Research Article Open Access
Electrochemical Nanosized Biosensors: Perspectives and Future of Biocatalysts
Jadwiga Soloducho* and Joanna Cabaj
Department of Medicinal Chemistry and Microbiology, Faculty of Chemistry, Wroclaw University of Technology, Poland
- *Corresponding Author:
- Jadwiga Soloducho
Department of Medicinal Chemistry and Microbiology
Faculty of Chemistry, Wroclaw University of Technology
Wybrzeze Wyspianskiego 27, 50-370 Wroclaw, Poland
Tel: +48-71-320-2891
Fax: +48-71-320-2427
E-mail: jadwiga.soloducho@pwr.wroc.pl
Received date: March 30, 2013; Accepted date: April 22, 2013; Published date: April 24, 2013
Citation: Soloducho J, Cabaj J (2013) Electrochemical Nanosized Biosensors: Perspectives and Future of Biocatalysts. J Anal Bioanal Techniques S7:005. doi: 10.4172/2155-9872.S7-005
Copyright: © 2013 Soloducho J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Electrochemical biological sensor device unite the sensitivity of classic instrumental techniques with the inseparable selectivity of the biological agent. The bio-element of the sensing device identifies the analyte following in a biocatalytic way and definitely generates an electrical response recorded by a transducing element. The signal is proportional to concentration of the analyzed substance. Certain of developed modern biosensors are commercial available and are regularly applied in clinical, environmental, or industrial arrangements. The enzymatic electrode is often the principal element of electrochemical biosensors. The choice of suitable composition of agents, in example: biocatalyst, mediators, semiconducting elements, supports, for design of enzymatic sensors directs an electrode activity according to electron transport rate, its stability, and vitality.
Presented article underlined also the variety of technologies for creation of sensors basing on carbon nanotubes and their application in detecting of a numerous of biological molecules.
Keywords
Biosensors; Nanostructures; Thin films; Electrochemical detection; Carbon nanotubes
Introduction
In the past few years, substantial research effort has been devoted to the development of organic biosensors, since they combine general merits of electronic sensors, like speed, size and system integration, with the ones of organic semiconductors, such as low cost production, facile integration with flexible substrates, and biocompatibility [1,2]. According to the fact, the design of modern biosensors is strictly combined with achievements of the nanotechnology.
Nanostructures and nanotechnology are connected with design and production of material and devices in range 1-100 nm. In this context, using of different type of nanoparticles or nanosized materials could improve i.e. sensitivity of device on the step of this construction already [3-5].
Molecular layers or other engineering materials in nano size affect with unique physic-chemical properties as surface effect or quantum size effect. Applying of different types of nanomaterials in biosensors enables using modern transducers in the technology of their fabrication. According to their size sensing nanounits can totally change the classical chemical and biological diagnostics, to allow fast in vivo analysis.
One of the frontiers in nanotechnology regards the precise organization of nanomaterials into two- and three-dimensional nano architectures for the production of new functional devices. To meet this challenge, high spatial control over the positioning of objects with dimensions on the nanometer scale has to be achieved on macroscopic surfaces. Among many others, the most general way to immobilize nanomaterials on different surfaces is achieved by the use of monolayers.
Control over the positioning and orientation of molecules on a surface allows the fabrication of nanostructures with new functionalities and a broad range of applications. Self-assembled monolayers of noncovalently bound molecules ensured the relevant path to form areas with peculiar functions, which allow tuning precisely the physicochemical properties of the surface.
It is extremely important for the device sensitivity that the recognizing elements have to improve surface density, stability, need to minimize some interactions with substances other than analyte. This type of materials can be designed by different molecular ways, i.e. Langmuir-Blodgett (LB) -type techniques [6,7], SAMs or LbL as well as electrolytic deposition [8,9].
The key factor of application of molecular layers to construct a biological sensor is the ability to reduce an immediate answer of the device.
Nanostructures
Modern molecular nanosized materials using in biological assemblies are in grown of interest. Different types of nano-sized units are developed according to specification of the character as well as application in biosensing technologies. This type of nanostructures contains graphene [10,11], nanoparticles (i.e. silver) [12], nanotubes [13], nanonofibers [14], nanorods [15] and thin molecular layers [6-9].
Modern nano-sized materials using in biological assays appear as a quickly progressing area. Varied type of structures is developed due to determination their characteristic features and ability of using in biosensing devices. The nanostructures embrace i.e. carbon nanotubes, nano-sized fibers, nanoparticles and molecular layers.
Nano-sized particles in biosensorics
Nanosized units have found a large number of applications in biosensorics. Exemplifying, functional nanoparticles can be used as drug delivers, as well as bounded with protein or nucleic acid has been applied as recognition part of biosensors (i.e. electrochemical biosensors).
Nanoparticles reveal unusual, dependent on the size chemical and physical features, as optical magnetic, catalytic, thermodynamic, and electrochemical [16].
Nanosized materials show distinctive physical features which much differ from those of their bulk counterparts. Simultaneously, intensive research efforts have been made to understand and control the collective properties of nanoparticles [17]. This progress in nanosized biological technologies requires techniques for observation, characterization as well as control nano-sized occurrences.
The biological application of nanoparticles were found in group of nanostructured conducting polymers [18,19], metal nanoparticles [11], metal oxides [20], semiconducting nanoparticles or quantum dots (QDs) [21] as well as organic-inorganic composites [22].
In example, nanosized conducting polymers (as is known from literature) show tunable porosity, high surface area, low energy optical transitions, low ionization potential, high electron affinity and unique electrical features [18,19]. Furthermore, polymers are rather cheap and can be functionalized by different patterning techniques to obtain demanded optical, electronic or mechanical properties, as well as biocompatibility. These unusual properties of nanomaterials conduct to a variety of applications in analytical sciences, biosensor devices or drug release systems, as reviewed by many scientists [18,19].
Because of their unusual catalytic features, nano-sized particles of some noble metals (i.e., Pt, Au, Ag) are engaged to design novel biosensors [23,24]. These nanoparticles often show remarkable catalytic activity in case of CO oxidation, hydrogenation of unsaturated alcohols, aldehydes and O2 reduction, when compare to the respective bulk metal elements [24].
Last time a huge amount of nano-size metal oxide (i.e., ZnO, TiO2, MnO2, SiO2) devices with new capabilities was fabricated [20]. The semiconducting, piezoelectric and pyroelectric features of the metal oxides were applied in optics, catalysis, sensor devices and as piezoelectrics. Nano-size metal oxides have broad band gaps and high binding energy. They are transparent and reflective, what make them ideal for construction of UV LEDs and lasers. Furthermore, nano-sized metal oxides possess high surface area, are non toxic, biocompatible, chemical stable.
Systems including of semiconducting quantum dots coupled to biological materials are of growing interest in the research area of both biotechnology and nanotechnology [21]. The conjugation of QDs yields hybrid materials, processes, and devices that can utilize both the unusual optical and magnetic features of quantum dots and high selective binding to oligonucleotides and polypeptides [21]. Particularly, the connection of polypeptide to QDs is in a great attention due to their using in construction more complex systems as well as in new sensing techniques [25].
Because of the tunable dependent on size optical properties, semiconducting quantum dots are greatly investigated in universal applications, which concerns LEDs [26], optical amplifier for telecommunication networks and biological labels [27]. It was demonstrated that covering the quantum dots with some inorganic semiconducting system (with higher band gap) may basically increase the photoluminescence as well as the chemical stability by passivating nonradioactive surface recombination sites [28]. Due to the fact, the core-shell-type of quantum dots, i.e., (CdSe)ZnS, are broadly applied in both optoelectronics and variety of biosystems [29].
As was recently reviewed by Prakash et al. [22] some metal nanoparticles and polymers frameworks (MNPPFs) have attracted extraordinarily scientific interest, as occurring porous material, because of the structural variety, flexibility and tenability, therefore, a broad range of applications was found (i.e. gas storage, selective catalysis, guest dependent luminescence) [30].
MNPPFs modified electrodes are attractive electrochemical sensors and biosensing devices. Selectivity of these devices extremely depends on the recognition component (biological molecule, i.e. enzyme) as well as hosting matrices and their reactions/interactions. MNPPFs are exceptional useful to obtain the appropriate sensitivity and stability, due to the fact inorganic parts behave as redox mediator of biological molecule, and organic polymer acts as selective adsorbate for biomolecules [31].
Generally, processes of sensor based on MNPPFs are classified as signal transduction and bio-recognition element techniques. Their signal transductions mainly are relied on electrochemical, optical, thermal, piezo-electric, cantilever and mass sensitive analysis. The bioelement method specifies antibodies, proteins, cells, nucleic acids and enzymes [32].
Novel sensors based on MNPPFs are effective, sensitive. Moreover, they show splendid selectivity when combined with biomolecules [33].
Biosensors based on molecular self-assembly
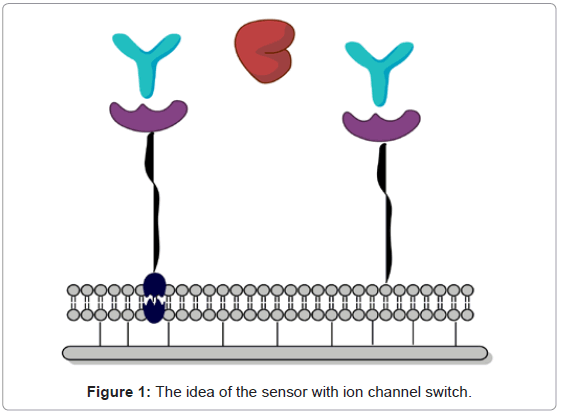
Self-assembled elements imitate natural patterns are the main factors linking biology, chemistry and physics. Molecular selfassembling is often applied to design modern molecular materials, and elements due to biological sensors [34]. According to design and production of biosensing devices molecular lipid layers as well as liposomes are of great interest, mainly because they are built of phospholipids or other lipid-like cell structures. The amphiphilic character enable them spontaneous formation of ordered systems. The lipid bilayered systems generate a neutral medium for immobilization of i.e. enzymes, receptors, cell elements, or even whole cells in a well-oriented pattern. An example of biomimetic system basing on spontaneous layer organization in natural medium is the sensor with ion channel switch reported by Cornell et al. [35]. The idea of the nanosystem was a mimetic membrane equipped with gramicidin (Figure 1).
Molecular self-assembly biomimetic systems are the joint effort in the sensorics field lying between biology, chemistry and physics. Molecular self-assembly techniques are very often used in designing of modern structures, materials, and devices for application in biosensorics [34]. Among the all SAMs systems, in a great of interest are ordered lipid layers and liposomes [34]. Due to their physicochemical character lipid, lipid-like films as well as liposomes are noticed to be in particular connection with biosensor devices. These amphiphilic structures composed mainly of phospholipids. Their hydrophilic/ hydrophobic characteristic enables them the spontaneous forming of ordered lipid systems. In example, supported bilayer lipid membranes can provide natural environment for embedded enzymes, receptors, cell fragments under mild conditions and in well-defined order [35].
One of the engineered devices designed due to bilayer lipid membranes was the biosensing device with ion channel switch presented by Cornell et al. [35]. The construction solution of the system was connected with well-oriented mimetic bilayer equipped with protein (gramicidin, Figure 1).
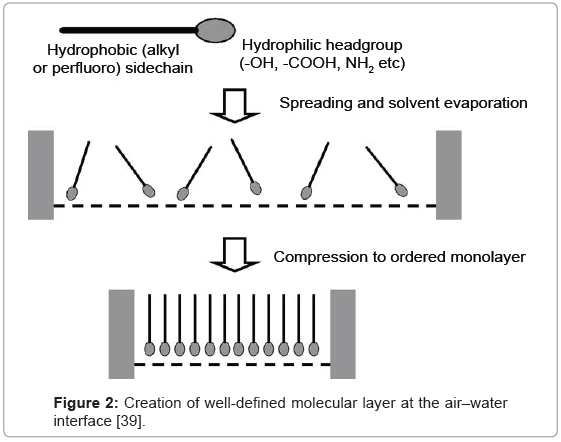
Monolayer engineering creating molecular assemblies is based also on Langmuir-Blodgett (LB) [36] or Langmuir-Schaefer (LS) [37] techniques, frequently united with self-assembly techniques [38]. Generally, single layers of amphipathic molecules arrange at the liquidair interface on the LB equipment. The process is connected with spreading - at the beginning, and subsequently with the compressing of monolayer to a demanded surface pressure (Figure 2).
The formed monolayer (in the next step) is transported onto solid substrate, the orientation of the monolayer is arranges as vertical. Consecutive layers are grown up by repeating of the transfer process. On the other hand, the protein monolayers are mainly formed by application of horizontal lift. The Langmuir-Schaefer (LS) technique allows transferring these types of monolayers on solid. In case of biocatalysts, an adsorption process seems highly valuable in maintaining of the protein activity in molecularly designed layer [39,40].
The LS technique of formation of monolayers is since it enables for a permanent monitoring of deposition data as i.e. surface quality, molecular density, and surface pressure. Especially, hybrid Langmuir monolayers composed of sterols, phospholipids, sphingo- and glycolipids, introduce a highly informative pathway for studying i.e. intermolecular interactions between membrane components and biomolecule [41].
Since peptides are not standard amphiphilic structures, some proper methods are adhered to use the chemical methods (e.g., derivatization [42] or modification of the subphase composition [43]) or by applying some artificial membranes, due to maintaining of the natural structure and activity of polypeptides in monolayer.
The quality of every protein-monolayer depends on the level of maintaining of the natural polypeptide features. The immensity of electrostatic forces retaining polypeptide can be compared to the surface tension one [44].
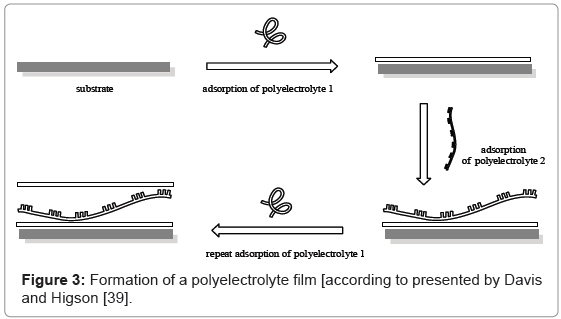
A more recently developed technique [45] due to organize the molecular layers makes use of the interaction between varied (in charge) polyelectrolytes. The process is presented in figure 3.
This method utilizes the positive/negative charge effect between supports and layers to form frequent layered film retained by electrostatic forces. Charged solid immersed in diluted solution of oppositively charged polymer species causes strong interactions and fabrication of thin polyelectrolyte layer, such as polystyrene sulphonate, which generates anionic character of polyelectrolyte. Rinsing the sample in the next electrolyte (i.e. polyallylamine hydrochloride) is connected with deposition of the next layer and reversing the charge of surface. Repeating of the procedure allows obtaining the designed multilayer with well-known thickness.
LbL method takes advantage of the charge–charge interaction between substrate and monolayers of polyelectrolytes to form multiple nano-size architecture maintained together by electrostatic forces. The formation of LBL systems are attributed to electrostatic interactions, hydrogen bonding, hydrophobic interactions and Vander Waals forces [46].
The adsorption outcomes of electrostatic interactions, thin layers may be eagerly deposited on different solids. The adsorption method applied in fabrication the layers was proved to be driven by the electrostatic interactions observed between doped chains of the polycation and the charged chains of the polyanion [47].
By merits of the weak attractions of a hydrogen-bonded layer on hydrophobic surface, it is potential to produce elastic films [48]. Hydrogen-bonded layer-by-layer systems give new solutions for LbL films, which were produce in much harder way than their electrostatically gathered counterparts.
Mukhopadhyay et al. [49] admitted a co-action between hydrophilic-hydrophobic interactions to determine the architecture of molecules in Langmuir-Blodgett layer processing.
Vander Waals forces as well maintain the arrangement of the oppositely charged layers. The layer growing is found by consecutive adsorption of differently charged systems and currently is broadly applied in biomedicine, and energy-related field [50].
Unlike the LB films LbL layers are much more amorphous and not tend to built crystalline structures [51]. They are stable according to charge interactions [45]. The LbL layers are strongly dependent on pH and ionic strength LbL films are not restricted to even surfaces, however may be also deposited on charged elements [52,53]. The different sorts of the method are adequate in deposition of biomolecules, which are mostly charged particles [54].
Electrochemical Detection
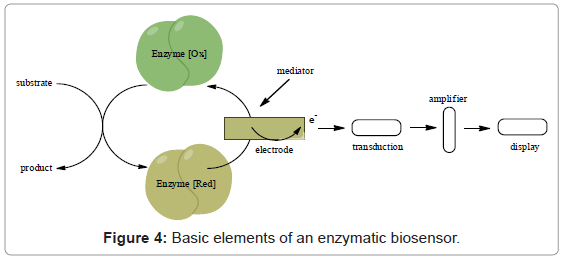
Majority of sensing devices apply electrochemical detection in transducer design due to many advantages as low cost, simplicity of production (Figure 4) [55]. The reaction being monitored electrochemically typically generates a measurable current (amperometry), a measurable charge accumulation or potential (potentiometry) or alters the conductive properties of the medium between electrodes (conductometry) [56]. Use of electrochemical impedance spectroscopy by monitoring both resistance and reactance in the biosensor is also becoming more common [56].
Electrochemistry ensures many merits as detection method. One of the main advantages of the technique is fact, that it is possible to use samples with small volume [57]. Electrochemical detection may be applied to obtain low detection limits in immunoassays and attoand zeptomole detecting electrochemical immunoassays are produced [58,59].
In order to homogeneous immunoassays, which do not separate the isolation of the antibody–antigen complex, electrochemical detection is not influenced by sample constituents (chromophores, fluorophores). Therefore electrochemical monitoring may be made on entire blood, without any disruption from fat globules, red blood cells, hemoglobin, and bilirubin [60,61].
Electrochemical techniques are generally organized into three main categories of measurement: current, potential and impedance.
Voltammetry/amperometry
Voltammetric and amperometric techniques are characterized by applying a potential to a working electrode versus a reference electrode and measuring the current. The current is a result of electrolysis by means of an electrochemical reduction or oxidation at the working electrode. The electrolysis current is limited by the mass transport rate of molecules to the electrode [62]. The current response is usually a peak or a plateau which is proportional to the concentration of analyzed substance. Voltammetric methods include many types i.e. linear sweep voltammetry, cyclic voltammetry, hydrodynamic voltammetry, differential pulse voltammetry, polarography, and stripping voltammetry [62]. These methods have a wide dynamic range, and are useful for low level quantitation.
In amperometry, changes in current generated by the redox processes are monitored directly with time while a constant potential is maintained at the working electrode as regards to a reference electrode. It is the absence of a scanning potential that distinguishes amperometry from voltammetry. The technique is implemented by stepping the potential directly to the desired value and then measuring the current, or holding the potential at the desired value and flowing samples across the electrode as in flow injection analysis. Current can be in proportion to the concentration of the electroactive species.
Amperometric biosensors have additional selectivity in that the oxidation or reduction potential used for detection is characteristic of the analyte species [55]. Amperometric detection is commonly used with biocatalytic and affinity sensors because of its simplicity [63].
Growing interest is paid also to electrochemical detection in flow systems, which can be used in permanent monitoring of environment as well as in industrial processes, since the flow conditions enable changing of the solution in easy way in multistep processes, as well as perfect in on-line control.
Electrochemical sensors are part of an electrochemical cell that consists of either three electrodes or two electrodes. A typical three electrode electrochemical cell consists of a working electrode of a chemically stable, conductive material, i.e. Pt, Au, or C (i.e. graphite); a reference electrode, and an auxiliary electrode.
One advantage of this system is that the charge from electrolysis passes through the auxiliary electrode instead of the reference electrode, which protects the reference electrode from changing its half-cell potential. Both three electrode systems and two electrode systems are used for sensors. However, two electrodes are generally preferred for disposable sensors because long-term stability of the reference is not needed and the cost is lower. The electrodes may be miniaturized, due to micrometres are well-known, while nano-sizes are not common [64]. According to the necessity of miniaturization of biosensing detectors the nanosized particles, carbon nanotubes are being of interest in these type devices construction [65].
Electrochemical biosensors
As is well known from literature [56] oxidoreductases are mainly used in fabrication of different sensor devices. The base of biocatalytic detection is catalytic reaction when the electrons are produce or are used up. The universal simplified a biocatalyst-based sensor using immobilized protein as chemiselective element and an electrode as classical transducer is shown in figure 4.
Although many types of biorecognition elements have been used in biosensing devices, electrochemical biosensors primarily use enzymes due to their high biocatalytic activity and specificity [56]. Biocatalytic sensors using enzymes as the recognition element often have relatively simple designs and do not require expensive instrumentation. Such sensors are typically easy to use, compact, and inexpensive devices.
According to data reported by Cai et al. [66], electrochemical biosensors are constructed often from metallic nanoparticles, which are applied in order to improve the amount of biomolecules immobilized on biosensing layer. In example the colloidal gold was used by Cai and co-workers to improve nucleic acid deposition on a gold electrode, and finally reduce the detection limit of designed sensor [66]. Metal nanoparticles are mainly used as catalyst of biochemical processes and can be easy employed in biosensing devices design.
It was earlier reported that gold-enzymatic protein colloids are applied on electrode surface to design and construct oxygen peroxide sensors as well as glucose or xanthine ones [67-69] due to study of electrochemistry of horseradish peroxidase [69]. In case of horseradish peroxidase deposited on carbon electrode covered with colloidal gold, Xu et al. reported rapid amperometric response and affinity to reduction of hydrogen peroxide without presence of any mediating species [69]. The biosensing device was found as sensitive, stable and able to detect H2O2 (detection limit 0.4 AM [69]).
According to Curri et al., it is also well known, that semiconducting nanocrystals are able to increase the effect of photoreactions and could be effectually linked with biomolecules creating modern photoelectrochemical systems [70] as in case nanocrystals of CdS deposited on gold electrode in mixture with formaldehyde dehydrogenase.
Electrochemical thin film biosensor
Thin films are known as thin layers in thickness scale ranging between a nanometer to several micrometres. There are a several well-known deposition methods for thin film fabrication, such as LB/ LS method, LbL assembly, evaporation, sputtering, chemical vapor depositions, electropolymerization, etc.
In a last time an interesting literature concerns polymeric materials applied in biosensing devices was found [71,72], in which polypyrrole was strongly favored. In group of conducting polymers, polypyrrole plays an important role in the electrochemical sensing devices because of its electrochemical activity and sensitivity as well as other features. Important is also fact that polypyrrole is easy to obtain from aqueous solutions by chemical or electrochemical pathway. In biosensor design, polypyrrole is often applied as a conducting layer and due to the fact the other materials can be incorporated into the support for correction the effect of sensor. Fiorito et al., in example, fabricated hybrid layers with proper stability and homogenity built of polypyrrole and copper hexacyanoferrate [73]. The biosensor was able to detect reduction of H2O2 (Na+ or K+ in environment of sample) [73].
The LbL method has been often applied in the immobilization of different biomolecules following the early reports of Lvov et al. [74]. The main advantage of the technique was the permanent monitoring in both film thickness and molecular architecture. According to the suitable multilayer production conditions, containing the application of aqueous solutions at optimized pH-dependent biological molecule activity over a determined time, the method is extremely useful for a wide range of bio-materials applied as sensing elements (DNA [75], enzymes [76] and antigen–antibody pairs [77,78]).
LbL technique was employed as well to construct biosensors based on polypyrrole by Shirsat et al. [78]. The polypyrrole film was obtained by electrochemical polymerization on platinum electrode coated with polyvinylidene fluoride membrane. Moreover, the system was equipped with carbon nanotube multilayer. This type of layered hybrid structure formed an excellent base for the biocalysts immobilization because of exceptional properties of both, polypyrrole and carbon nanotubes [78].
Immobilization of glucose oxidase on this hybrid matrix by crosslinking resulted with sensitive, fast response time glucose biosensor [79].
As was reported earlier by Siqueira et al. [80] enzymatic electrodes fabricated with LbL layers could exhibit electrochemical stability, and this type of sensing devices can be used in medical diagnostics. Actually, the LbL technique can enable to improve the biocatalytic properties of sensor. The method allows obtaining hybrid molecular materials with better transferring of charge as well as maintenance of the bioactivity of protein [80].
To compare, using the LB technique the molecular layers are not inevitably soluble in water, the fact does this techniques supplementary. As is well known, LB layers form due to transport of Langmuir monolayer onto substrate by vertical immersing in the subphase. Multilayered film is obtained during repeated processes of immersion and withdrawal. Demanding of not soluble layer may be limited, however, this limitation may be omitted due to adsorption process of soluble molecules–(i.e. polypeptides) from subphase on pre-formed Langmuir molecular layer [36,80].
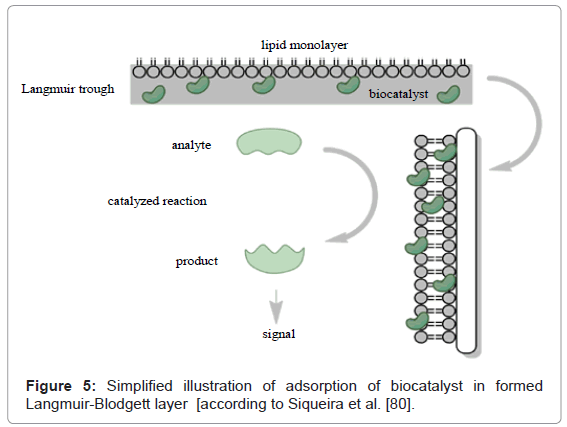
As was reported i.e. by Girard-Egrot et al. [81], phospholipid supports are employed to introduce biological molecules which are in co-deposition on solids, i.e. LB layers [81], (Figure 5). Moreover, to improving transport of the biomolecules, the phospholipid support may be employed as proper arrangement and improve sensitivity [81].
However, to construct effective biosensor, exceptionally weighty is design and well-organization of biomolecules on the sensor surface. According to that, well-oriented monolayers built of semiconducting units formed by LB method may perhaps be employed for uniform sensing properties. Well-oriented LB layers are taken into account to be extremely suitable, as the ordered position of molecules. As was reported earlier, the thickness in nano-size can affect in a strongly sensitive device with fast response [6,82,83]. These type semiconducting LB films can be applied as conducting molecular wires between i.e. an enzyme and the electrode surface. That sort of connection significantly affects on the electron transfer. Employing of well-oriented molecular architectures obtained by LB technique can implement an ability to produce molecular biosensors [83].
Mainly, biological molecules which can be used in sensors design are soluble in water. This fact directly relates to slim possibility of immediate deposition by the LB method, but, they are charged molecules, able to dissolving in the sub phase. When the monolayer of relevant charged molecule is covered the water surface and built Langmuir-Blodgett layers, biological molecule is annexed in LB layer. Anzai et al. [84] co-deposited penicillinase with stearic acid onto an Ion-Sensitive Field Effect Transistor (ISFET) to construct a penicillin biosensor. Another technique of introduction of biological molecules to Langmuir-Blodgett system was offered by Ohlsson et al. [85]. The method was connected with obtaining LB films of phospholipids, which then were employed as supports for liposomes deposition. A layer of phospholipid may be as well applied as a matrix for liposomes, and then transported on a solid during maintaining activity [86].
LB molecular layers can be used in construction of electrodes for cholesterol detection, as the well-oriented arrangement of molecules and nano-sized thickness are likely to appear in extremely sensitive device with ultra-fast response [87,88]. Matharu et al. [89] reported the covalent immobilization of cholesterol oxidase onto LB monolayer film. It has been shown that these well-ordered polymer chains behave as molecular wires affecting in improved electronaccepting conduct over O2 from the reduced biocatalyst. The LB layers were also used in fabrication of layer-biosensor for detection of phenolic compounds what was reported earlier [6].
The quality of creation of protein-layer on subphase is directly connected with the keeping of the native proteins features. Significance of electrostatic forces preserving the peptide structure can be compared to surface tension. Proteins are polystructures which incline to build up layers characterized by stability due to their amphiphilic character. Frequently proteins spreading at the suphase may result in change of the conformation of biomolecule (forming unfolding) [90]. According to Caseli et al. [91] is known, that insulin or ovalbumin unfold entirely while i.e. cytochrome C partially. This is probably connected with a relation of polar and non-polar residues of amino acids. Stable protein monolayers are not produced by highly polar proteins. In all these circumstances the adequate matrix support may be demanded. In example, biocatalyst activity in lipid LB films reported by Girard- Ergot et al. [81] is maintained due to the lipid molecular arrangement which protects the protein, placing the protein feature to permit the recognition and signal occurrence. Mainly the phospholipids are used as the protecting agents [91-93].
Biocatalytic Sensors
Enzyme-based electrodes
The biocatalyst is the main determined element of the enzyme electrode because of the selective properties and catalyzing production of the electrically active substance to detect [94,95]. The electroactive product can be monitored directly using amperometry, in which the produced current is measured in response to an applied, constant voltage.
Alternatively, the disappearance of the redox active species in an enzyme-catalyzed reaction can be monitored by the electrode. The activity of the immobilized enzyme depends on solution parameters and electrode design. The rapid enzymatic catalysis can also sometimes provide significant signal amplification in a biosensor [95]. The shelf life and stability of an enzyme generally determine the life-time of the biosensor. The use of enzyme electrodes as biosensors continues to increase because of their simplicity, and inexpensive in construction. These devices are able to provide rapid analysis, can easy regenerate, and are reusable [95]. However, the number of available enzymebased biosensors is still smaller than the number of potential analytes. Another disadvantage of enzyme electrodes is that the enzyme layer in the biosensor has to be replaced periodically since it gradually loses activity. Also, sophisticated electrochemical detection strategies or membranes are sometimes required to prevent interference from other redox active species at certain detection potentials [96].
Biosensors based on biocatalysts are shared in three generations. Biosensors of first generation were oxygen-based, whereas the secondgeneration are mediator-based. Third-generation of the biosensors is directly coupled enzyme electrodes.
Electrodes coated with glucose oxidase are broadly applied in detection of glucose since invention of Clark and Lyons (1950s and 1960s) [97]. These amperometric sensors became known as the firstgeneration biosensors or Clark oxygen electrodes and were soon implemented by Updike and Hicks, who constructed the first functional biocatalytic sensor for glucose [98].
Direct redox processes between biocatalysts and electrodes are rare since most polypeptide element tends to denaturation at the electrode surface. A lot of direct electron transfer reactions are rather slow and not reversible [99]. However, a restricted amount of biocatalysts such as HRP have proven ability to direct electron transfer between the enzyme active site’s prosthetic group and electrode [100]. An active site of enzyme that allows the selective targeting of an analyte is usually buried within the enzyme’s tertiary protein structure, near the centre of the protein.
Therefore, the electrons obtained in the biocatalyzed process cannot every time be directly transported to the surface of electrode thus restricting the charge communication between the protein and the transducer. The broadly approved Marcus theory of electron transfer reports that electron transport decays exponentially with distance [101].
Then biocatalysts frequently need some service with electron transport to the surface of transducer. According to the fact the artificial redox mediators are suitable shuttle agents in transport of electron between active center of enzyme and surface of electrode. In example, mediators have substituted molecular oxygen as the electron shuttle (equation 1). In contact with working electrode mediator’s reoxidiz at rather low potentials producing a current while keep contact with the working electrode (equation 2).
GOx–FADH2+2MediatorOx–Gox → FAD+2MediatorRed+2H+ (1)
2MediatorRed → 2MediatorOx + 2e- (2)
Mediators should ideally be nontoxic, independent of the pH, stable in both the oxidized and reduced forms, and unreactive with oxygen [99]. Examples of formerly applied mediators contain for example quinones, conducting organics, ruthenium complexes, ferrocene derivatives. Mediated enzyme electrodes had a much better sensor performance than the first-generation biosensors mainly due to eliminating the O2 dependence and being able to control the concentration of the oxidizing agent in the biosensor [99]. Hand selecting the oxidizing agent for the sensor also allowed more suitable oxidation potentials to be used for the amperometric sensors. By carefully selecting a mediator and a suitable redox potential, the transduction event at the second-generation biosensor could be measured in a potential range where other possible sample components such as ascorbate, urate, and paracetamol are not oxidized or reduced thereby minimizing interferences [95]. Incorporating redox mediators also allowed other oxidoreductase enzymes such as peroxidases and dehydrogenases to be used as the biorecognition element in the sensor thereby expanding the list of possible target analytes.
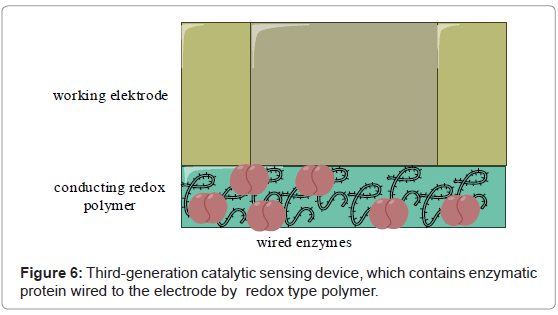
Third-generation of biosensors has the biorecognition component coupled with the electrode by immobilization of the protein and mediating agent together at the electrode surface. This can be achieved by direct electrical contact biocatalyst-electrode, immobilizing of enzyme and mediator in a conducting polymer, or wiring the enzyme to the electrode by immobilizing in the redox polymer (Figure 6) as first described by Ohara et al. [102].
The co-immobilization prevents the mediators from diffusing out of the biosensor film. The co-immobilized mediators, or the flexible surrounding redox polymers, improve the transport of electrons between the enzyme’s active site and the surface of working electrode and hence generation of high current densities.
Optimization of enzyme electrodes
The biocatalyst is the fundamental factor of enzyme electrode construction. This biological catalyst is mainly selected to produce electrodes for sensor and fuel cells while specificity/selectivity has to be crucial to sample examination. Although, the enzymes generally are restricted in the amount of processes they are able to biocatalyze that consequently reduced the applications. Additionally, enzyme electrodes limit activity mainly in area of physiological environment according to exceptional lack of stability of biocatalysts in other than aqueous conditions as well as at higher temperatures.
A subsequent problem of biocatalyst to be applied in sensor device is inhibition of its activity according to different agents, also chemicals. As is known from literature, biocompatible materials can be extremely valid in design of enzyme electrodes. Moreover, a perfect material for electrodes is described by conducting properties for providing proper electron transfer.
Carbon-Nanotube based enzyme electrodes: Carbon nanotubes (CNTs) are by far the most broadly used nano-sized materials to product porous, semi-conducting electrodes. Carbon nanotubes are characterized as single-walled nanotubes and multi-walled nanotubes. These agents can be applied as an electron mediator due to design of protein electrodes. It is possible to use them both directly as well as past functionalization [103,104]. Kandimalla and Ju [103] immobilized acetylcholinesterase by covalent binding to a multi-walled carbon nanotube functionalized with chitosan. The obtained immobilized matrix was characterized by homogeneous porous structure. This type of enzymatic electrode may biocatalyze the process of oxidation of thiocholine, improving sensitive features of electrode.
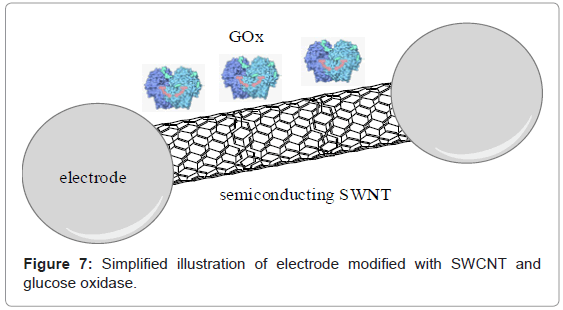
The carbon semi-conducting materials are strongly attractive for dehydrogenase and oxidase biosensors because of the low-potential detection limit of H2O2 as well as NADH. In example Sarma et al. [105] reported an amperometric sensor for glucose detection. The device was built of glucose oxidase immobilized on glass carbon electrode modified with carbon nanotubes and platinum nanoparticles (Figure 7). In comparison to other glucose oxidase electrodes, the activity of device was much higher, according to better transfer of electrons from active center of enzyme to surface of electrode. Successive example of glucose amperometric biosensor was shown by Muguruma et al. [106], they design bioelectrode based on composite of carbon nanotubes, whereas Xu et al. [107] described the pH-sensitive electrode modified with single-walled nanotubes. Recently, Liu et al. [108] reported electrochemical glucose biosensor based on carbon nanotubes and quantum dots (CdTe) as well as glucose oxidase-modified glassy carbon electrode.
It is worth to emphasize carbon nanotubes have a permanent capacity to mediate rapid electron-transfer. Furthermore, carbon nanotubes chemical functionalization may be applied to connect nearly any chemical group to them, which enable to improve the solubility and biocompatibility of the carbon nanotubes.
The appearance of carbon nanotubes with impurities (metals) is the key disadvantage when applying in the process of modification of electrode [109,110]. These types of metallic particles are able to dominate the electrochemistry of carbon nanotubes. According to Pumera [109], when impurities represent at 50 ppm may be toxicological threat when they are able to take a part in redox processes with the biological molecules.
Graphene adhesion to biological molecules is brought about by the π-stacking interactions according to hexagonal system and the ring structures broadly current in biological nano-sized molecules.
When applied the graphene it is possible to evade the simple problems related to metal species and CNTs. These remarkable features of graphene (rapid electron transfer, good thermal conductivity, splendid flexibility, biocompatibility) are responsible for prospective using in electrochemical biological sensors [109-112].
The electrochemistry of biocatalysts causes direct electron transfer from the electrode to active center of the protein without any mediating system or other species [113]. Although, the redox center of every biological molecule is often hidden deeply in the large 3D systems. Recent scientific data has shown that graphene is able to enhance direct electron transfer between enzymes and electrodes [114].
Direct electron transfer in enzymatic electrodes electrode surfaces can be applied to develop enzyme-catalyzed reactions in biological systems and to provide the electrochemical ground for the study of biocatalyst kinetics and thermodynamics of redox transformations [114].
The using of graphene in modern cost-attractive sensors may assist some diseases [115]. Different scientific data indicate that glucose biosensors based on graphene state reproducibility, selectivity and sensitivity. The electrochemical behaving of glucose oxidase sensor has been acknowledged by electrochemical measurements in the potential of 0.8-0 V indicating formerly suggested processes [116] when applying reduced films of graphene in detection of glucose. The attendance of CdS nano-sized crystal with graphene stated low detection limit of 0.7 mM [117]. A polyvinylpyrrolidone-protected graphene/polyethyleniminefunctionalized glucose oxidase biosensor was applied also by Shan et al. [118] to study direct electron transfer of glucose oxidase. There was also reported that graphene-modified electrodes were used successfully for selectivity between dopaminic and ascorbic signals, responsible for dopamine detection [119].
Conclusions, Prospects and Challenges
Nanomaterials and technologies closely related to them are growingly being applied to design novel sensing devices. Eagerly, nanosize-based sensors should be combined with biochips with modern electronic elements, sample processing as well as analysis. That can strongly highlight utility, when ensuring the smallest measuring systems, mobile, inexpensive, and widely convenience in diagnostics.
Study on protein electrodes to effective diagnostic applications is an enormous challenge. Relevant use of varied binder as well as immobilization agents, mediators, biological materials, enzymes, or solids as electron collectors for designing of enzymatic sensing tools is crucial to generate appropriate current from biocatalytic processes. Lately, developing in the field of advanced nanomaterials is strongly affected on the redox processes as well as kinetic of transport of electrons. Other crucial points in the design and construction of novel attractive sensor devices are the life-time of enzymatic electrode, rate of electron transfer, and miniaturization of enzyme electrode.
Design of mixed-biocatalyst pathways to comprehensive oxidation of substrate also in the same time to record frequent answers to various constituents with an individual application for biosensorics is still in frequently growing interest. Complex sensing arrangements including single and complex selective agents can be awaited to have a valid contribution in environmental monitoring as well as in broad clinical chemistry.
Acknowledgements
Financial support from the Wroclaw University of Technology and Polish Ministry of Science and Higher Education Grant No. NR05-0017-10/2010, 2012/05/B/ST5/00749 authors are gratefully acknowledged.
References
- Lin P, Yan F (2012) Organic thin-film transistors for chemical and biological sensing. Adv Mater 24: 34-51.
- Khan HU, Jang J, Kim JJ, Knoll W (2011) In situ antibody detection and charge discrimination using aqueous stable pentacene transistor biosensors. J Am Chem Soc 133: 2170-2176.
- Vo-Dinh T, Cullum BM, Stokes DL (2001) Nanosensors and biochips: frontiers in biomolecular diagnostics. Sens Actuators B 74: 2-11.
- Haruyama T (2003) Micro- and nanobiotechnology for biosensing cellular responses. Adv Drug Deliv Rev 55: 393-401.
- Jain KK (2003) Nanodiagnostics: application of nanotechnology in molecular diagnostics. Expert Rev Mol Diagn 3: 153-161.
- Cabaj J, Soloducho J, Nowakowska-Oleksy A (2010) Langmuir-Blodgett film based biosensor for estimation of phenol derivatives. Sens Actuators B: Chemical 143: 508-515.
- Cabaj J, Soloducho J, Swist A (2010) Active Langmuir-Schaefer films of tyrosinase—Characteristic. Sesns Actuators B: Chemical 150: 505-512.
- Cabaj J, Soloducho J, Chyla A, Jedrychowska A (2011) Hybrid phenol biosensor based on modified phenoloxidase electrode. Sens Actuators B: Chemical 157: 225-231.
- Cabaj J, Chyla A, Jedrychowska A, Olech K, Soloducho J (2012) Detecting platform for phenolic compounds - characteristic of enzymatic electrode. Optical Materials 34: 1677-1681.
- Shao Y, Wang J, Wu H, Liu J, Aksay I A, et al. (2010) Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 22: 1027-1036.
- Shan C, Yang H, Han D, Zhang Q, Ivaska A, et al. (2010) Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosens Bioelectron 25: 1070-1074.
- Wu S, Zhao H, Ju H, Shi C, Zhao J (2006) Electrodeposition of silver-DNA hybrid nanoparticles for electrochemical sensing of hydrogen peroxide and glucose. Electrochemistry Communications 8: 1197-1203.
- Yang M, Yang Y, Liu Y, Shen G, Yu R (2006) Platinum nanoparticles-doped sol-gel/carbon nanotubes composite electrochemical sensors and biosensors. Biosens Bioelectron 21: 1125-1131.
- Wang J, Lin Y (2008) Functionalized carbon nanotubes and nanofibers for biosensing applications. Trends Analyt Chem 27: 619-626.
- Lu X, Wen Z, Li J (2006) Hydroxyl-containing antimony oxide bromide nanorods combined with chitosan for biosensors. Biomaterials 27: 5740-5747.
- Sanvicens N, Marco MP (2008) Multifunctional nanoparticles--properties and prospects for their use in human medicine. Trends Biotechnol 26: 425-433.
- Lu Y, Liu GL, Lee LP (2005) Nanowell surface enhanced Raman scattering arrays fabricated by soft-lithography for label-free biomolecular detections in integrated microfluidics. Appl Phys Lett 87: 074-101.
- Chen Y, Luo Y (2009) Precisely defined heterogeneous conducting polymer nanowire arrays-fabrication and chemical sensing applications. Adv Mater 21: 2040-2044.
- Hatchett DW, Josowicz M (2008) Composites of intrinsically conducting polymers as sensing nanomaterials. Chem Rev 108: 746-769.
- Ansari AA, Solanki PR, Kaushik A, Malhotra BD (2009) Recent advances in nanostructured metal oxides based electrochemical biosensors for clinical diagnostics. In: Yogeswaran U, Kumar S, Chen S, Nanostructured Materials for Electrochemical Biosensors. Nova Science Publishers; Hauppauge, NY, USA.
- Bruchez M Jr, Moronne M, Gin P, Weiss S, Alivisatos AP (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281: 2013-2016.
- Prakash S, Chakrabarty T, Singh AK, Shahi VK (2013) Polymer thin films embedded with metal nanoparticles for electrochemical biosensors applications. Biosens Bioelectron 41: 43-53.
- Yogeswaran U, Chen SM (2008) Recent trends in the application of carbon nanotubes-polymer composite modified electrodes for biosensors: a review. Anal Lett 41: 210-243.
- Pingarron JM, Sedeno PY, Cortes AG (2008) Gold nanoparticle-based electrochemical biosensors. Electrochim Acta 53: 5848-5866.
- Constantine CA, Gattas-Asfura KM, Mello SV, Crespo G, Rastogi V, et al. (2003) Layer-by-layer biosensor assembly incorporating functionalized quantum dots. Langmuir 19: 9863-9867.
- Tessler N, Medvedev V, Kazes M, Kan S, Banin U (2002) Efficient near-infrared polymer nanocrystal light-emitting diodes. Science 295: 1506-1508.
- Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, et al. (2002) In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 298: 1759-1762.
- Hines MA, Guyot-Sionnest PJ (1996) Synthesis and characterization of strongly luminescing ZnS-capped CdSe nanocrystals. J Phys Chem 100: 468-471.
- Ebenstein Y, Mokari T, Banin U (2004) Quantum-dot-functionalized scanning probes for fluorescence-energy-tranfser-based microscopy. J Phys Chem B 108: 93-99.
- Liu B (2012) Metal-organic framework-based devices: separation and sensors. J Mat Chem 22: 10094-10101.
- Gopalan AI, Lee KP, Ragupathy D, Lee SH, Lee JW (2009) An electrochemical glucose biosensor exploiting a polyaniline grafted multiwalled carbon nanotube/perfluorosulfonate ionomer-silica nanocomposite. Biomaterials 30: 5999-6005.
- Monosik R, Streansky M, Surdik E (2012) Biosensors - classification, characterization and new trends. Acta Chimica Slovaca 5: 109-120.
- Liu A (2008) Towards development of chemosensors and biosensors with metal-oxide-based nanowires or nanotubes. Biosens Bioelectron 24: 167-177.
- Boozer C, Yu Q, Chen S, Lee C, Homola J, et al. (2003) Surface functionalization for self-referencing surface plasmon resonance (SPR) biosensors by multi-step self-assembly. Sens Actuators B 90: 22-30.
- Cornell BA, Braach-Maksvytis VL, King LG, Osman PD, Raguse B, et al. (1997) A biosensor that uses ion-channel switches. Nature 387: 580-583.
- Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38: 2221-2295.
- Langmuir I, Schaefer VJ (1938) Activities of urease and monolayers. J Am Chem Soc 60: 1351-1360.
- Netzer L, Sagiv J (1983) A new approach to construction of artificial monolayer assemblies. J Am Chem Soc 105: 674-676.
- Davis F, Higson SP (2005) Structured thin films as functional components within biosensors. Biosens Bioelectron 21: 1-20.
- Nicolini C (1998) Engineering of enzyme monolayer for industrial biocatalysis. An overview. Ann N Y Acad Sci 864: 435-441.
- Gaines GL (1966) Insoluble monolayers at the liquid-gas interfaces. Interscience, New York.
- Riccio A, Lanzi M, Antolini C, De Nitti C, Tavani C, et al. (1996) Ordered monolayer of cytochrome c via chemical derivatization of its outer arginine. Langmuir 12: 1545-1549.
- Erokhin V, Facci P, Nicolini C (1995) Two-Dimensional Order and Protein Thermal Stability: High Temperature Preservation of Structure and Function. Biosens Bioelectron 10: 25-34.
- Soloducho J, Cabaj J (2011) Langmuir monolayers in biosensors. In: Langmuir monolayers in thin film technology, Edition, Sherwin JA, Nova Science Publishers, Inc, New York.
- Decher G, Hong JD, Schmitt J (1992) Buildup of ultrathin multilayer films by a self-assembly process. 3. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 210-211: 831-835.
- de Villiers MM, Otto DP, Strydom SJ, Lvov YM (2011) Introduction to nanocoatings produced by layer-by-layer (LbL) self-assembly. Adv Drug Deliv Rev 63: 701-715.
- Cheung JH, Stockton WB, Rubner MF (1997) Molecular-level processing of conjugated polymers. Layer-by-layer manipulation of polyaniline via electrostatic interactions. Macromolecules 30: 2712-2716.
- Kim BS, Park SW, Hammond PT (2008) Hydrogen-bonding layer-by-layer-assembled biodegradable polymeric micelles as drug delivery vehicles from surfaces. ACS Nano 2: 386-392.
- Mukhopadhyay MK, Sanyal MK, Datta A, Webster J, Penfold J (2005) Interplay between hydrophilic and hydrophobic interactions for deciding the molecular orientation in Langmuir-Blodgett film deposition. Chem Phys Lett 407: 276-282.
- Song W, He Q, Möhwald H, Yang Y, Li J (2009) Smart polyelectrolyte microcapsules as carriers for water-soluble small molecular drug. J Control Release 139: 160-166.
- Decher G (1997) Fuzzy nanoassemblies: toward layered polymeric nanocomposites. Science 277: 1232-1237.
- Sukhorukov GB, Donath E, Lichtenfeld H, Knippel E, Knippel M, et al. (1998) Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloids and Surfaces A: Physicochemical and Engineering Aspects 137: 253-266.
- Donath E, Sukhorukov GB, Caruso F, Davis SA, Mohwald H (1998) Novel hollow polymer shells by colloid templated assembly of polyelectrolytes. Angew Chem Int Ed 37: 2201-2205.
- Wilchek M, Bayer EA (1988) The avidin-biotin complex in bioanalytical applications. Anal Biochem 171: 1-32.
- Wang J (2006) Analytical Electrochemistry. John Wiley & Sons VCH, Hoboken, New Jersey, USA.
- Grieshaber D, MacKenzie R, Vörös J, Reimhult E (2008) Enzymatic Biosensors - Sensor Principles and Architectures. Sensors 8: 1400-1458.
- Ronkainen-Matsuno NJ, Thomas JH, Halsall HB, Heineman WR (2002) Electrochemical immunoassay moving into the fast lane. TrAC Trends Anal Chem 21: 213-225.
- Bauer CG, Eremenko AV, Ehrentreich-Förster E, Bier FF, Makower A, et al. (1996) Zeptomole-detecting biosensor for alkaline phosphatase in an electrochemical immunoassay for 2,4-dichlorophenoxyacetic acid. Anal Chem 68: 2453-2458.
- Jenkins SH, Heineman WR, Halsall HB (1988) Extending the detection limit of solid-phase electrochemical enzyme immunoassay to the attomole level. Anal Biochem 168: 292-299.
- Wijayawardhana CA, Halsall HB, Heineman WR (2002) Electroanalytical Methods of Biological Materials. Brajter-Toth A, Chambers JQ, edition, Marcel Dekker, New York.
- Yao H, Jenkins SH, Pesce AJ, Halsall HB, Heineman WR (1993) Electrochemical homogeneous enzyme immunoassay of theophylline in hemolyzed, icteric, and lipemic samples. Clin Chem 39: 1432-1434.
- Kissinger PT, Heineman WR (1996) Laboratory Techniques in Electroanalytical Chemistr. Marcel Dekker Inc, New York, USA.
- Halsall HB, Heineman WR (1990) Electrochemical immunoassay: an ultrasensitive method. J Int Fed Clin Chem 2: 179-187.
- Heinze J (1993) Ultramicroelectrodes in Electrochemistry. Angew Chem Int Ed Engl 32: 1268-1288.
- Farrell S, Ronkainen-Matsuno NJ, Halsall HB, Heineman WR (2004) Bead-based immunoassays with microelectrode detection. Anal Bioanal Chem 379: 358-367.
- Cai H, Xu C, He P, Fang Y (2001) Colloid Au-enhanced DNA immobilization for the electrochemical detection of sequence-specific DNA. J Electroanal Chem 510: 78-85.
- Crumbliss AL, Perine SC, Stonehuerner J, Tubergen KR, Zhao J, et al. (1992) Colloidal gold as a biocompatible immobilization matrix suitable for the fabrication of enzyme electrodes by electrodeposition. Biotechnol Bioeng 40: 483-490.
- Zhao J, O’Daly JP, Henkens RW, Stonehuerner J, Crumbliss AL (1996) A xanthine oxidase/colloidal gold enzyme electrode for amperometric biosensor applications. Biosens Bioelectron 11: 493-502.
- Xu X, Liu S, Ju H (2003) A novel hydrogen peroxide sensor via the direct electrochemistry of horseradish peroxidase immobilized on colloidal gold modified screen-printed electrode. Sensors 3: 350-360.
- Curri ML, Agostiano A, Leo G, Mallardi A, Cosma P, et al. (2002) Development of a novel enzyme/ semiconductor nanoparticles system for biosensor application. Mater Sci Eng C Biomim Mater Sens Syst 22: 449-452.
- Zhang B, Zhang ZJ, Wang B, Yan J, Li JJ, et al. (2001) Preparation of gold nano-arraied electrode on silicon substrate and its electrochemical properties: Probe into biosensor based on electroluminescence of porous silicon. Acta Chimi Sin 59: 1932- 1936.
- Teles FRR, Fonseca LP (2008) Applications of polymers for biomolecule immobilization in electrochemical biosensors. Materials Science & Engineering C Biomimetic and Supramolecular Systems 28: 1530-1543.
- Fiorito PA, Brett CM, Córdoba de Torresi SI (2006) Polypyrrole/copper hexacyanoferrate hybrid as redox mediator for glucose biosensors. Talanta 69: 403-408.
- Lvov Y, Decher G, Sukhorukov G (1993) Assembly of thin films by means of successive deposition of alternate layers of DNA and poly(ally1amine). Macromolecules 26: 5396-5399.
- Sukhorukov GB, Montrel MM, Petrov AI, Shabarchina LI, Sukhorukov BI (1996) Multilayer films containing immobilized nucleic acids. Their structure and possibilities in biosensor applications. Biosens Bioelectron 11: 913-922.
- Yoon HC, Kim HS (2000) Multilayered assembly of dendrimers with enzymes on gold: thickness-controlled biosensing interface Anal Chem 72: 922-926.
- Trau D, Yang W, Seydack M, Caruso F, Yu NT, et al. (2002) Nanoencapsulated microcrystalline particles for superamplified biochemical assays. Anal Chem 74: 5480-5486.
- Shirsat MD, Too CO, Wallace GG (2008) Amperometric glucose biosensor on layer-by-layer assembled carbon nanotube and polypyrrole multilayer film. Electroanalysis 20: 150-156.
- Gade VK, Shirale DJ, Gaikwad PD, Savale PA, Kakde KP, et al. (2006) Immobilization of GOD on electrochemically synthesized Ppy-PVS composite film by cross-linking via glutaraldehyde for determination of glucose. Reactive & Functional Polymers 66: 1420-1426.
- Siqueira JR Jr, Caseli L, Crespilho FN, Zucolotto V, Oliveira ON Jr (2010) Immobilization of biomolecules on nanostructured films for biosensing. Biosens Bioelectron 25: 1254-1263.
- Girard-Egrot AP, Godoy S, Blum LJ (2005) Enzyme association with lipidic Langmuir-Blodgett films: interests and applications in nanobioscience. Adv Colloid Interface Sci 116: 205-225.
- Ohnuki H, Honjo R, Endo H, Imakubo T, Izumi M (2009) Amperometric cholesterol biosensors based on hybrid organic-inorganic Langmuir-Blodgett films Thin Solid Films 518: 596-599.
- Matharu Z, Pandey P, Pandey MK, Gupta V, Malhotra BD (2009) Electrochemical Cholesterol Sensor Based on Tin Oxide-Chitosan Nano-biocomposite Film. Electroanalysis 21: 1587-1596.
- Anzai J, Hashimoto J, Osa T, Matsuo T (1988) Penicillin sensors based on an ion-sensitive field-effect transistor coated with stearicacid Langmuir-Blodgett membrane. Anal Sci 4: 247-250.
- Ohlsson P, Torbjorn T, Herbai E, Lofas S, Puu G (1995) Liposome and proteoliposome fusion onto solid substrates, studied using atomic force microscopy, quartz crystal microbalance and surface Plasmon resonance: biological activities of incorporated components. Bioelec Bioenerg 38: 137-148.
- Pizzolato F, Morelis RM, Coulet PR (2000) Enzymatic nanolayers from protoelipidic Langmuir-Blodgett films for biosensors. Quim Anal 19: 32.
- Ohnuki H, Saiki T, Kusakari A, Endo H, Ichihara M, et al. (2007) Incorporation of glucose oxidase into Langmuir-Blodgett films based on Prussian blue applied to amperometric glucose biosensor. Langmuir 23: 4675-4681.
- Zasadzinski JA, Viswanathan R, Madsen L, Garnaes J, Schwartz DK (1994) Langmuir-Blodgett films. Science 263: 1726-1733.
- Matharu Z, Sumana G, Arya SK, Singh SP, Gupta V, et al. (2007) Polyaniline Langmuir-Blodgett film based cholesterol biosensor. Langmuir 23: 13188-13192.
- Cabaj J, Soloducho J (2011) Hybrid film biosensor for phenolic compounds detection in Environmental biosensors. InTech 213-236.
- Caseli L, Zaniquelli MED, Furriel RPM, Leone FA (2002) Enzymatic activity of alkaline phosphatase adsorbed on dimyristoylphosphatidic acid Langmuir-Blodgett films. Colloid Surf B-Biointefaces 25: 119-128.
- Pavinatto FJ, Caseli L, Pavinatto A, dos Santos DS Jr, Nobre TM, et al. (2007) Probing chitosan and phospholipid interactions using Langmuir and Langmuir-Blodgett films as cell membrane models. Langmuir 23: 7666-7671.
- Caseli L, Nobre TM, Silva DAK, Loh W, Zaniquelli MED (2001) Flexibility of the triblock copolymers modulating their penetration and expulsion mechanism in Langmuir monolayers of dihexadecyl phosphoric acid. Colloid Surf B-Biointerfaces 22: 309-321.
- Wang J (2001) Glucose Biosensors: 40 Years of Advances and Challenges. Electroanalysis 13: 983-988.
- Bartlett PN (2008) Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications. John Wiley & Sons, West Sussex, England.
- Ronkainen NJ, Halsall HB, Heineman WR (2010) Electrochemical biosensors. Chem Soc Rev 39: 1747-1763.
- Updike SJ, Hicks GP (1967) The enzyme electrode. Nature 214: 986-988.
- Mikkelsen SR, Corton E (2004) Bioanalytical Chemistry, John Wiley & Sons, Hoboken, New Jersey, USA.
- Eggins BR (2002) Chemical Sensors and Biosensors, John Wiley & Sons, West Sussex, England.
- Gorton L, Lindgren A, Larsson T, Muteanu F, Ruzgas T, et al. (1999) Direct electron transfer between heme-containing enzymes and electrodes as basis for third generation biosensors. Anal Chim Acta 400: 91-108.
- Bard AJ, Faulkner LR (2000) Electrochemical Methods: Fundamentals and Applications. 2nd Edition, John Wiley & Sons, Hoboken, New Jersey, USA.
- Ohara TJ, Rajagopalan R, Heller A (1993) Glucose electrodes based on cross-linked [Os(bpy)2Cl]+/2+ complexed poly(1-vinylimidazole) films. Anal Chem 65: 3512-3517.
- Kandimalla VB, Ju H (2006) Binding of acetylcholinesterase to multiwall carbon nanotube-cross-linked chitosan composite for flow-injection amperometric detection of an organophosphorous insecticide. Chemistry 12: 1074-1080.
- Yang X-Y, Tian G, Jiang N, Su BL (2012) Immobilization technology: a sustainable solution for biofuel cell design. Energy Environ Sci 5: 5540-5563.
- Sarma AK, Vatsyayan P, Goswami P, Minteer SD (2009) Recent advances in material science for developing enzyme electrodes. Biosens Bioelectron 24: 2313-2322.
- Muguruma H, Shibayama Y, Matsui Y (2008) An amperometric biosensor based on a composite of single-walled carbon nanotubes, plasma-polymerized thin film, and an enzyme. Biosens Bioelectron 23: 827-832.
- Xu Z, Chen X, Qu X, Jia J, Dong S (2004) Single-wall carbon nanotube-based voltammetric sensor and biosensor. Biosens Bioelectron 20: 579-584.
- Liu Q, Lu X, Li J, Yao X, Li J (2007) Direct electrochemistry of glucose oxidase and electrochemical biosensing of glucose on quantum dots/carbon nanotubes electrodes. Biosens Bioelectron 22: 3203-3209.
- Pumera M (2009) Electrochemistry of graphene: new horizons for sensing and energy storage. Chem Rec 9: 211-223.
- Pumera M (2010) Graphene-based nanomaterials and their electrochemistry. Chem Soc Rev 39: 4146-4157.
- Allen MJ, Tung VC, Kaner RB (2010) Honeycomb carbon: a review of graphene. Chem Rev 110: 132-145.
- Brownson DAC, Banks CE (2011) Graphene electrochemistry: Surfactants inherent to graphene inhibit metal analysis. Electrochem Commun 13: 111-113.
- Shao Y, Wang J, Wu H, Liu J, Aksay IA, et al. (2010) Graphene based electrochemical sensors and biosensors: a review. Electroanalysis 22: 1027-1036.
- Kuila T, Bose S, Khanra P, Mishra AK, Kim NH, et al. (2011) Recent advances in graphene-based biosensors. Biosens Bioelectron 26: 4637-4648.
- Wang Y, Shao Y, Matson DW, Li J, Lin Y (2010) Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 4: 1790-1798.
- Yang MH, Choi BG, Park HS, Hong WH, Lee SY, et al. (2010) Development of a Glucose Biosensor Using Advanced Electrode Modified by Nanohybrid Composing Chemically Modified Graphene and Ionic Liquid. Electroanalysis 22: 1223-1228.
- Wang K, Liu Q, Guan QM, Wu J, Li HN, et al. (2011) Enhanced direct electrochemistry of glucose oxidase and biosensing for glucose via synergy effect of graphene and CdS nanocrystals. Biosens Bioelectron 26: 2252-2257.
- Shan C, Yang H, Song J, Han D, Ivaska A, et al. (2009) Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal Chem 81: 2378-2382.
- Li F, Chai J, Yang H, Han D, Niu L (2010) Synthesis of Pt/ionic liquid/graphene nanocomposite and its simultaneous determination of ascorbic acid and dopamine. Talanta 81: 1063-1068.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17086
- [From(publication date):
specialissue-2013 - Nov 21, 2025] - Breakdown by view type
- HTML page views : 12234
- PDF downloads : 4852