Epidemiological Status of Visceral Leishmaniasis in Iran: Experiences and Review of Literature
Received: 12-Dec-2011 / Accepted Date: 13-Feb-2012 / Published Date: 15-Feb-2012 DOI: 10.4172/2161-0681.S3-003
Abstract
Abstract
Background: The Mediterranean form of visceral leishmaniasis (VL), or kala-azar, is a potentially fatal vector-borne
zoonotic disease. At the present time, VL caused by Leishmania infantum is recognized as an important parasitic disease in Iran.
Objective: The aim of the study was to review the epidemiological aspects of VL in Iran during 1996-2010.
Methods: Twenty seven eligible journal articles on VL epidemiological aspects were indexed in electronic
databases (ISI and MEDLINE) from 1996 to 2010 and selected for this review accompanied by my personal
experiences over two decades. For the detection of VL in humans and animal reservoir hosts, anti-Leishmania
antibodies were detected using direct agglutination test (DAT). Parasitological examinations were performed
on suspected VL patients and all captured wild canines and rodents. Different molecular methods were
used for identification of Leishmania species isolated from infected humans, animal reservoirs, and vectors.
Results: More than 3000 cases of symptomatic VL were detected in 31 provinces of Iran from 1996 to
2010. The majority of VL cases (92.8%) were found among children up to 12 years old. DAT surveillance
could help decrease mortality and morbidity in VL endemic areas of Iran. The principal animal reservoir
hosts of the infection were domestic and wild canines. Infections of man and desert rodents were accidental.
Phlebotomus kandelakii; P. perfiliewi in northwest Iran; and P. major, P. keshishiani, and P. alexandri
in southern parts of Iran were parasitologically or molecularly shown to be the principal VL vectors.
Conclusion: The Mediterranean form of VL is a serious zoonotic disease that occurs sporadically
in all geographical zones of Iran except northwestern and southern Iran, where the disease is endemic
Keywords: Visceral leishmaniasis; Epidemiological aspects; Iran
316450Introduction
Visceral leishmaniasis (VL), also known as kala-azar, is a systemic parasitic disease transmitted by female sand flies and caused by various Leishmania species [1], which are found throughout parts of the Old and New Worlds and can infect humans as well as domestic and wild animals [1]. Domestic dogs (Canis familiaris) are principal VL reservoir hosts that can carry either L. infantum and/or L. chagasi [1]. These Leishmania species are responsible for a wide spectrum of clinical manifestations in humans, particularly in children up to 12 years of age and immunocompromised patients [2]. Unlike cutaneous leishmaniasis, which accounts for almost 20,000 new cases per year [3], VL has been reported sporadically in Iran, but the disease is endemic in northwestern and southern areas of the country [4-6] with about 100–300 new cases reported annually. The objective of this study was to describe the epidemiological status of VL in Iran from 1996 to 2010.
Materials and Methods
Twenty seven eligible journal articles on VL epidemiological aspects were indexed in electronic databases (ISI and MEDLINE) from 1996 to 2011 and selected for this review accompanied by my personal experiences. As VL diagnosis and treatment delay lead to high patient mortality, a serological surveillance using the direct agglutination test (DAT) has been established through cooperation of the Provincial Health Services in the endemic VL foci in Iran since 1996. Investigations were conducted in northwestern, northeastern, southern, and southeastern Iran, where human VL is endemic in some areas. The criteria for VL endemic areas were based on annual reports documenting the disease in indigenous people, especially in children. The sample collection was performed either passively or actively.
For passive sampling, blood was collected by trained healthcare workers from all suspected VL patients, who reported at least three clinical signs, including abdominal distension, paleness, and fever for at least a two-week duration. For active sampling, a VL serosurvey was carried out in areas where at least five VL cases were confirmed during the previous three years. Children were approached either by a home visit or in nurseries, preschools, or primary schools, as infantile VL occurs more often in the endemic areas of Iran.
Dog populations in villages were selected by simple random or probable sampling. Wild canines and rodents were shot and captured around the areas where cases of human VL had been identified previously. Field laboratories equipped with direct agglutination tests (DATs) were set up at each location for the purpose of providing a base where patients, especially children up to 12 years of age, and suspected animals could be examined.
In these studies, serological tests (particularly DAT), parasitological examinations including direct microscopy and culture, and occasionally animal inoculation and molecular characterization techniques were used.
For DAT, blood samples were prepared from humans and animal reservoir hosts using the following procedures. For humans, blood samples were collected by a finger prick (two drops at 30 μl per drop and were spotted on filter paper (Whatman no.4), allowed to dry, and stored at -20 °C until tested. Alternatively, 100 μl of finger-prick blood samples were collected from suspected patients into two heparincoated microhematocrit tubes and transferred to local laboratories in a cold box at 4°C. The collected blood was centrifuged at 800g for 5-10 min and the sera were separated and tested. For canines, blood samples were collected after physical examinations by veterinary doctors. Blood samples (~2.5 ml) were acquired by venipuncture, poured into 10 ml polypropylene tubes, and processed 4-10 h after collection. The collected blood samples were centrifuged at 800 g for 5-10 min, and the sera were separated and stored at 20°C until assayed by DAT.
DAT performance
The DAT antigen used in these studies were prepared in the parasitology department at the School of Health at Tehran University of Medical Sciences. All DAT batches were made as previously described by Harith et al. [7]. All samples were assayed by DAT in remote laboratories and confirmed by the leishmaniasis laboratory at the School of Public Health, Tehran University of Medical Sciences [4-6]. Antibody titers ≥ 1:3200 were considered as positive results in humans [8-10] and ≥1:320 for canines [7,11-13].
Parasitological examination
Parasitological examinations were performed on suspected cases in humans, rodents, and domestic and wild canines. Smears were prepared from bone marrow materials of serologically suspected humans and from skin lesions, livers, spleens, and large lymph nodes of the animals. All prepared smears were stained with 10% Giemsa stain solution and examined microscopically for the presence of amastigote forms of Leishmania spp. Some biopsy specimens were collected aseptically and cultured using Novy MacNeal and Nicolle medium. The cultures were incubated at 26°C for up to six weeks and examined weekly for the presence of promastigotes. For mass production of promastigotes, monophasic culture media, such as RPMI1640, was used.
Molecular characterization
Some Leishmania promastigotes, which were isolated from infected humans and dogs, were analyzed by isoenzyme analysis and molecular techniques including RAPD-PCR [14,15], PCR-RFLP [16], nested PCR and sequencing with internal transcribed spacer (ITS) [13] or the Leishmania nagt gene, encoding N-acetylglucosamine-1-phosphate transferase.
Results
Agents
Based on studies that were carried out over the last decade by isoenzyme analysis and molecular techniques on 30 Leishmania spp. isolated from bone marrow materials of infected humans and spleens of 50 infected domestic and wild canines throughout Iran showed that L. infantum Lon49 was the principal agent of human and canine VL [4-6,11-19]. Isoenzyme analysis was performed under the supervision of Dr. D. Evans at the London School of Hygiene and Tropical Medicine, United Kingdom, and Dr. Gh. R. Hatam at Shiraz University of Medical Sciences, Iran. Molecular results, particularly PCR-RFLP using the nagt gene, were confirmed by Prof. K. P. Chang from the University of Chicago, USA.
L. tropica is typically dermatotropic [20] and has been confirmed as a causative agent of VL infection in domestic dogs and humans, particularly in two patients with an HIV/leishmaniasis co-infection with generalized and multiple lesions of skin and visceral involvement and also in an immune-suppressed patient from south-central Iran [18,19].
Conclusion: L. infantum was found to be the principal agent of human and canine VL in Iran. L. tropica was the second most common etiological agent of VL, particularly in immunosuppressed patients.
Humans
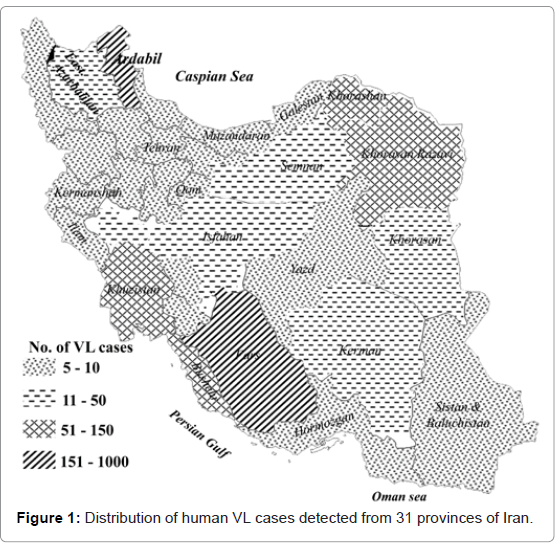
The first case of human VL in Iran was reported by Pouya (1949) in a boy from the Caspian area in northern Iran [21]. Since then, 4300 cases of human VL have been diagnosed in at least 113 cities and districts in Iran throughout the end of 1993 [5]. Altogether, more than 3000 cases of VL had been diagnosed in 31 Iranian provinces up to 2010 (Figure 1). Almost 40% of the diagnosed kala-azar cases are reported from northwestern Iran. The average annual number of the diagnosed cases of VL in Iran during last decade was 0.449 cases/100,000 inhabitants. The highest incidence rate of VL was 57 cases/100,000 inhabitants from Ardabil province, northwestern Iran. Based on a prospective survey that was carried out by Davies and Mazloumi Gavgani [8] on 5671 people in northwestern Iran, the average incidence rate of VL infection was found to be 2.8% in all ages equally at risk in 1985.
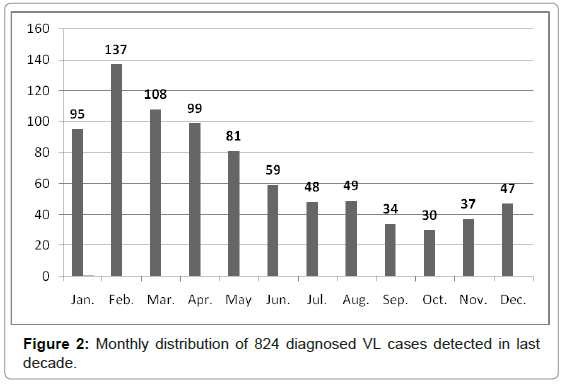
The DAT was developed and modified as a simple, practical, reliable, and economical technique for the diagnosis and seroepidemiological surveys of human and animal VL in Iran [4-9,22-23]. Active and passive VL serosurveys were performed on a total of 40,838 human serum samples, collected from four distinct geographical zones of Iran from 1996 to 2010. Altogether, 2265 (5.5%) samples showed anti- Leishmania antibodies at a ratio of 1:3200 or higher (Table 1), which were considered seropositive results. Almost 75% of DAT-positive cases (≥1:3200) showed clinical signs and symptoms in physical examinations. Predominant signs and symptoms in 217 hospitalized patients with DAT positive (≥1:3200) results included paleness (99.5%), fever (96.9%), splenomegaly (91.5%), hepatomegaly (53.6%) and lymphadenopathy (21.1%). From 246 symptomatic cases of kala-azar, diagnosed in northwestern Iran, 91% were ≤ 5 years-old and only 9% of patients were > 5 years-old. A statistically significant difference was found between males (58%) and females (42%) (p < 0.01). Moreover, 95.0% of the cases lived in rural areas while only 5% lived in urban areas. The annual monthly distribution of VL was determined by examining 824 patients living in northwestern and southern Iran. Five hundred and twenty of cases (63.1%) were diagnosed during cold months (January to May) (Figure 2). This finding associates with second phase of sand fly activities which are initiated from August. [24-27]. This distribution showed that the average incubation period of human VL ranged from 2 to 6 months after being bitten by a phlebotomine vector.
| Zones | No. of tested | No. of DAT+ | 95% CI | sero-prevalence (%) |
|---|---|---|---|---|
| North-west | 31499 | 1877 | 5.7-6.2 | 6.0 |
| North-east | 3341 | 85 | 2.0-3.1 | 2.5 |
| Central* | 1431 | 88 | 4.9-7.4 | 6.1 |
| South | 4567 | 215 | 3.9-5.1 | 4.7 |
| Total | 40838 | 2265 | 5.3-5.7 | 5.5 |
*Majority of samples were only prepared from hospitalized patients.
Table 1: Sero-prevalence of human visceral Leishmania infection by direct agglutination test (DAT) with anti-Leishmania infantum antibodies at ≥1:3200 titers by geographical zones during 1996 -2010.
There are no current records for the mortality rate of kala-azar in Iran. In a few old reports, the mortality rates of the disease among hospitalized patients varied from 10 to 30% [6,9]. However, based on our retrospective study carried out on 602 hospitalized kala-azar patients in northwestern Iran, only 2.8% of the patients died due to VL [5,6]. It seems that high mortality rates in hospitalized patients were mainly due to the delay in referral to medical centers.
Based on an intervention study, conducted in northwestern Iran from 2001 to 2007, a VL surveillance system using DAT was established for children aged ≤ 12 years in the primary health system. All cases with clinical VL signs and symptoms and positive DAT results were referred to remote hospitals for physical examinations and treatment. The mean annual incidence of VL significantly decreased from 1.88 (1985–2000) to 0.77 per 1000 children post-intervention (2001–2007). In a control area with no surveillance establishment, VL incidence increased from 0.11 to 0.23 per 1000 inhabitants [23].
During the intervention period, there were only two confirmed VL deaths in the intervention area (including an 8-year-old boy who did not tolerate meglumine antimoniate or amphotericin B regimens for his treatment), while at least 10 VL deaths were registered in the intervention area before the surveillance intervention. Thus, the annual VL mortality rate in children in the intervention area significantly decreased from 0.16 to 0.009/1000 children aged ≤12 years before and after the intervention, respectively (p < 0.001) [23], whereas 20 VL deaths in children up to 15 years old were registered in the control villages during this period [23].
Discussion and conclusion: Infantile VL occurs in various parts of Iran. Ardabil and east Azerbaijan, bordering Armenia and Azerbaijan, are VL endemic areas with a 6.0% seropositivity rate. DAT is a practical and reliable serological technique for the diagnosis and seroepidemiological surveys of VL in human as well as animal reservoirs with high validity and reliability. Almost 99.0% of infantile VL cases were found among children up to 12 years old.
In some rural areas of Iran, the rate of active kala-azar cases in males was higher than females, but these differences were not statistically significant. It appears that various weather and humidity conditions in the four distinct established geographical zones could influence serological detection of L. infantum infections in both humans and dogs, because sand fly activities have been correlated with weather and humidity in northwestern and southern Iran. Increased human Leishmania infection has occurred in northwestern Iran where at least 3.0% of children and 13.0% of domestic dogs showed L. infantum infection. It seems that the number of infected dogs from each zone have the greatest potential for disease transmission, due to a large dog population (7 dogs/100 inhabitants in the Meshkin-Shahr district of northwestern Iran). High canine infection rates should be considered as the most important risk factor of VL in northwestern Iran [6,11-13] The dog population and canine L. infantum infections in northeastern locations were low, thus we found low human seropositivity rates (2.5%). A high seropositivity rate (6.1%) in central Iran can be related to our sampling method because all serum samples were prepared from hospitalized patients with clinical VL manifestations.
Animal reservoir hosts
Of 5605 serum samples collected from domestic dogs in villages that are known as endemic foci of human VL, 597 (10.6%) were seropositive by DAT analysis with titers of ≥1:320 (Table 2). Up to 25% of seropositive dogs showed clinical VL signs [11-13].
| Zones CI | No. of tested | No. of DAT + | Sero-prevalence (%) | 95% CI Agents | Principal |
|---|---|---|---|---|---|
| Northwest | 2148 | 376 | 17.5 | 16.7-19.1 | L. infantum L. tropica |
| North & Northeast | 476 | 24 | 5.0 | 3.0-6.9 | L. infantum |
| Central | 2117 | 130 | 6.1 | 5.1-7.1 | L. infantum L. tropica |
| South | 864 | 67 | 7.7 | 5.9-9.5 | L. infantum L. tropica |
| Total | 5605 | 597 | 10.6 | 9.8-11.4 | L. infantum L. tropica |
Table 2: Sero-prevalence of canine visceral Leishmania infection by direct agglutination test (DAT) with anti-Leishmania infantum antibodies at ≥1:320 titers by geographical zones during 1999 -2010.
Parasitological and serological examinations were performed on 30 wild canines (foxes, jackals and wolves). The results showed that 10% of the captured wild canines were infected by L. infantum Lon 49 [12] (Table 3). A total of 680 rodents (Gerbillidae and Cricetidae) were caught during 1998 to 2004 in northwestern Iran. Leishmania parasites were detected in livers or spleens of 77 (11.3%) rodents by direct microscopic examination [28-30] .
| Type of animal positive | No.of animal | Parasitological positive | Serological | Leishmania sp. | ||
|---|---|---|---|---|---|---|
| DAT | ELISA | IFA | ||||
| Foxes | 10 | 1 | 1 | 1 | 0 | L. infantum |
| Jackals | 10 | 1 | 1 | 1 | 1 | L. infantum |
| Wolves* | 10 | 1 | 1 | 1 | 1 | L. infantum |
| Total | 30 | 3 | 3 | 3 | 2 | L. infantum |
*L. infantum was isolated and identified from an infected wolf in Iran for the first time in 2005.
Table 3: Results of parasitology and serology tests in wild canines collected in northwestern parts of Iran (2002-2003).
Leishmania spp. was isolated from five Meriones persicus specimens, three Mesocricetus auratus specimens and three Cricetulus migratorius specimens in culture media. Using isoenzyme techniques, the promastigotes isolated from M. persicus were characterized as L. donovani zymodeme LON 50 and those from M. auratus and C. migratorius were identified as L. infantum by conventional PCR assay (Table 4).
| Rodent species | No.of tested | No. of Positive microscopy | No. of Positive on culture media | Leishmania species (isoenzyme & PCR) |
|---|---|---|---|---|
| Cricetulus migratorius | 23 | 5 | 3 | L. infantum |
| Mesocricetus auratus | 4 | 3 | 3 | L. infantum |
| Meriones persicus | 469 | 69 | 5 | L. infantum |
| L. donovani LON50 | ||||
| Mus musculus | 124 | 0 | 0 | 0 |
| Rattus norvegicus | 60 | 0 | 0 | 0 |
| Total | 680 | 77 | 11 | L. infantum L. donovani |
Table 4: Leishmania species isolates from rodents caught in northwest of Iran during 1998–2004.
L. infantum may be transmitted from infected rodents to humans in endemic areas. This species of Leishmania is zoonotic and has been isolated from humans, domestic dogs, wild canines, and foxes in endemic areas of Iran. In a study carried out in the Semeskandeh area of Mazanderan province in northern Iran, Leishmania spp. infection was reported in internal organs of R. rattus, but the parasites were not isolated and characterized [31].
Conclusion: Domestic dogs and wild canines are the principal reservoir hosts for L. infantum in all four distinct Iranian geographical zones. Determination of the prevalence of canine Leishmania infection is necessary to control zoonotic VL in canines, particularly domestic dogs. Wild canines including jackals, foxes and wolves were infected by L. infantum and may serve as secondary reservoirs in endemic areas, particularly in villages located in mountainous regions where the transmission sylvatic cycle of VL occurs.
L. infantum infection was reported for the first time in a wolf, which was shot and examined with nine others in northwest Iran where human and canine VL are endemic. Also, desert rodents (gerbils) harbor Leishmania spp. infection and may, therefore, play a role in transmission of Leishmania spp. to humans, particularly children. Further ecological and biological studies of rodents and sand flies are necessary in endemic foci of zoonotic VL of Iran until the exact role of the rodents as animal reservoirs is completely elucidated.
Vectors
Based on epidemiological, parasitological and molecular studies carried out in VL endemic areas of Iran during the last two decades, Phlebotomus kandelakii [24,25,29] P. perfiliewi transcucasicus [26,27] in northwestern Iran, P. major [32,33], P. keshishiani [34] and P. alexandri [35] in southern parts of Iran are recognized as the main VL vectors.
Conclusion: In recent studies, natural leptomonas infections have been observed in P. kandelakii (0.34%), P. perfiliewi (1.09%), P. major (3.3%), P. keshishiani (2%) and P. alexandri (1.7%) [25,27,32,34].
To control VL in Iran, we suggest eliminating stray dogs; identifying suspect leashed dogs by periodic DAT and eliminating those found infected; vector control; identifying human cases using practical serological tests such as DAT; treating infected individuals early to decrease the disease’s mortality rate; and developing public health education.
Acknowledgements
I wish to thank Dr. M. M. Gooya, Director of Diseases Management Center, Ministry of Health, Treatment and Medical Education, Tehran, Iran; Dr. Shirzadi, Director of Zoonoses of Iran; the staff at District Health Centers in endemic VL areas in Iran; and finally, all of my colleagues in local leishmaniasis laboratories in the studied areas for their sincere collaboration. I also wish to thank my laboratory colleagues in the leishmaniasis lab at the School of Public Health, Tehran University of Medical Sciences: Dr. H. Hajjaran, Dr. B. Akhoundi, Mr. Z. Zarei, and Mrs. S. Charehdar .
This study received financial support from Tehran University of Medical Sciences and Center of Diseases Control of Iran (Project No: 130/6/10447) and the National Institute of Health Research (NIHR), Islamic Republic of Iran (Projects No: 88-03-27-9223).
References
- World Health Organization (2010) Control of leishmaniases. Technical report series 793 of WHO Expert Committee, Geneva.
- World Health Organization (2000) Leishmaniasis and Leishmania/HIV coinfection, WHO/CDC/CSR/ISR.
- Diseases Management Center, Ministry of Health, Treatment and Medical Education of Iran (2002–2010) Annual Communicable Diseases Report.
- Edrissian Gh H, Nadim A, Alborzi AV, Ardehali S (1998) Visceral leishmaniasis: the Iranian experiences. Arch Iranian Med 1: 22-26.
- Edrissian Gh H (1996) Visceral leishmaniasis in Iran and the role of serological tests in diagnosis and epidemiological studies. In: Ozcel MA, Alkan MZ Parasitology for the 21st century, CAB International.
- Mohebali M, Edrissian Gh H, Nadim A, Hajjaran H, Akhoundi B, et al. (2006) Application of direct agglutination test (DAT) for the diagnosis and seroepidemiological studies of visceral leishmaniasis in Iran. Iranian J Parasitol 1: 15-25.
- el Harith A, Slappendel RJ, Reiter I, van Knapen F, de Korte P, et al. (1989) Application of a direct agglutination test for detection of specific anti-Leishmania antibodies in the canine reservoir. J Clin Microbiol 27: 2252-2257.
- Davies CR, Mazloumi Gavgan AS (1999) Age, acquired immunity and the risk of visceral leishmaniasis: a prospective study in Iran. Parasitology 119: 247-257.
- Soleimanzadeh G, Edrissian GH, Movahhed-Danesh AM, Nadim A (1993) Epidemiological aspects of kala-azar in Meshkin-Shahr, Iran: human infection. Bull World Health Organ 71: 759-762.
- Edrissian Gh H, Hajjaran H, Mohebali M, Soleimanzadeh G, Bokaei S (1996) Application and evaluation of direct agglutination test in sero-diagnosis of visceral leishmaniasis in man and canine reservoirs in Iran. Iranian J Med Sci 21: 119-124.
- Bokai S, Mobedi I, Edrissian Gh H, Nadim A (1998) Seroepidemiological study of canine visceral leishmaniasis in Meshkin-Shahr, northwest of Iran. Arch Inst Razi 48-49.
- Mohebali M, Hajjaran H, Hamzavi Y, Mobedi I, Arshi S, et al. (2005) Epidemiological aspects of canine visceral leishmaniosis in the Islamic Republic of Iran. Vet Parasitol 129: 243-251.
- Moshfe A, Mohebali M, Edrissian G, Zarei Z, Akhoundi B, et al. (2009) Canine visceral leishmaniasis: asymptomatic infected dogs as a source of L. infantum infection. Acta Trop 112: 101-105.
- Motazedian H, Noyes H, Maingon R (1996) Leishmania and Sauroleishmania: the use of random amplified polymorphic DNA for the identification of parasites from vertebrates and invertebrates. Exp Parasitol 83: 150-154.
- Mohebali M, Parsa B, Motazedian MH, Yaghoobi-Ershadi MR, Hajjaran H (2002) Identification of Leishmania species from different parts of Iran using a random amplified polymorphism DNA in human, animal reservoirs and vectors. Med J Islamic Rep Iran 15: 243-246.
- Kazemi Rad E, Mohebali M, Hajjaran H, Rezaei S, Mamishi S (2008) Diagnosis and characterization of Leishmania species in Giemsa-stained slides by PCR-RFLP. Iranian J Publ Health 37: 54-60.
- Mohebali M, Edrissian GH, Shirzadi MR, Akhoundi B, Hajjaran H, et al. (2011) An observational study on the current distribution of visceral leishmaniasis in different geographical zones of Iran and implication to health policy. Travel Med Infect Dis 9: 67-74.
- Jafari S, Hajiabdolbaghi M, Mohebali M, Hajjaran H, Hashemian H (2010) Disseminated leishmaniasis caused by Leishmania tropica in HIV-positive patients in the Islamic Republic of Iran. East Mediterr Health J 16: 340-343.
- Alborzi A, Rasouli M, Shamsizadeh A (2006) Leishmania tropica-isolated patient with visceral leishmaniasis in southern Iran. Am J Trop Med Hyg 74: 306-307.
- Yaghoobi-Ershadi MR, Hanafi-Bojd AA, Javadian E, Jafari R, Zahraei-Ramazani AR, et al. (2002) A new focus of cutaneous leishmaniasis caused by Leishmania tropica. Saudi Med J 23: 291-294.
- Abass E, Mahamoud A, Mansour D, Mohebali M, El Harith A (2011) Validation of a β-ME ELISA for detection of anti Leishmania donovani antibodies in Eastern Sudan. Iran J Immunol 8: 150-158.
- Mohebali M, Edrissian GH, Shirzadi MR, Hosseingholizadeh G, Pashaei MH, et al. (2010) Integrated visceral leishmaniasis surveillance system in primary care for children in Meshkin-Shahr district, north-western Islamic Republic of Iran. East Mediterr Health J 16: 1050-1054.
- Absavaran A, Rassi Y, Parvizi P, Oshaghi M A, Abaie M R, et al. (2009) Identification of sand flies of the subgenus Larroussius based on molecular and morphological characters in north western Iran. Iranian J Arthropod-Borne Dis 3: 22-35.
- Rassi Y, Javadian E, Nadim A, Zahraeii AR, Vatandoost H (2005) Phlebotomus (Larroussius) kandelakii, the principal and proven vector of visceral leishmaniasis in north west of Iran. Pakistan J Biol Sci 80: 1-5.
- Sanei Dehkordi A, Rassi Y, Oshaghi MA, Abaie MR, Rafizadeh S, et al. (2011) Molecular detection of Leishmania infantum in naturally infected Phlebotomus perfiliewi transcaucasicus in Bilesavar district, northwestern Iran. Iran J Arthropod-Borne Dis 5: 20-27.
- Rassi Y, Javadian E, Nadim A, Rafizadeh S, Zahraii A, et al. (2009) Phlebotomus perfiliewi transcaucasicus, a vector of Leishmania infantum in northwestern Iran. J Med Entomol 46: 1094-1098.
- Mohebali M, Edrissian GH, Shirzadi MR, Hosseingholizadeh G, Pashaei MH, et al. (2010) Integrated visceral leishmaniasis surveillance system in primary care for children in Meshkin-Shahr district, north-western Islamic Republic of Iran. East Mediterr Health J 16: 1050-1054.
- Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjaran H, et al. (2004) Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. East Mediterr Health J 10: 591-599.
- Fallah E, Farshchian M, Mazlomi A, Majidi J, Kusha A, et al. (2006) Study on the prevalence of visceral Leishmaniasis in rodent’s of Azarshahr district (new focus), northwest of Iran. Arch Razi Inst 61: 27-33.
- Gholami Sh, Shahabi S, Mobedi I (1999) Prevalence of leishmaniasis in rodents’population in Jangle area of Semeskandeh district from Sari township, Mazandaran Province. Proceeding of 8th Congress of Infectious and Tropical Diseases, Tehran, Iran.
- Sahabi Z, Seyedi Rashti MA, Nadim A, Javadian E, Kazemeini M, et al. (1992) A preliminary study on the natural leptomonad infection of Phlebotomus major in an endemic focus of visceral leishmaniasis in Fars Province, south of Iran. J Publ Health 21: 87-93.
- Azizi K, Rassi Y, Javadian E, Motazedian MH, Asgari Q, et al. (2008) First detection of Leishmania infantum in Phlebotomus (Larroussius) major (Diptera: Psychodidae) from Iran. J Med Entomol 45: 726-731.
- Seyedi Rashti M A, Sahabi Z (1995) Phlebotomus (Larroussius) Keshishiani, Shchurenkova 1936, another vector of visceral leishmaniasis in Iran. J Publ Health 24: 25-30.
- Azizi K, Rassi Y, Javadian E, Motazedian MH, Rafizadeh S, et al. (2006) Phlebotomus (Paraphlebotomus) alexandri: a probable vector of Leishmania infantum in Iran. Ann Trop Med Parasitol 100: 63-68.
Citation: Mohebali M (2012) Epidemiological Status of Visceral Leishmaniasis in Iran: Experiences and Review of Literature. J Clinic Experiment Pathol S3:003. DOI: 10.4172/2161-0681.S3-003
Copyright: © 2012 Mohebali M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15042
- [From(publication date): 0-2012 - Nov 25, 2024]
- Breakdown by view type
- HTML page views: 10375
- PDF downloads: 4667