Review Article Open Access
Endosonographic Diagnosis of Chronic Pancreatitis
| Akane Yamabe, Athushi Irisawa*, Goro Shibukawa, Yoko Abe, Akiko Nikaido, Ko Inbe and Koki Hoshi | |
| Department of Gastroenterology, Fukushima Medical University Aizu Medical Center, Japan | |
| Corresponding Author : | Athushi Irisawa Department of Gastroenterology Fukushima Medical University Aizu Medical Center 21-2, Maeda, Yazawa, Kawahigashi Aizuwakamatsu, 969-3492, Japan Tel: 81-0242-75-2100 E-mail: irisawa@fmu.ac.jp |
| Received May 09, 2013; Accepted May 30, 2013; Published June 01, 2013 | |
| Citation: Yamabe A, Irisawa A, Shibukawa G, Abe Y, Nikaido A, et al. (2013) Endosonographic Diagnosis of Chronic Pancreatitis. J Gastroint Dig Syst S2:005. doi:10.4172/2161-069X.S2-005 | |
| Copyright: © 2013 Yamabe A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Chronic pancreatitis (CP) is characterized by irreversible damage that engenders fibrosis and necrosis of pancreatic tissue, with the consequent loss of endocrine and exocrine function of the pancreas. The clinical course of CP engenders a high rate of morbidity and mortality over a 20-25-year period. In view of this fact, current efforts emphasize the establishment of early diagnosis to commence intervention that can positively affect the natural course of the disease. Endoscopic ultrasonography (EUS) is a well-established and less-invasive modality for CP diagnosis. The higher imaging resolution provided by EUS enables detection of subtle pancreatic abnormalities, not only parenchymal but also ductal changes, that are undetectable using other modalities. This review presents an overview of the endosonographic diagnosis of chronic pancreatitis.
| Keywords |
| Chronic pancreatitis; EUS; Early chronic pancreatitis; ERP |
| Introduction |
| Chronic pancreatitis (CP) is characterized by irreversible damage that engenders fibrosis and necrosis of the pancreatic tissue [1], with the loss of endocrine and exocrine function of the pancreas [2]. Alcohol is the most common etiology of CP in 64.8% and idiopathy (unidentified) in 18.2%. Sarles et al. reported that 60-70% of patients with CP have a 6-12 year history of alcohol abuse [3]. In addition, regarding the relation between smoking and CP, smoking rates of CP patients are high (74.7% in men, 26.0% in women). Furthermore, lifestyles that include drinking and smoking are strongly associated with the onset and progress of CP. The clinical course of CP engenders a high rate of morbidity and mortality over a 20-25 year period. Moreover, CP is recognized as a risk factor of pancreatic cancer: patients with CP are 16 times more likely to develop pancreatic cancer than normal individuals are [4]. In view of these facts, current efforts emphasize establishment of early diagnosis to commence intervention that can positively affect the natural history of the disease: follow-up of CP in a rigorous manner is necessary using various modalities. |
| Endoscopic ultrasonography (EUS) is a well-established and less-invasive modality for CP diagnosis [5-10]. Although endoscopic retrograde pancreatigraphy (ERP) has been regarded as the gold standard for CP diagnosis, ERP cannot investigate the parenchymal changes. In that respect, the higher imaging resolution provided by EUS enables detection of subtle pancreatic abnormalities, not only parenchymal, but also ductal changes that are undetectable using other modalities. In this review, the authors describe the role of EUS in CP diagnosis, especially in the early stage. |
| Normal Pancreas on EUS |
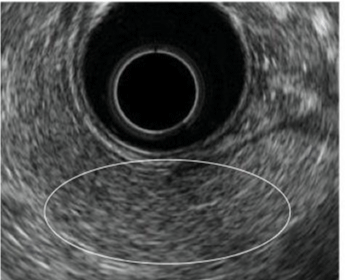
| An understanding of normal pancreas on EUS is extremely important for making EUS diagnosis of CP. The normal pancreatic parenchyma is uniformly depicted in equal or a slightly less hyperecho to the liver; it presents a so-called fine reticular pattern. The main duct dilation, duct irregularity and side branch ectasia are not visualized within the parenchyma [8]. Furthermore, the main ductal wall is observed as a uniform and slightly hyperlinear echo: 2.4 mm diameter in the head, 1.8 mm in the body, and 1.2 in the tail [6]. The EUS image of CP is defined based on these views. The EUS image of a normal pancreas is presented in figure 1. |
| EUS Features of Chronic Pancreatitis |
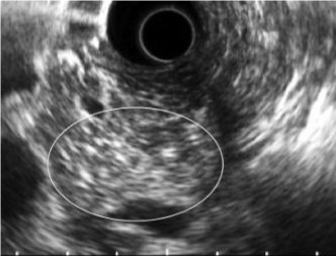
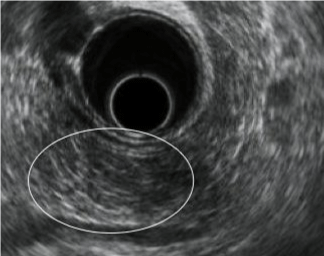
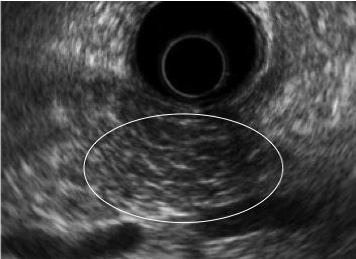
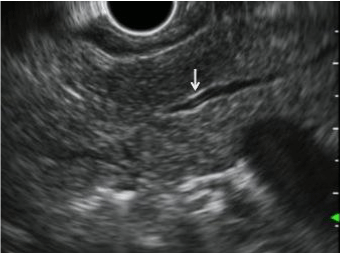
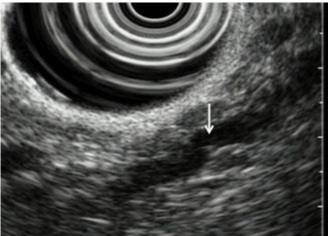
| CP is characterized by fibrosis of parenchyma along with ductal changes in the pancreas. These changes of fibrosis have been characterized as hyperechoic using ultrasound [11-13]. The superiority of EUS is not so high in the case of advanced CP because typical CP findings such as atrophy, calcification, main duct dilation, duct irregularity, cyst are readily observed even using other modalities. However, it is difficult to observe minute changes of pancreatic parenchyma using means other than EUS. Among patients with chronic pancreatitis, EUS will reveal all pancreatic abnormalities. Typical EUS findings are shown in figures 2-6. |
| Diagnosis of CP using EUS |
| Traditional EUS criteria for diagnosis of CP |
| Several studies have compared EUS findings with ERP (Cambridge classification) for CP evaluation [14]. Results of these studies suggest that ductal and parenchymal abnormalities detected using EUS correlate with the presence of CP. From these studies, the traditional EUS criteria for CP are recognized as follows: hyperechoic foci, hyperechoic strands, parenchymal lobularity, irregular pancreatic duct margins, hyperechoic pancreatic duct margins, visible pancreatic side branches, pancreatic duct dilation, shadowing calcifications and cysts. Opinions vary among researchers, but the presence of CP was diagnosed when EUS revealed at least 2-3 of the features described above. Our investigation also showed the over 80% patient who showed changes over ‘equivocal’ in Cambridge classification had more than three EUS findings [12]. In addition, the EUS is able to evaluate the severity of CP depending on the number of criteria present with high sensitivity and specificity. The disease severity was classified as mild (2 or 3-4 features), moderate (5-6 features), and severe (more than 7 features), based on ERP findings as a gold standard [8-10]. |
| New EUS criteria for CP diagnosis |
| Several investigators have reported the total number of EUS criteria, which is not only useful for diagnosis of CP but also for assessing severity. However, individual EUS criteria are considered to have their own importance in each ERP grading. Consequently, to discriminate against EUS features in every stage of CP, each EUS criterion was made as a point of reference for comparison between normal and varying degrees of CP. Irisawa et al. [12] reported the important EUS features in each severity: hyperechoic foci in mild CP, hyperechoic foci/visible side-branches/duct dilatation in moderate CP, and visible side-branches/duct dilatation/duct irregularity/calcification in severe CP (p<0.05). These EUS features described above were found to be independent predictors for assessing CP severity. |
| In 2009, the Rosemont classification is proposed as new EUS diagnostic criteria. New directionality of CP diagnosis by EUS was provided in an attempt to improve EUS reliability for CP. This classification system was an attempt to standardize and define more explicitly the endosonographic features and thresholds for the diagnosis of chronic pancreatitis, with grouping of criteria into major and minor importance categories (Tables 1 and 2) [15]. |
| As for the traditional EUS diagnostic criteria described above, the disease severity classification is accomplished merely from the number of CP findings. However, these diagnostic criteria are noticed as contrary when observing CP by EUS. Therefore, the value of each EUS finding differs in each level of CP severity. As an extreme example, findings having both calcification and cysts are equal to findings including both hyperechoic foci and strands in value as mild CP using the total number of findings in traditional criteria. In other words, the EUS findings observed on each practical CP stage are not considered in traditional criteria. However, in the Rosemont classification, each finding is assigned a grade (major or minor) in consideration of the value of findings. |
| In the Rosemont classification, the pancreas parenchyma findings are based on four typical findings: Hyperechoic foci, Lobularity, Cyst, Hyperechoic strands. In addition, Hyperechoic foci are subclassified as ‘with shadowing’ or ‘without shadowing’; Lobularity is subclassified as ‘with honeycombing’ and ‘without honeycombing’. However, as pancreatic duct findings, Calcification/stone in main duct are added to findings proposed previously such as Duct irregularity and Side branch dilation and Main duct dilation and Hyperechoic duct margins. Including subclassification, diagnostic criteria are produced based on 12 items described above about EUS findings and its grade. Creation of the newer Rosemont classification carried the hope of accomplishing the following: higher accuracy, better interobserver agreement, more well-received descriptors used by referring doctors (‘consistent with’, ‘suggestive of ’, etc.) than the more conventional descriptors of ‘high probability’, ‘indeterminate’, or ‘intermediate probability’, etc.). However, it was reported that most patients had the same diagnosis irrespective of the criteria that had been used [16]. No clear conclusion has been provided yet for whether traditional criteria or Rosemont classification is superior for CP diagnosis. |
| Proposed diagnostic criteria for early stage CP |
| Since the establishment of the landmark classification of pancreatitis at the Marseille symposium in 1963 [17], many classifications and diagnostic criteria have been proposed for chronic pancreatitis. However, general diagnostic criteria for CP are set for diagnosing advanced CP. They are unlikely to improve patients’ prognoses. In 2009, the Research Committee on Intractable Pancreatic Diseases supported by the Ministry of Health, Labour and Welfare of Japan, the Japan Pancreas Society and the Japanese Society of Gastroenterology revised the criteria, including through the standpoint of diagnosing early CP using EUS [18]. It is a challenge aimed at improvement of the long-term prognosis of CP patients by early diagnosis and therapeutic intervention in this disease. |
| This criteria comprise six items: (1) characteristic imaging findings, (2) characteristic histological findings, (3) repeated upper abdominal pain, (4) abnormal pancreatic enzyme levels in the serum or urine, (5) abnormal pancreatic exocrine function, and (6) continuous heavy drinking of alcohol equivalent to or more than 80 g/day of pure ethanol, as determined in accordance with criteria for alcoholic CP reported by Ammann [19]. According to these criteria, early CP is diagnosed by the presence of characteristic imaging findings for early CP together with more than two items among (3)-(6). The characteristic imaging findings for early CP were set as the following seven EUS features (five parenchymal and two ductal features): (1) lobularity with honeycombing, (2) lobularity without honeycombing, (3) hyperechoic foci without shadowing, (4) stranding, (5) cysts, (6) dilated side branches, and (7) hyperechoic main pancreatic duct (MPD) margin [15] (Table 3). More than two among the seven features including any of (1)-(4), which are most likely to reflect fibrous changes in pancreatic parenchyma, are judged to be sufficient for the EUS findings of early CP [9-12,20,21]. It remains as a challenge for future study to ascertain whether early CP diagnosed using these criteria progress to advanced CP. |
| Correlation between EUS and histological findings |
| Several researchers have investigated whether EUS features of CP are correlated with histology. Specific criteria such as hyperechoic foci, hyperechoic strands, and lobularity, which are identified only by EUS, were estimated as correlated respectively with histological findings as follows: focal fibrosis, bridging fibrosis, and interlobular fibrosis (Table 4) [11]. |
| Varadarajulu et al. [22] reported that EUS features were significantly associated with histologic abnormalities in a prospective study of 42 non-calcific CP (NCCP) patients. Parenchymal EUS features that were significantly associated with histopathologic NCCP were echogenic foci (p<0.0001), stranding (p<0.001), and lobulations (p=0.04); ductal features significantly associated with histopathologic NCCP were dilated (p<0.0001) or irregular main pancreatic duct (p<0.0001), side branches (p<0.001), and hyperechoic duct margins (p=0.03). Significant correlation was found between the number of EUS criteria and severity of NCCP on histology (r=0.85; p<0.0001). Although this study is limited by the small number of patients, results demonstrate the capability of EUS to detect early histological changes of CP. Albashir et al. [23] showed the meaningful correlation between the EUS and histological findings in the surgical case. They set that EUS scoring was performed using nine standard criteria, with ≥ 4 considered abnormal. Reportedly, the fibrosis score significantly correlated with the EUS score (r=0.72; 95% confidence interval (CI)=0.43, 0.87; p<0.001), and EUS had a sensitivity of 84% (95% CI=69, 100) and specificity of 100% (95% CI=40, 100) compared with histology. |
| Bhutani et al. [24] developed an animal model for studying EUS changes of early chronic pancreatitis, and described the serial changes of early chronic pancreatitis by EUS and correlates results with histology. In this study, a CP model was made by indwelling a 5-Fr pancreatic stent into pancreatic duct of dog. Animals were divided into two survival groups: 2 weeks and 4 weeks. Sequential pancreatic sections were taken from the head, body, and tail of the pancreas. EUS findings were correlated with histologic results with respect to degree of fibrosis, inflammation, and edema. As a result, the pancreas appeared homogeneous with only a few echogenic septations and echogenic margins of the main pancreatic duct at baseline EUS. At 2 and 4 weeks post-stenting, EUS images showed the following changes: lobularity, hyper and hypoechoic foci, increased echogenic septations, visible pancreatic duct side branches, and irregular margins of the main pancreatic duct. These EUS findings were supported by correlating results with histology. |
| Issues of CP diagnosis by EUS |
| Previous reports suggest that EUS can be a sensitive modality for diagnosing chronic pancreatitis when compared to ERCP findings [7,9,10,12,25]. However, currently inter observer variation might affect EUS diagnosis of chronic pancreatitis. Wiersema et al. [26] compared the degree of agreement among three experienced endosonographers in individual features of CP. The agreement was 88% for hyperechoic foci, 94% for focal reduced echogenicity, 94% for lobularity, 83% for hyperechoic duct margins, and 94% for duct irregularity. This study showed that EUS findings of CP might have good reliability, however, because no gold standard for diagnosis of early CP exists, the subjective assessment of endosonographic findings might vary among endosonographers, even among expert endosonographers, and standardized diagnosis of CP by EUS might be difficult. In relation to this issue, Irisawa et al. [13] conducted computer analysis to assess quantitation of EUS changes of chronic pancreatitis, demonstrating that, with respect to hyperechoic findings reflecting to fibrous changes, significant differences were found between normal pancreas and mild/ moderate/severe CP, and between mild and moderate/severe CP. These findings indicated the possibility of diagnosing CP objectively. In addition, results of our study suggested that computer-assisted image analysis might distinguish the appearance of early CP from that of normal pancreas. |
| Aging is regarded as a factor of parenchymal/ductal changes on EUS similar to chronic pancreatitis, in fact, many patients have pancreatic parenchymal and ductular changes that are identifiable on EUS in individuals without history or symptoms of pancreaticobiliary disease [27]. Many endosonographers have the impression that aging can cause variations in ductal and parenchymal pancreatic anatomy. Rajan et al. [28] reported that isolated EUS abnormalities are frequent in adults >60 without pancreatic disease. They reported that the presence of more than three EUS abnormalities, ductal or intra-parenchymal stones, ductal narrowing or ductal dilation is more likely to represent disease than age-related changes. However, whether these changes represent early pancreatic disease or simply represent normal variation remains unknown. |
| In addition, several investigators have reported that not only aging but also gender, smoking, alcohol drinking, and BMI level can influence EUS findings. Yusoff et al. [29] demonstrated in a much larger study (n = 1157) that heavy ethanol intake (OR 5.1, 95% CI: 3.1-8.5), male sex (OR 1.8, 95% CI: 1.3-2.6), clinical suspicion of pancreatic disease (OR 1.7, 95% CI: 1.2-2.3), and heavy smoking (OR 1.7, 95% CI: 1.2- 2.4) are independent predictors of severe EUS changes. In another study, Al-Haddad et al. [30] reported that a fatty pancreas causes hyperechogenicity of the parenchyma that can imitate echogenic changes of CP. In a logistic regression analysis, obesity, fatty liver disease, and alcohol intake were shown to be independent predictors of fatty infiltration of the pancreas. As described above, mild parenchymal and ductal EUS features can have poor specificity, resulting in the ‘over diagnosis’ of CP. All endosonographers should remain apprised of these issues. |
| Role and Future Development of EUS in CP Diagnosis |
| A CP diagnosis produced based on EUS entails several problems, but EUS is a useful modality that can reveal abnormalities of pancreatic parenchyma and duct that are not detected using other diagnostic imaging modalities. Abdominal pain is common and frequently debilitating in patients with chronic pancreatitis, particularly from an early stage. Chang et al. [31] demonstrated that EUS abnormalities are found at high frequencies in patient with abdominal pain, and that EUS is superior to transabdominal US for detecting CP. Consequently, they recommended that esophagogastroduodenoscopy combined with EUS should be considered in the first-line diagnostic evaluation of patients with upper abdominal pain, to detect mild/early CP. In addition, follow-up of CP for occurrence of pancreatic cancer is extremely important. Reportedly, patients with chronic pancreatitis are 16 times more likely to develop pancreatic cancer than normal individuals are. Strong evidence indicates the association of hereditary pancreatitis and pancreatic cancer [4]. Therefore, the physician is frequently faced with difficult decisions about how to manage the risk. Nevertheless, the identification of pancreatic cancer in advanced CP is extremely difficult. Some reports in the literature demonstrate that EUS detectability of pancreatic cancer in patients with CP is around 60% [32], and 54-74%, even if using combined EUS-FNA [33-36]. Therefore, the follow-up of CP from early stages, which is not detected using traditional modalities (e.g., CT, ERCP), will be important to detect smaller cancer nodes. |
| Conclusion |
| EUS is a useful and safe technique for detecting pancreatic parenchymal and ductal abnormalities, which are suggestive of CP. Medical intervention for CP in the early stage, can improve the CP prognosis. |
References
- Etemad B, Whitcomb DC (2001) Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology 120: 682-707.
- Clain JE, Pearson RK (1999) Diagnosis of chronic pancreatitis. Is a gold standard necessary? Surg Clin North Am 79: 829-845.
- Sarles H, Cros RC, Bidart JM (1979) A multicenter inquiry into the etiology of pancreatic diseases. Digestion 19: 110-125.
- Simon B, Printz H (2001) Epidemiological trends in pancreatic neoplasias. Dig Dis 19: 6-14.
- Dancygier H (1995) Endoscopic ultrasonography in chronic pancreatitis. Gastrointest Endosc Clin N Am 5: 795-804.
- Catalano MF, Geenen JE (1998) Diagnosis of chronic pancreatitis by endoscopic ultrasonography. Endoscopy 30: 111-115.
- Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, et al. (1993) Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy 25: 555-564.
- Bhutani MS (1999) Endoscopic ultrasound in pancreatic diseases. Indications, limitations, and the future. Gastroenterol Clin North Am 28: 747-770
- Sahai AV, Zimmerman M, Aabakken L, Tarnasky PR, Cunningham JT, et al. (1998) Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc 48: 18-25.
- Catalano MF, Lahoti S, Geenen JE, Hogan WJ (1998) Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc 48: 11-17.
- Raimondo M, Wallace MB (2004) Diagnosis of early chronic pancreatitis by endoscopic ultrasound. Are we there yet? JOP 5: 1-7.
- Irisawa A, Katakura K, Ohira H, Sato A, Bhutani MS, et al. (2007) Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. Journal of Gastroenterol 42: 90-94.
- Irisawa A, Mishra G, Hernandez LV, Bhutani MS (2004) Quantitative analysis of endosonographic parenchymal echogenicity in patients with chronic pancreatitis. J Gastroenterol Hepatol 19: 1199-1205.
- Sarner M, Cotton PB (1984) Classification of pancreatitis. Gut 25: 756-759.
- Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, et al. (2009) EUS-based criteria for the diagnosis of chronic pancreatitis: The Rosemont classification. Gastrointest Endosc 69: 1251-1261.
- Kalmin B, Hoffman B, Hawes R, Romagnuolo J (2011) Conventional versus Rosemont endoscopic ultrasound criteria for chronic pancreatitis: comparing interobserver reliability and intertest agreement. Can J Gastroenterol 25: 261-264.
- Sarles H (1963) Pancreatitis: Symposium of Marseille. Karger: 1965.
- Shimosegawa T, Kataoka K, Kamisawa T, Miyakawa H, Ohara H, et al. (2010) The revised Japanese clinical diagnostic criteria for chronic pancreatitis. J Gastroenterol 45: 584-591.
- Ammann RW (1997) A clinically based classification system for alcoholic chronic pancreatitis: summary of an international workshop on chronic pancreatitis. Pancreas 14: 215-221.
- Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, et al (2007) Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc 65: 808-814.
- Gleeson FC, Topazian M (2009) Endoscopic retrograde chlangiopancreatography and endoscopic ultrasound for diagnosis of chronic pancreatitis. Curr Gastroenterol Rep 9: 123-129.
- Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA (2007) Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc 66: 501-509.
- Albashir S, Bronner MP, Parsi MA, Walsh RM, Stevens T (2010) Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol 105: 2498-2503.
- Bhutani MS, Ahmed I, Verma D, Xiao SY, Brining D (2009) An animal model for studying endoscopic ultrasound changes of early chronic pancreatitis with histologic correlation: a pilot study. Endoscopy 41: 352-356.
- Bhutani MS (1999) Endoscopic ultrasonography: changes of chronic pancreatitis in asymptomatic and symptomatic alcoholic patients. J Ultrasound Med 18: 455-462.
- Wallace MB, Hawes RH, Durkalski V, Chak A, Mallery S (2001) The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc 53: 294-299.
- Nattermann C, Goldschmidt AJ, Dancygier H (1993) Endosonography in chronic pancreatitis: A comparison between endoscopic retrograde pancreatography and endoscopic ultrasonography. Endoscopy 25: 565.
- Rajan E, Clain JE, Levy MJ, Norton ID, Wang KK, (2005) Age-related changes in the pancreas identified by EUS: a prospective evaluation. Gastrointest Endosc 61: 401-406.
- Yusoff IF, Sahai AV (2004) A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol 2: 405-409.
- Al-Haddad M, Khashab M, Zyromski N, Pungpapong S, Wallace MB, et al. (2009) Risk factors for hyperechogenic pancreas on endoscopic ultrasound: a case-control study. Pancreas 38: 672-675.
- Chang KJ, Erickson RA, Chak A, Lightdale C, Chen YK, et al. (2010) EUS compared with endoscopy plus transabdominal US in the initial diagnostic evaluation of patients with upper abdominal pain. Gastrointest Endosc 72: 967-974.
- Fritscher-Ravens A, Brand L, Knöfel WT, Bobrowski C, Topalidis T, et al. (2002) Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol 97: 2768-2775.
- Barthet M, Portal I, Boujaoude J, Bernard JP, Sahel J (1996) Endoscopic ultrasonographic diagnosis of pancreatic cancer complicating chronic pancreatitis. Endoscopy 28: 487-491.
- Ardengh JC, Lopes CV, Campos AD, Pereira de Lima LF, Venco F, et al. (2007) Endoscopic ultrasound and fine needle aspiration in chronic pancreatitis: differential diagnosis between pseudotumoral masses and pancreatic cancer. JOP 8: 413-421.
- Varadarajulu S, Tamhane A, Eloubeidi MA (2005) Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc 62: 728-736.
- Krishna NB, Mehra M, Reddy AV, Agarwal B (2009) EUS/EUS-FNA for suspected pancreatic cancer: influence of chronic pancreatitis and clinical presentation with or without obstructive jaundice on performance characteristics. Gastrointest Endosc 70: 70-79.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 16191
- [From(publication date):
specialissue-2011 - Nov 14, 2025] - Breakdown by view type
- HTML page views : 11372
- PDF downloads : 4819
