 1 mW cm-2) for 60 min. After photo-irradiation, the sample solution was analyzed with high-performance liquid chromatography (HPLC). The HPLC system consisted of an intelligent HPLC pump (PU2080 plus, JASCO Co., Tokyo, Japan) and a column oven (Shodex OVEN AO-30, SHOWA DENKO K.K., Tokyo, Japan) equipped with an ODS column (InertSustain C18, GL Science Inc., Tokyo, Japan). The mobile phase, containing 40 mM acetate including 73 mM sodium acetate and methanol (20/80, v/v), was prepared according to the literature [19]. The analysis was carried out at a column temperature of 25°C and a flow rate of 0.5 ml/min. The HPLC eluate was routed directly into a photodiode array UV-Visible detector (SPD-M20A, Shimadzu). The concentrations of tryptophan, tyrosine, and phenylalanine were determined from the absorbance at 280 nm, 275 nm, and 260 nm, respectively.
Evaluation of the activity of catalase photosensitized by DDP (V) TPP
The inhibitory effect on protein function by the photosensitized reaction of DDP (V) TPP was evaluated using the measurement of catalase activity. The catalytic activity of damaged catalase should be decreased. This activity was determined by the fluorometry of H2O2 using folic acid [20]. Although folic acid itself is less fluorescent, the folic acid decomposed by H2O2 strongly fluoresces. To detect H2O2, the sample solution containing 10 μM folic acid, 20 μM CuCl2, and 100 μM H2O2 with or without catalase is incubated for 30 min at 37°C to measure the fluorescence intensity of folic acid (Ex: 350 nm, Em: 450 nm). The sample solutions (1.0 mL) containing 1.0 unit of catalase with or without 10 μM DDP (V) TPP were irradiated with LED (λmax=519 nm, 1 mW cm-2) for 30 min. For the control experiment, the sample solution containing catalase and DDP (V) TPP without irradiation was also prepared.
Results and Discussion
Evaluation of photosensitized damage of human serum albumin by DDP (V) TPP
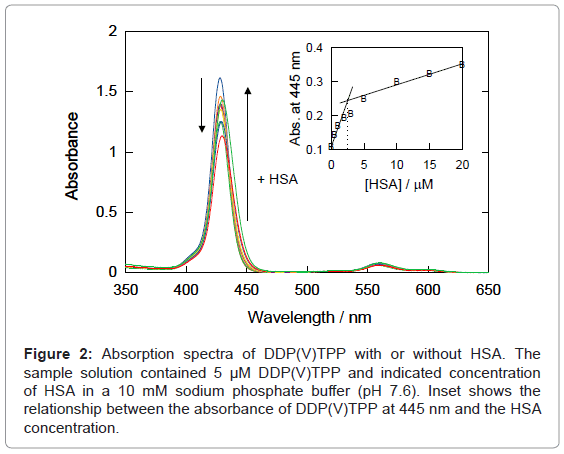
In the presence of HSA, the hyperchromic effect and red-shift were observed in the UV-Vis absorption spectra of DDP (V) TPP (Figure 2), indicating the static interaction between DDP (V) TPP and the protein. Analysis of the absorbance showed that the intersection point appeared at ca. 2.5 μM HSA, which is half of the DDP (V) TPP concentration (inset of Figure 2), suggesting the 2:1 complex formation between DDP (V) TPP and HSA.
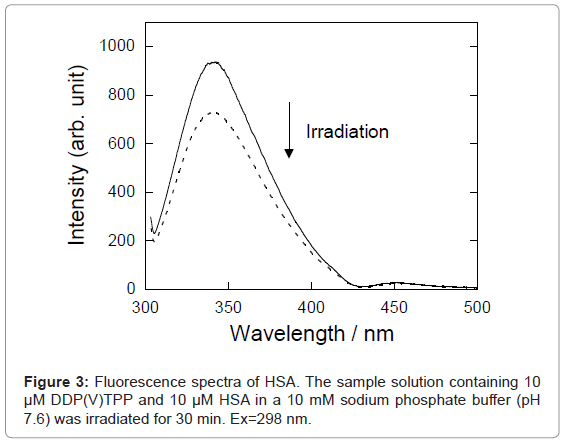
Figure 3 shows the fluorescence spectra of HSA in buffer solution. The intensity of HSA fluorescence around 350 nm, assigned to the tryptophan residue, was decreased by photo-irradiation in the presence of DDP (V) TPP. The fluorescence decrement of HSA can be explained by the amino acid oxidation through the photosensitized reaction [17,18]. The concentration of damaged HSA, [Damaged HSA], is determined as follows:
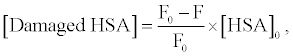 (1)
(1)
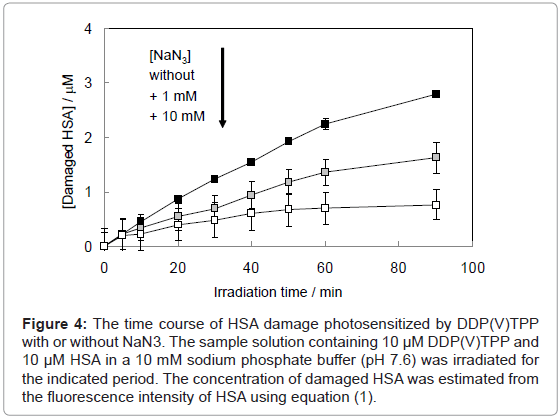
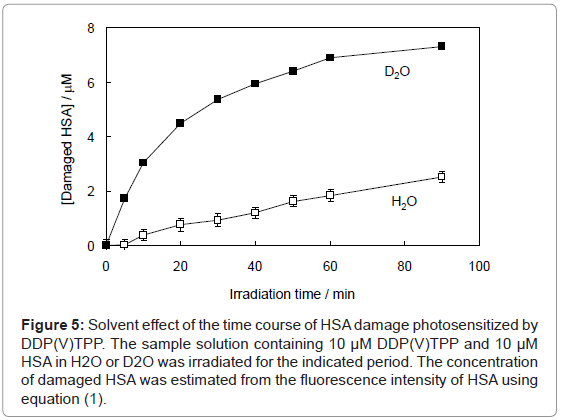
where F and F0 are the fluorescence intensities of HSA with or without the treatment of photosensitized reaction by DDP (V) TPP, respectively and [HSA]0 is the initial concentration of HSA (10 μM) (Figure 4). This HSA damage was partially inhibited by NaN3, a physical quencher of 1O2 (Figure 4). Furthermore, HSA damage was enhanced in D2O (Figure 5), in which the lifetime of 1O2 is markedly elongated (about 2 ~ 4 μs in H2O to 70 μs in D2O) [21]. These findings suggest HSA oxidation by1O2. It has been reported that the quantum yield of 1O2 generation (ΦΔ) by DDP (V) TPP is relatively large (ΦΔ=0.73) [15], indicating that the 1O2 mechanism is also important for photosensitized biomolecule damage in the presence of a sufficient concentration of molecular oxygen.
Figure 4: The time course of HSA damage photosensitized by DDP(V)TPP with or without NaN3. The sample solution containing 10 μM DDP(V)TPP and 10 μM HSA in a 10 mM sodium phosphate buffer (pH 7.6) was irradiated for the indicated period. The concentration of damaged HSA was estimated from the fluorescence intensity of HSA using equation (1).
Figure 5: Solvent effect of the time course of HSA damage photosensitized by DDP(V)TPP. The sample solution containing 10 μM DDP(V)TPP and 10 μM HSA in H2O or D2O was irradiated for the indicated period. The concentration of damaged HSA was estimated from the fluorescence intensity of HSA using equation (1).
On the other hand, HSA damage was not completely inhibited by an excess amount of NaN3 (~10 mM) (Figure 4), suggesting that the electron transfer mechanism is partly responsible for HSA photodamage, as is the 1O2 mechanism. The quenching efficiency of 1O2 by NaN3 (Efq) can be calculated from the following equation using the lifetime of 1O2 (τΔ = 3.5 μs):
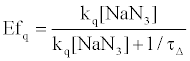 (2)
(2)
where [NaN3] is the concentration of NaN3 and kq is the quenching rate coefficient of 1O2 by NaN3. The kq is almost the same as the diffusion control limit (kdif), which is calculated as follows:
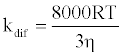 (3)
(3)
where R is the gas constant, T is the absolute temperature, and η is the viscosity of water (8.91x10-4 kg m-1 s-1). Thus, the Efq becomes 0.996 in the presence of 10 mM NaN3. This value suggests that almost all the 1O2 can be quenched by 10 mM NaN3. Consequently, the damage of HSA photosensitized by DDP (V) TPP in the presence of 10 mM NaN3 should be due to the electron transfer mechanism. The inhibited ratio of the HSA damage by NaN3 indicates the contribution of the 1O2 mechanism. The estimated contributions of HSA damage through the electron transfer and the 1O2 generation mechanisms for 60 min irradiation were 0.31 and 0.69, respectively.
Lifetime of photoexcited DDP (V) TPP with human serum albumin
The time-resolved fluorescence intensity of DDP (V) TPP could be fitted by a single exponential function in the case without HSA, and the estimated lifetime (τf ) was 4.67 ns. Similarly, the single exponential function was well fitted in the case with HSA, and the obtained value of τf (4.56 ns) was slightly shorter than that without HSA, suggesting the electron transfer quenching. The quenching rate constant of photoexcited DDP (V) TPP through electron transfer (ket) in the HSA microenvironment should be estimated from the following equation:
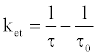 (4)
(4)
where τ is 4.56 ns and τ0 is 4.67 ns. The obtained ket value (5.2x106 s-1) is much less than the total quenching rate constant, which equals the reciprocal of τ(2.2x108 s-1). It suggests that the protein damage through electron transfer from the amino acid residue to the S1 state of DDP (V) TPP gradually proceeds. In the cases of other porphyrin P(V) complexes, the electron transfer from the amino acid to the porphyrin S1 state has been reported [18]. The electron transfer in the triplet excited state (T1) of porphyrin is rarely reported because the oxidative activity of the porphyrin T1 is weak.
Evaluation of photosensitized damage of amino acids by DDP (V) TPP
The amino acid damage photosensitized by 10 μM DDP (V) TPP was also examined using 100 μM tryptophan, tyrosine, and phenylalanine. In an HPLC analysis, the signal assigned to tryptophan was observed at the retention time of around 5.4 min. The decrease of the absorption signal showed that tryptophan was decomposed by the photoexcited DDP (V) TPP, whereas tyrosine and phenylalanine were hardly damaged by the photosensitized reaction of DDP (V) TPP. In these experimental conditions (λmax=519 nm, 1 mW cm-2), 62% of tryptophan was damaged by DDP (V) TPP photo-irradiated for 60 min. The quantum yield of the decomposed tryptophan was estimated to be 0.035 from the absorbed photon number by DDP (V) TPP and the detected concentration of tryptophan.
Evaluation of catalytic activity of catalase photosensitized by DDP (V) TPP
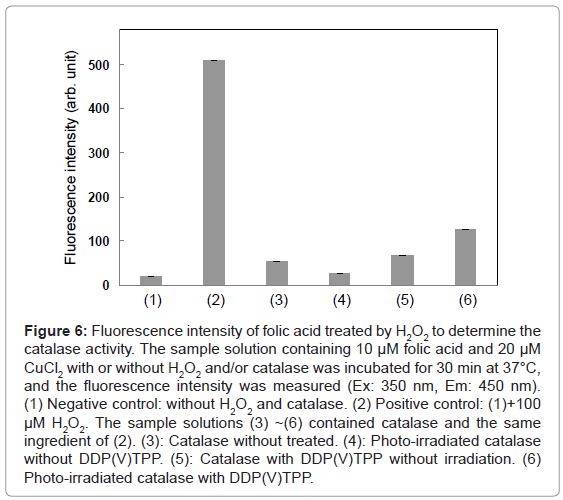
As mentioned above, DDP (V) TPP can photosensitize the damage of isolated tryptophan and tryptophan residue of HSA. To examine the effect of photosensitization by DDP (V) TPP on the enzyme activity, catalase was used as the target enzyme model. Catalase activity was determined by the fluorometry of H2O2. The fluorescence intensity of the sample solution was enhanced by the formation of a fluorescent compound through folic acid decomposition (Figure 6). In the presence of catalase, the fluorescence enhancement was inhibited. Photo-irradiation to catalase without DDP (V) TPP showed little effect on the catalase activity. The fluorescence intensity was recovered in the following cases, indicating the deactivation of catalase. DDP(V) TPP showed a slight deactivation effect on the catalase activity without irradiation. Photo-irradiated DDP (V) TPP demonstrated a larger deactivation effect than that without irradiation. These results indicate that the photosensitized reaction by DDP (V) TPP can deactivate the catalase activity. To evaluate the effect of photosensitization of DDP (V) TPP, the following analysis was carried out.
Figure 6: Fluorescence intensity of folic acid treated by H2O2 to determine the catalase activity. The sample solution containing 10 μM folic acid and 20 μM CuCl2 with or without H2O2 and/or catalase was incubated for 30 min at 37°C, and the fluorescence intensity was measured (Ex: 350 nm, Em: 450 nm). (1) Negative control: without H2O2 and catalase. (2) Positive control: (1)+100 μM H2O2. The sample solutions (3) ~(6) contained catalase and the same ingredient of (2). (3): Catalase without treated. (4): Photo-irradiated catalase without DDP(V)TPP. (5): Catalase with DDP(V)TPP without irradiation. (6) Photo-irradiated catalase with DDP(V)TPP.
The fluorescence enhancement without catalase (ΔI0) is proportional to the initial concentration of H2O2 ([H2O2]0) and the reaction time (t) as follows [20]:
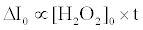 (5)
(5)
In the presence of catalase, the concentration of H2O2 ([H2O2]) can be expressed as the following equation, which is based on the assumption that H2O2 is decomposed by catalase linearly with the t:
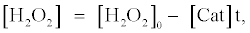 (6)
(6)
where [Cat] indicates activity of catalase (arbitrary unit). The fluorescence enhancement is in proportion to the average concentration of [H2O2] ([H2O2]0/2) and the t when the [H2O2] decreases with the t. In the presence of catalase, the t indicates the period in which H2O2 is decomposed entirely by catalase. The t in the presence of catalase can be obtained from equation (6) as follows:
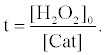 (7)
(7)
Therefore, the fluorescence enhancement with catalase (ΔI) is
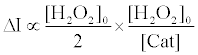 (8)
(8)
Using equations (5) and (8), the [Cat] is expressed as equation (9),
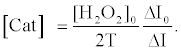 (9)
(9)
The relative activity of catalase photosensitized by DDP (V) TPP can be obtained from equation (10) as follows:
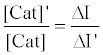 (10)
(10)
where [Cat]’ is the activity of photosensitized catalase and ΔI’ is the fluorescence enhancement in the case of photosensitized catalase. Using this equation, the relative activity was calculated to be 0.32, indicating that 68% of the catalase activity was decreased by the photosensitized reaction of DDP (V) TPP under these experimental conditions.
Conclusion
DDP (V) TPP could induce protein photodamage through 1O2 generation and the electron transfer mechanism. 1O2 generation is a well-known mechanism for porphyrin photosensitization (Figure 7) [22-24]. The electron transfer mechanism is hardly observed in the case of protein or DNA damage by a usual visible-light photosensitizer. The fluorescence quenching of DDP (V) TPP through an interaction with HSA suggests that the electron abstraction from the tryptophan residue to the S1 of DDP (V) TPP contributes to the electron transfer mechanism of HSA photodamage. The formed radical cation of the tryptophan residue through electron transfer should undergo further reaction with the surrounding elements, such as water and oxygen. An oxidized product, such as N-formylkynurenine, should finally be formed [25]. Singlet oxygen generated by HSA-binding DDP (V) TPP also oxidizes tryptophan, resulting in the formation of oxidized products including N-formylkynurenine [26]. The analysis of amino acid decomposition also showed that DDP (V) TPP can oxidize only tryptophan, which is easily oxidized compared with tyrosine and phenylalanine. In this study, a method to analyze deactivation of the enzyme by photosensitized reaction was also proposed. Using this method, it was demonstrated that the activity of catalase was inhibited by the photosensitized reaction of DDP (V) TPP. These results indicate that damage of the amino acid residue, possibly tryptophan, photosensitized by DDP (V) TPP leads to deactivation of the enzyme. The activity of DDP (V) TPP may be preserved under a lower oxygen condition such as a tumor through an electron transfer mechanism. These characteristics of DDP (V) TPP are advantageous for the PDT photosensitizer.
Acknowledgements
This work was partially supported by JSPS KAKENHI Grant Number 23750186.
References
- Dolmans DE, Fukumura D, Jain RK (2003) Photodynamic therapy for cancer. Nat Rev Cancer 3: 380-387.
- Castano AP, Mroz P, Hamblin MR (2006) Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer 6: 535-545.
- Wilson BC, Patterson MS (2008) The physics, biophysics and technology of photodynamic therapy. Phys Med Biol 53: R61-109.
- Kawanishi S, Hiraku Y, Oikawa S (2001) Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res 488: 65-76.
- Cló E, Snyder JW, Ogilby PR, Gothelf KV (2007) Control and selectivity of photosensitized singlet oxygen production: challenges in complex biological systems. Chembiochem 8: 475-481.
- Cadet J, Douki T, Ravanat JL (2010) Oxidatively generated base damage to cellular DNA. Free Radic Biol Med 49: 9-21.
- Bratasz A, Kulkarni AC, Kuppusamy P (2007) A highly sensitive biocompatible spin probe for imaging of oxygen concentration in tissues. Biophys J 92: 2918-2925.
- Hirakawa K, Segawa H (1999) Excitation energy transfer and photo-induced electron transfer in axial bispyrenyl phosphorus porphyrin derivatives: factors governing the competition between energy and electron transfer processes under the existence of intramolecular p-p interaction. J Photochem Photobiol A: Chem 123: 67-76.
- Hirakawa K, Segawa H (2010) Acid dissociation of the axial hydroxyl group of hydroxy(1-pyrenebutoxy)phosphorus(V) porphyrin controls the intramolecular excitation energy transfer. Photochem Photobiol Sci 9: 704-709.
- Hirakawa K, Kawanishi S, Hirano T, Segawa H (2007) Guanine-specific DNA oxidation photosensitized by the tetraphenylporphyrin phosphorus(V) complex via singlet oxygen generation and electron transfer. J Photochem Photobiol B 87: 209-217.
- Shiragami T, Matsumoto J, Inoue H, Yasuda M (2005) Antimony porphyrin complexes as visible-light driven photocatalyst. J Photochem Photobiol C: Photochemistry Rev 6: 227-248.
- Inoue H, Okamoto T, Kameo Y, Sumitani M, Fujiwara A, et al. (1994) Photochemical epoxidation of cyclohexene sensitized by tetraphenylporphyrinatoantimony(V) in the presence of water acting both as an electron and an oxygen donor. J Chem Soc Perkin Trans 1: 105-111.
- Andou Y, Ishikawa K, Shima K, Shiragami T, Yasuda M, et al. (2002) O-Alkylation of dihydroxo(tetraarylporphyrinato)phosphorus(V) and antimony(V) complexes with alkyl halides. Bull Chem Soc Jpn 75: 1757-1760.
- Hirakawa K, Kawanishi S, Matsumoto J, Shiragami T, Yasuda M (2006) Guanine-specific DNA damage photosensitized by the dihydroxo(tetraphenylporphyrinato)antimony(V) complex. J Photochem Photobiol B 82: 37-44.
- Matsumoto J, Shinbara T, Tanimura S, Matsumoto T, Shiragami T, et al. (2011) Water-soluble phosphorus porphyrins with high activity for visible light-assisted inactivation of Saccharomyces cerevisiae. J Photochem Photobiol A: Chem 218: 178-184.
- He XM, Carter DC (1992) Atomic structure and chemistry of human serum albumin. Nature 358: 209-215.
- Hirakawa K, Ebara Y, Hirano T, Segawa H (2009) Photo-damage of human serum albumin by porphyrin P(V) complex through electron transfer and singlet oxygen generation. J Jpn Soc Laser Surgery and Medicine 29: 372-382.
- Hirakawa K, Fukunaga N, Nishimura Y, Arai T, Okazaki S (2013) Photosensitized protein damage by dimethoxyphosphorus(V) tetraphenylporphyrin. Bioorg Med Chem Lett 23: 2704-2707.
- Sánchez-Machado DI, Chavira-Willys B, López-Cervantes J (2008) High-performance liquid chromatography with fluorescence detection for quantitation of tryptophan and tyrosine in a shrimp waste protein concentrate. J Chromatogr B Analyt Technol Biomed Life Sci 863: 88-93.
- Hirakawa K (2006) Fluorometry of hydrogen peroxide using oxidative decomposition of folic acid. Anal Bioanal Chem 386: 244-248.
- Ogilby PR, Foote CS (1983) Chemistry of singlet oxygen. 42. effect of solvent, solvent isotopic substitution, and temperature on the lifetime of singlet molecular oxygen (1.DELTA.g). J Am Chem Soc 105: 3423-3430.
- Hirakawa K (2009) Fluorometry of singlet oxygen generated via a photosensitized reaction using folic acid and methotrexate. Anal Bioanal Chem 393: 999-1005.
- DeRosa MC, Crutchley RJ (2002) Photosensitized singlet oxygen and its applications. Coordination Chem Rev 233-234: 351-371.
- Lang K, Mosinger J, Wagneroviá DM (2004) Photophysical properties of porphyrinoid sensitizers non-covalently bound to host molecules; models for photodynamic therapy. Coord Chem Rev 248: 321-350.
- Ehrenshaft M, Silva SO, Perdivara I, Bilski P, Sik RH, et al. (2009) Immunological detection of N-formylkynurenine in oxidized proteins. Free Radic Biol Med 46: 1260-1266.
- Gracanin M, Hawkins CL, Pattison DI, Davies MJ (2009) Singlet-oxygen-mediated amino acid and protein oxidation: formation of tryptophan peroxides and decomposition products. Free Radic Biol Med 47: 92-102.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15306
- [From(publication date):
specialissue-2013 - Apr 10, 2025] - Breakdown by view type
- HTML page views : 10575
- PDF downloads : 4731