Research Article Open Access
Estimation of Pharmacokinetics of Propofol in Indian Pateints by HPLC Method
A Puri1*, B Mehdi1, N B Panda1 and G D Puri S Dhawan21Post Graduate Institute of Medical Education and Research, India
2Panjab University, Chandigarh, 160012,India
- *Corresponding Author:
- Dr. Puri
Post Graduate Institute of Medical Education
and Research# India
E-mail: avinash_puri@hotmail.com
Received date: March 26, 2011; Accepted date: May 16, 2011; Published date: May 17, 2011
Citation: Puri A, Mehdi B, Panda NB, Dhawan GDPS(2011) Estimation of Pharmacokinetics of Propofol in Indian Pateints by HPLC Method. J Anal Bioanal Tech 2:120. doi: 10.4172/2155-9872.1000120
Copyright: © 2011 Puri A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A quick and sensitive reversed phase high performance liquid chromatography (HPLC) method has been developed in Indian surgical patients to determine the concentration of Propofol In human plasma. Propofol can be isolated from human plasma by adding 1ml precipitating solution which consists of acetonitrile and perchloric acid (67:33) mixture, which also contains dibutylpthalate (1mg/ml) as an internal standard. The sample is mixed for two minute on a vortexer. The plasma substance precipitated by acetonitrile and perchloric acid are further separated by centrifugation. The supernatant is directly injected into the HPLC system with the help of autosampler.
The analysis was carried out using column 250 × 4.6 mm column packed with10-μm Spherisorb reversed phase octadecyl silane particles (C 18 ). The 500ml of mobile phase (67:33:0.04) consisted of 335ml of acetonitrile and 165ml of distilled water and 200μl of acetic acid maintaining the pH 4.0.The flow rate of the mobile phase was 1.5ml/ min. propofol was monitored by a UV detector at a 270nm wavelength. The limit of detection of propofol (in human plasma) was found to be 0.0001μg/ml while limit of quantification was found to be 0.001μg/ml for a 20ul injection volume.
Keywords
Propofol; HPLC; Plasma concentration
Introduction
Propofol is an intravenous sedative hypnotic agent which is used for both induction as well as maintenance of general anesthesia. The pharmacokinetics of propofol has been evaluated extensively in the west in different patients groups after either bolus doses or continuous infusions and presently there are multiple models available based on that data. So far, the pharmacokinetics of propofol has not been studied in the Indian. In our experience, previous methods based on HPLC and UV detection was not proved to be fast one for research or clinical purposes. [1-3] The method we are decscribed here is fast and simple and detection limit is 0.0001 µg/ml.
Experimental
Chemicals and equipments
All chemicals were used of HPLC grade. Acetonitrile (Rankem, India), DibutylPthalate and 65% Perchloric acid were obtained from Merck Company. We used Perkin Elmer model for this estimation [3]. A Gilson Model 302 solvent delivery pump equipped with autosampler model 231 was used. The UV visible LC 90 detector was used. The signal from the UV detector was recorded on a Hewlett-Packard 3396A integrator. The column was carried out by 250 × 4.6 mm column packed with 10-µm Spherisorb reversed phase octadecyl silane particles (C18) [4]. A model 220 Branson sonicator was used to degas the mobile phase and solvents.
Preparation of stock solution
The concentration of Propofol stock solution of Propofol was 1 gm/ ml and then this solution is diluted in different concentration of 0.001, 0.003, 0.01, 0.05, 0.075, 0.1, 0.5, 1, 2, 5, 8, 10 and 12 µg/ ml in 10 ml of acetonitrile. This solution was prepared to plot the calibration curve and this stock solution can be stored at 4°C till the time of analysis (almost more than month).
Preparation of precipitating solution
Precipitating solution was prepared to precipitate the proteins. To obtain the precipiataing solution, a solution of DibutylPthalate 1 gm/ ml diluted into 1 mg/ ml in acetonitrile: then 0.1 µl of this solution was diluted in 10 ml of acetonitrile and 65% Perchloric acid in the ratio of 67:33 to obtain the precipitating solution.
HPLC conditions
The 500ml of mobile phase (67:33:0.04) consisted of 335ml of acetonitrile and 165 ml of distilled water and 200 µl of acetic acid maintaining the pH 4.0.The flow rate of the mobile phase was 1.5 ml/ min. propofol was monitored by a UV detector at a 270 nm wavelength [5].
Method
After approval from the Institutional Ethics Committee and written informed consent, ASA grade 1 old Indian patient was included. All patients underwent surgeries (laparoscopic cholecystectomy, Hernioplasty, Hysterectomy, Septopolasty and parotidectomy) requiring general anesthesia for less than two hours and expected blood loss of less than 10% of total blood volume. Patients with hepatitis, HIV infection, hepatic, renal, hematological and cardiovascular diseases were excluded from the study. No pregnant patient and no patient with history of smoking or alcohol intake were included in the study. Patients were premedicated with Tab Diazepam 5mg night before as well as 2 hrs before induction. Before induction of anaesthesia two large bore intravenous lines were secured, one each in the antecubital vein and vein on dorsum of the contralateral hand. The antecubital vein was used for blood sampling. Blood was centrifuged at 2000 rpm at 10 minutes to separate the plasma. After that precipitating solution (1ml) was added to 1ml of plasma sample, with the help of vortex mixer, stirred it for 2 minutes. The plasma substance precipitated by acetonitrile and Perchloric acid were separated by centrifuged at 20,000 rpm for 10 minutes. The supernatant was separated and inserted into the autosampler of HPLC system [5], after running baseline for an hour of mobile phase. The concentration of Propofol in test samples were calculated by using the regression parameters obtained from the area of the calibration graph.
| Concentration (µg/ml) | Area |
|---|---|
| 0.001 | 20.8687 |
| 0.003 | 21.7461 |
| 0.01 | 24.817 |
| 0.05 | 42.365 |
| 0.075 | 49.215 |
| 0.1 | 106.82 |
| 0.5 | 199.1 |
| 1 | 447.4 |
| 2 | 912 |
| 5 | 2213.93 |
| 8 | 3530.03 |
| 10 | 4407.42 |
| 12 | 5284.83 |
Table 1: Showing different dilutions of Propofol showing areas.
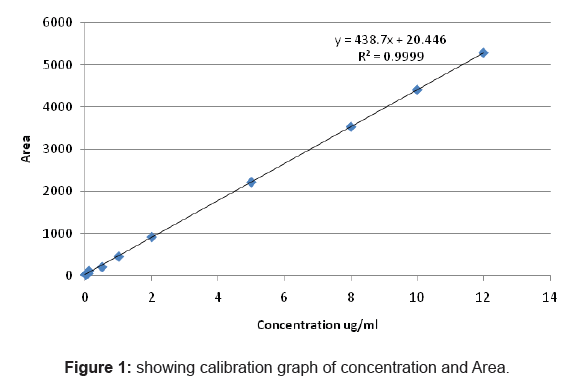
Linearity was validated by measuring area responses at the concentration range of 0.001µg/ml to 12µg/ml. Two separate stock solution were prepared, the same serial dilutions were made and each sample was injected in duplicate. A linear regression analysis was performed.
The calibration curve was found to be linear in the range 0.001µg/ ml to 12µg/ml
And the equation was (y= 438.7x+20.44)
Y= Area
X= concentration
R2= 0.999
The minimum detectable concentration of the analyte (is the smallest concentration that can be detected reliably) the Limit of detection is related to both the signal and the noise of the system as usually is defined as whose signal to noise ratio S/N ratio is at least 3 to 1. The minimum quantifiable amount often known as the limit of quantification is the concentration that can be quantitaed reliably with specify level of accuracy and precision.
The limit of detection was found to be 0.0001µg/ml while limit of quantification was found to be 0.001µg/ml as evident from the calibration curve.
Results and Discussion
This method was applied to carry out a pharmacokinetic study of Propofol in 26 Indian patients using single bolus dose of 2 mg/kg BW. The sampling time was from 2 min to 24 hrs. Mean Propofol value was obtained at 2 min was 5.5 ± 0.8 and at 24 hrs it was 0 ± 0 µg/ml. Plasma levels were stables during other time intervals. The reversed phase HPLC method described here is simple, reproducible and can be used for routine clinical and reaserch purposes. This method can also be used to measure plasma levels every hour from the pharmacological induction of the patients during prolonged anesthesia, and therefore allows propofol infusion to be modified during surgical operations. By this method we can detect plasma concentrations in a very short time.
Acknowledgements
Mr. Thomas (Senior technician), department of pharmacology, PGIMER, Chandigarh.
References
- Douglas EJ, Plummer Gf, Cosgrove MB (1981) Estimation of ICI 35,868 (Diprivan) in blood by high performance liquid chromatography. J Chromatogr 7: 223:232.
- Fan SZ, Yu HY, Chen YL, Liu CC (1995) Propofol concentration monitoring in plasma or whole blood by gas chromatography and high-performance liquid chromatography. Anesth Analg 8: 81-175.
- Yu HY, LiauJK (1993) Quantitaition of propofol in plasma by capillary gas chromatography. J Chromatography 615: 77-81.
- Plummer GF (1987) An improved method for the determination of propofol (ICI 35868) in blood. Journal of Chromatography 421: 171-176.
- Schuttler J, Schwilden H, Stoeckel H (1985) Pharmacokinetic and Pharmacodynamic modeling of propofol (Diprivan) in volunteers and surgical patients. Postgrad Med J 61: S53-S54.
- Pavan I, Buglione E (1992) Monitoring Propofol serum levels by rapid and sensitive reversed phase high performance liquid chromatography during prolonged sedation in ICU patients. J Chromatogr Sci 30: 164-166.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14522
- [From(publication date):
April-2011 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 9861
- PDF downloads : 4661