Research Article Open Access
Evaluation of Stability Factors in the Anaerobic Treatment of Slaughterhouse Wastewater
| Edith Padilla-Gasca, Alberto López-López* and Juan Gallardo-Valdez | |
| Unidad de Tecnología Ambiental, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, Guadalajara, México | |
| Corresponding Author : | Dr. Alberto López-López Unidad de Tecnología Ambiental Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, Guadalajara, México E-mail: alopez103@yahoo.com, allopez@ciatej.net.mx |
| Received: October 01, 2010; Accepted: February 08, 2011; Published: February 10, 2011 | |
| Citation: Padilla-Gasca E, López-López A, Gallardo-Valdez J(2011) Evaluation of Stability Factors in the Anaerobic Treatment of Slaughterhouse Wastewater. J Bioremed Biodegrad 2:114. doi:10.4172/2155-6199.1000114 | |
| Copyright: © 2011 Padilla-Gasca E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The main environmental factors that assured the stability of an anaerobic batch process for the treatment of slaughterhouse wastewater were evaluated. The reactor was inoculated with anaerobic sludge from a distillery wastewater treatment plant. The volume of produced CH4 was proportional to the quantity of organic matter removed matter (measured as Chemical Oxygen Demand, COD). The overall organic matter removal was 75%. The acetic, propionic and butyric acids concentration profiles were similar, where acetic acid had the highest concentration throughout the study period. The response time recorded between the acetogenic and methanogenic stages in this study was two days. The process showed an elevated acidogenic activity of 1.62 g of VFA/g of COD removed and a high Methane Production Rate (MPR) of about 450 ml of CH4/g of COD removed. The anaerobic treatment of slaughterhouse wastewater generated a buffer system which produced sufficient alkalinity to neutralize the effects of Volatile Fatty Acids (VFA) generated during the process.
| Keywords |
| Acetogenic; Acidogenic; Methanogenic; Slaughterhouse; Volatile fatty acids; Wastewater |
| Introduction |
| The slaughtering, processing and preserving activities required for meat production in municipal slaughterhouses generate large quantities of wastewater and solid waste. It is estimated that for every cow and pig processed, 700 and 330 liters of wastewater are generated, respectively, with an increase of 25% if further processing is carried out to produce edible products [1]. |
| Slaughterhouse wastewater is a complex mixture of proteins, complex organic compounds and fats. The organic load of 5,000-10,000 mg l-1, as measured as Chemical Oxygen Demand (COD), includes fats and oils at concentrations of around 100 to 300 mg l-1. In addition, the presence of pathogenic microorganisms has been reported [2]. |
| Usually these residual effluents are discharged into the municipal sewer system or directly into water bodies (streams, rivers, or lakes) [3,4], putting these ecosystems at risk. The lack of a treatment system in slaughterhouse facilities is primarily due to the lack of financial and technical resources. Current commercial technologies for treatment are based on aerobic and physiochemical processes which have high investment and operating costs [5]. |
| Treatment of slaughterhouse effluent by means of an anaerobic process is a potential alternative. In addition to lower costs of operation, the methane generated with anaerobic process may provide an energy source. Despite efforts to develop and implement anaerobic treatment systems for slaughterhouse wastewater, problems persist at the operational and process level [6,7]. |
| The anaerobic digestion process is based on the ability of microorganisms to remove, by assimilation and decomposition, the biodegradable organic matter present in wastewater [8]. Generally, this process consists of four stages; the first one is called hydrolysis and consists of the transformation of organic matter into simple soluble products like carbohydrates, fatty acids and amino acids. In the second one, called the acidogenic stage, fermentative bacteria use the hydrolysis products to form intermediate compounds like organic acids, including Volatile Fatty Acids (VFAs), in addition to hydrogen and carbon dioxide. In the third stage organic acids are oxidized partially by another group of bacteria called acetogenic, which produce additional quantities of hydrogen and acetic acid. Finally, in the fourth stage, both acetic acid and hydrogen are the raw material for the growth of methanogenic bacteria, converting acetic acid and hydrogen to biogas composed mainly of methane, carbon dioxide and hydrogen sulfide [9]. |
| The stability of the anaerobic process is affected by diverse environmental factors, i.e. temperature, pH, alkalinity, VFAs concentration, among others. The anaerobic treatment process is much more susceptible than the aerobic one for the same degree to deviations from the optimum environmental conditions. The successful operation of anaerobic reactors, therefore, demands meticulous control of environmental factors required to maintain viability of the microorganisms involved in the process [9,10]. |
| Several methods have been proposed for evaluating anaerobic processes, with the acidogenic and methanogenic activity study being most commonly used for that purpose [10,11]. The aforementioned stages are utilized to evaluate the behavior of the biomass under the influence of potentially inhibitory compounds such as VFAs and in order to establish the degree of biodegradability of the substrate [12]. The study of these elements and environmental factors are useful tools in the evaluation and optimization of the anaerobic process in the treatment of wastewater. |
| The objective of this investigation was to evaluate the main environmental factors for anaerobic treatment process in a batch reactor using slaughterhouse wastewater in order to understand the organic matter decomposition kinetics in these types of effluents and to assure the stability of process. |
| Materials and Methods |
| Wastewater sampling and analyses |
| The wastewater used in this research was obtained from a municipal slaughterhouse, Wastewater samples of a liter during intervals of two hours were obtained during a work day of 16 hours to form a composite sample of 8 liters, which was transferred to the laboratory and kept refrigerated at 4°C, for analysis and use in the experiment. The physicochemical and microbiological characterization analyses of the slaughterhouse wastewater were carried out according to APHA [13], and the results are presented in (table 1). |
| The monitoring of the main VFAs concentrations (acetic, propionic and butyric acids) was carried out by Gas Chromatography with Flame Ionization Detector (GC-FID). The samples were prepared and conserved based on the methodology proposed by Park et al. [14]. The chemical analysis was carried out in a GC-FID by Agilent Technologies, Model G1530A with a capillary column DB-FAB (0.25mm x 30m) by the same company, using helium as the carrier gas. A sample volume of 0.5 ml was injected with a gas flow rate of 1 ml min-1 and a split ratio of 20:1. The initial temperature was 80°C, held for one minute, and then increased at a rate of 20°C per minute until a temperature of 120°C was reached. Temperature was then increased at 6°C per minute until it reached 205°C. The VFAs were identified in a retention time range between 5 and 11 minutes. The injector temperature was 210°C and the detector temperature was 240°C. |
| Experimental System |
| The experimental device is shown in (Figure 1). A glass Pyrex reactor with a 2 litres capacity coupled to a heating and mixing system was used. Mixing was controlled at 10 rpm in order to maintain a homogeneous mixture and temperature was maintained at 30 ± 2ºC during the experimentation. Methane gas production in the system was measured by the method of Mariotte [15]. This method consists of passing the biogas through a solution of 3M NaOH to capture and convert the CO2 present in the biogas to Na2CO3 and then measuring volumetrically the fraction of methane (CH4) produced. The Methane Production Rate (MPR) in ml of CH4/g of COD was determined by a linear regression analysis of the experimental data adjusted by the method of least squares. |
| Setup of reactor |
| The reactor was inoculated with 400 ml of anaerobic sludge from an operating vinasse treatment plant and stabilized with a biomass measured as Volatile Suspended Solids (VSS) of 5,900 mg l-1. Sludge or biomass was exposed to the wastewater substrate for 7 days for acclimatization. During this period organic matter concentration, measured as COD, was increased gradually until it reached 100% of COD concentration present in the wastewater. Once the final concentration of organic matter contained in the effluent was reached, the process was evaluated during 27 days, Samples of 3 mL were removed every 24 hours to measure pH, alkalinity, VFAs, COD and CH4 production. The extracted volume was replaced by an equivalent volume of deionized water. In accordance with Del Real-Olvera et al. [16], the experiment was terminated once the methane production rate was constant and the formation of biomass pellets was observed at the bottom of the reactor. |
| Results and Discussion |
| Organic matter decomposition and methane production |
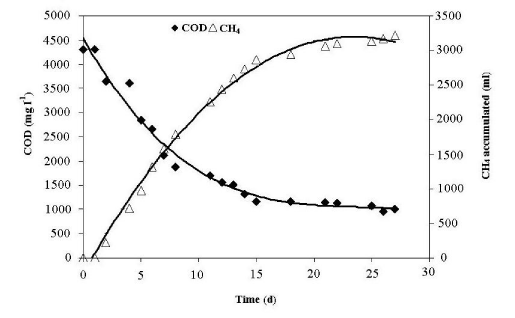
| (Figure 2) shows the decomposition kinetics of organic matter and the CH4 cumulative volume production profile, as a function of time. The volume of CH4 produced was proportional to the quantity of organic matter removed, which is consistent with the ideal behavior of an anaerobic process [16]. |
| A total of 75% organic matter removal was achieved during the 27 day experiment. This percentage correlates with the high biodegradability of the organic matter and demonstrate the high metabolic activity and biodegradability of the anaerobic biomass used [17]. Similar results have been reported by Pacheco and Magaña [18] for domestic wastewater, by Del Real-Olvera et al. [19] for vinasse effluent and by Caldera et al. [20] for slaughterhouse wastewater. |
| The process produced a total volume of 3,215 ml of methane during 27 days, coupled with the removal of 3,347 mg COD l-1. The fall in methane production at the end of the study correlated with the reduction in the methanogenic activity resulting from the exhaustion of bioavailable substrate [21]. |
| pH and alkalinity |
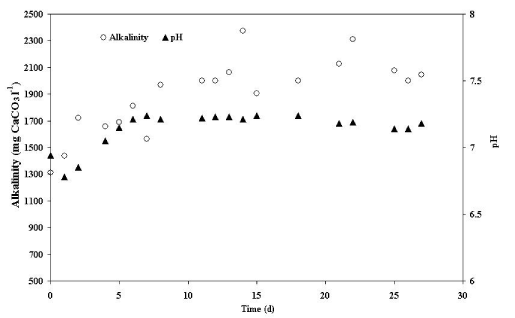
| An evaluation of pH and alkalinity was conducted during this research. (Figure 3) shows the changes in pH and alkalinity during the experimental period. The process started with an alkalinity of 1,310 mg CaCO3 l-1 which increased to an average value of 2,000 mg CaCO3 l-1 from the eighth day to the end of the study. There was a similar increase at the same time in pH from 6.8 to a constant 7.2. These values are within the optimal limits suggested for the development of anaerobic processes [22,23]. |
| The behavior of the alkalinity and pH showed that the treatment process produced an equivalent alkalinity to 690 mg of CaCO3 by liter of wastewater, sufficient to neutralize the VFAs generated during the process of anaerobic digestion, acting as a buffer system [24]. This phenomenon can be attributed to a high concentration of organic nitrogen present in the slaughterhouse wastewater. In agreement with Khanal [9], the organic matter (CHONS), composed mainly of proteins (like blood), reacts to form free ammonia. Subsequently, this compound reacts with the CO2 produced during the anaerobic process, resulting in ammonium bicarbonate, which contributes to the increase of system alkalinity according to the following reactions: |
| RCHNH2COOH + 2H2O → RCOOH + NH3 + CO2 + 2H2 |
| NH3 + H2O + CO2 → NH4+ + HCO3- |
| Volatile fatty acids |
| (Figure 4) shows the evolution of the VFAs concentration during the anaerobic treatment. The main VFA profiles showed a similar behavior. The maximum concentrations occurred on the fourth day, with acetic acid being the predominant VFA in the process. Sawyer et al. [23] suggested that an acetic acid concentration range of 50 to 250 mg l-1 in the effluent is optimal for the anaerobic digestion process. Our research recorded a maximum concentration of acetic acid of 448.3 mg l-1 during the acetogenic stage without observing any change in the treatment system stability, presumably due to the neutralization of the VFAs due to the alkalinity of the system. Khanal [9] suggested that the neutralization of VFAs in an anaerobic process can be explained according to the following reaction: |
| HCO3- + CH3COOH ↔ H2O+ CO2 + CH3COO- |
| Other authors have suggested that propionic acid is responsible for system acidification and the low efficiency in the methanogenic stage [24,25]. In our research, the highest concentration of propionic acid was 298 mg l-1, a value that was not high enough to destabilize the anaerobic process. |
| Zhao et al. [26] found that butyric acid concentration greater than 10,000 mg l-1 generated elevates concentrations of hydrogen in an anaerobic process. The maximum butyric acid concentration in the present study was 148 mg l-1. However, this value was not sufficient to change the pH value in the process. |
| Methane production rate |
| The relationship between methane production and organic matter decomposition is shown in (Figure 5). The MPR was 450 ml of CH4/g of COD removed at 30ºC and 0.82 atm. This value is within the range reported by Rivera et al. [27], which varied between 280 and 380 ml of CH4/g of COD removed at conditions for temperature and pressure of 25ºC and 1 atm respectively and to the value obtained by Del Real- Olvera et al. [19], which was 350 ml of CH4/g of COD removed for environmental conditions similar to the previous study. Both previous studies were performed using distillery effluent and control of alkalinity. The high MPR value obtained in our study is attributed to the different temperature and pressure conditions and to the elevated methanogenic activity. Therefore, due to the high MPR obtained in this study, the capture and use of CH4 as an energy source seems feasible. |
| VFAs production rate |
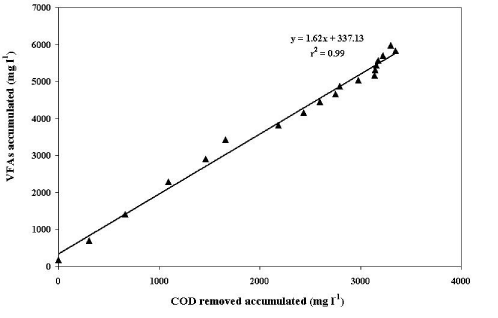
| The VFA production rate was determined graphically from the results of the VFA generation and organic matter decomposition. As shown in (Figure 6), we obtained a value of 1.62 g of VFA/g of COD removed. According to Colmenarejo et al. [28], this value can be associated with an elevated acidogenic activity. The high linear correlation coefficient showed that there is no VFA accumulation or there is a greater consumption of these intermediary compounds during the anaerobic process despite the elevated acidogenic activity. |
| Acetogenic and methanogenic stages |
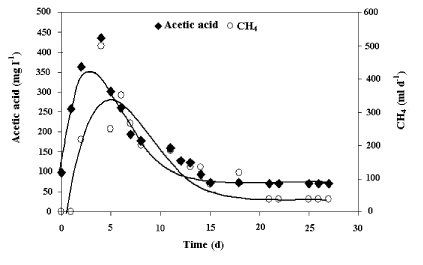
| The VFA concentration profiles showed a similar trend to that observed in methane production. (Figure 7) shows that acetic acid had the highest concentration during the treatment process, representing an important factor as an indicator of acetogenic activity [29]. |
| The response time between the acetic acid production stage (acetogenic) and the biogas production stage (methanogenic) was 2 days. This is consistent with that reported by Chaisri et al. [30], who indicated that the acetoclastic methanogenic pathway contributes in an important manner to the anaerobic digestion process. In this pathway, acetic acid can generate up to 72% of the total production of biogas of the methanogenic stage. |
| Relationship of VFAs/ALK |
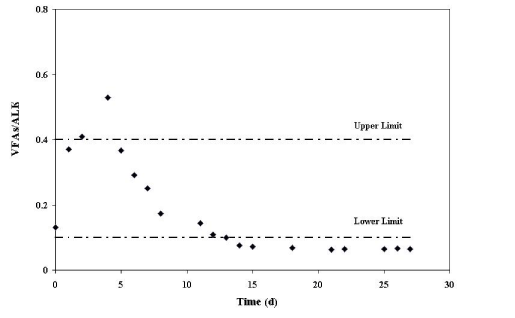
| The stability of an anaerobic process can be evaluated by the VFA/ ALK ratio (Volatile Fatty Acids/Alkalinity) [9]. Barampouti et al. [31] suggest that the ideal ratio of VFA/ALK is in the range of 0.1 to 0.3 to avoid the acidification of the process. A value above 0.4 is an indicator of instability. The ratios for this study are shown in (Figure 8). |
| Our results demonstrate that the VFA/ALK ratio exceeded the optimal upper limit on the fourth day (acidogenic stage). Despite this, the pH did not decrease during this period. This can be attributed to the buffer system described earlier. (Figure 8) shows that the VFA/ ALK ratio was below the lower limit suggested by day 14. This suggests that the VFAs concentration in the anaerobic process is linked to the easily assimilated organic matter by microorganisms and to methane production. |
| Conclusions |
| In this research the main factors that assured the stability of an anaerobic process for the treatment of slaughterhouse wastewater were evaluated. The stability of the treatment system was due to the alkalinity produced during the anaerobic process and by the characteristics of the wastewater. These conditions generated a buffer system that suppressed the effects of the acidogenic stage, maintaining the process pH at 7.2. |
| This study has shown the feasibility of treating slaughterhouse wastewater using a one-step anaerobic process, achieving removals of 75% of organic matter and the opportunity of use the CH4 generated as energy. The organic matter decomposition kinetics showed an ideal behavior of an anaerobic process and was highly associated with methane production. |
| The methanogenic activity developed in this anaerobic process is evidenced by a high MPR of approximately 450 ml CH4/g of COD removed at 30ºC and 0.82 atm. This study shows the technical viability of producing CH4 from the anaerobic treatment of slaughterhouse wastewater; nevertheless, it is necessary to do an economic study that assures the utilization of the CH4 as source of energy. |
| Acknowledgements |
| We are thankful for the support for this work provided by the state of Hidalgo (HGO FOMIX. Code 72697) and the scholarship funded by CONACYT for the pursuit of postgraduate studies for the first author. The authors are also grateful to visiting investigator Elizabeth Sugg of the United States Peace Corps/Mexico for his assistance in revising this paper. |
References
|
Tables and Figures at a glance
| Table 1 |
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14796
- [From(publication date):
January-2011 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10110
- PDF downloads : 4686
