Research Article Open Access
Efficacy of Endoscopic Mucosal Resection after Circumferential Mucosal Incision of Small Rectal Carcinoid Tumors
| Yosuke Mochizuki1*, Yasuharu Saito1, Osamu Inatomi2, Shigeki Bamba2, Yoshihide Fujiyama2, Mitsuaki Ishida3, Tomoyuki Tsujikawa4 and Akira Andoh5 | |
| 1Division of Digestive Endoscopy, Shiga University of Medical Science, Japan | |
| 2Department of Internal Medicine, Shiga University of Medical Science, Japan | |
| 3Department of Clinical Laboratory Medicine, Shiga University of Medical Science, Japan | |
| 4Division of Comprehensive Internal Medicine, Shiga University of Medical Science, Japan | |
| 5Division of Mucosal Immunology, Graduate School of Medicine, Shiga University of Medical Science, Japan | |
| Corresponding Author : | Yosuke Mochizuki Division of Digestive Endoscopy Shiga University of Medical Science Seta-Tukinowa, Otsu 520-2192, Japan Tel: 81-77-548-2217 Fax: 81-77-548-2219 E-mail: yousuke@belle.shiga-med.ac.jp |
| Received March 28, 2013; Accepted April 05, 2013; Published April 07, 2013 | |
| Citation: Mochizuki Y, Saito Y, Inatomi O, Bamba S, Fujiyama Y, et al. (2013) Efficacy of Endoscopic Mucosal Resection after Circumferential Mucosal Incision of Small Rectal Carcinoid Tumors. J Gastroint Dig Syst S6:006. doi:10.4172/2161-069X.S6-006 | |
| Copyright: © 2013 Mochizuki Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background: Endoscopic mucosal resection is widely used for treating rectal carcinoid tumors. However, histopathology has revealed that submucosal invasion leads to incomplete resection. Endoscopic submucosal dissection, which enables en bloc resection regardless of tumor size, has recently been reported to be useful in treating rectal carcinoid tumors; however, it is not widely used as standard treatment because of technical demands. We use endoscopic mucosal resection after circumferential mucosal incision, which is performed after mucosal resection around the lesion to treat rectal carcinoid tumors. To our knowledge, this is the first report of endoscopic mucosal resection after circumferential mucosal incision for colorectal carcinoid tumors. Objective: To evaluate the efficacy of this method. Design: Single-center retrospective clinical trial. Setting: Shiga University of Medical Science. Patients: We retrospectively studied 6 patients with rectal carcinoid tumors ≤ 10 mm treated by endoscopic mucosal resection after circumferential mucosal incision, between August 2010 and December 2012 at Shiga University of Medical Science. Interventions: Endoscopic mucosal resection after circumferential mucosal incision. Main outcome measures: En bloc resection rate, procedure time, complications. Results: The mean tumor size was 6.8 ± 1.8 mm (range 4-9 mm). The mean procedure time was 19.7 ± 5.1 min (range, 12-26 min). The en bloc and complete resection rates were 100% (6/6) and 50% (3/6), respectively. All tumor depths were contained in the submucosa, and clear resection margins were pathologically confirmed in all 6 patients. Three patients with lymphovascular involvement required additional radical surgical therapy. There were no complications or distant/local recurrence during the follow-up period (median, 4 months; range 4-26 months). Limitations: This study was limited by its retrospective nature. Conclusions: Endoscopic mucosal resection after circumferential mucosal incision was effective for treating ≤ 10-mm-diameter rectal carcinoid tumors.
| Keywords |
| Carcinoid; Mucosal incision; Submucosa; Lymphovascular |
| Introduction |
| The incidence of carcinoid tumors of the gastrointestinal tract has increased over the past 5 decades due to increased use of screening colonoscopies [1,2]. In Japan, carcinoid tumor of the digestive tract is frequently observed in the colorectal region, particularly in the rectum, within 10 cm of the dentate line [3]. Rapid advances in screening colonoscopy have enabled detection of small rectal carcinoids without symptoms. It has been reported that 86% of rectal carcinoids were ≤ 10 mm [4]. Although carcinoid tumors with invasion to the muscular layer increase lymph node metastases [5], the depth of tumors ≤ 10 mm in diameter is often limited to the submucosal layer [6]. In addition, tumors ≤ 10 mm in diameter have a low rate of lymph node metastases [7], and endoscopic local resection is selected to treat these lesions. Endoscopic Mucosal Resection (EMR) is widely used for treating colorectal superficial tumors [8-11], but incomplete resection of carcinoid tumors is not rare because a majority of the tumor occupies the submucosal layer. Concurrently, Endoscopic Submucosal Dissection (ESD), which enables en bloc resection regardless of tumor size, has recently been reported to be useful for treating colorectal superficial tumors [12-14]. There are many reports on the efficacy of ESD to treat colorectal carcinoids [15-18]. However, because ESD has the disadvantages of a high frequency of complications and need for advanced technical skills, this technique is not yet a standard treatment. At our hospital, endoscopic mucosal resection after circumferential mucosal incision (C-EMR) instead of ESD is performed to treat rectal carcinoids. Although positive resection margins can often be achieved with conventional EMR, C-EMR allows en bloc resection of the entire lesion as compared with conventional EMR. This is possible because circumferential mucosal incision and trimming to the submucosa facilitates grasping of the deep subumucosal layer under the lesion, which ensures that clear lateral and vertical margins are achieved. No studies have reported the efficacy of C-EMR for rectal carcinoid tumors <10 mm in diameter. This retrospective study evaluated the efficacy and outcomes of C-EMR. |
| Materials and Methods |
| Patient selection |
| We retrospectively included 6 patients with rectal carcinoid tumors of ≤ 10 mm that were all detected during screening colonoscopy and endoscopically treated by C-EMR between August 2010 and December 2012 at Shiga University of Medical Science. The following parameters were retrospectively evaluated: Tumor location, presence of a depression on the top of the tumors, tumor size, en bloc resection rate, complete resection rate, perforation rate, histopathological findings, presence of additional therapy, local recurrence rate, delayed bleeding rate, and perforation rate. |
| Definitions |
| The tumors were measured according to the maximum diameter of the resected specimen. En bloc resection was defined as one-piece endoscopic resection of the entire lesion with free lateral and vertical margins on histopathological examination. Complete resection was achieved when the following criteria were fulfilled: tumor-free lateral and vertical margins on histopathological examination, and no evidence of lymphatic or vascular invasion. Cases in which the resection margins could not be evaluated due to the burn effect were considered to be incomplete resections even though en bloc resection was achieved. In addition, delayed bleeding was defined as hematochezia ≥ 24 h after resection that required endoscopic hemostasis. Moreover, perforation was defined as a defect in the muscle layer detected during treatment or abdominal pain and fever with air leak on computed tomography (CT). |
| Endoscopic treatment |
| The following endoscopes were used for diagnosis: CF-H260AI, CFH260AZI, and PCF-Q260AI (Olympus Optical Co., Ltd., Tokyo, Japan). All diagnoses were confirmed by an endoscopist (Yosuke Mochizuki) highly experienced in diagnostic and therapeutic colonoscopy, and treatments were administered by 3 endoscopists (Mizuta Hiroo, 2 years of experience with endoscopy; Ayako Kobori and Yosuke Mochizuki, 9 years of experience with endoscopy). All 6 cases were assessed by CT for the absence of distant metastases and lymph node metastases before endoscopic resection. |
| C-EMR method |
| The endoscopes used for C-EMR included PCF-Q260JI and PCFQ260AZI with the transparent disposable attachment (Olympus) attached onto the tip of the endoscope to obtain a constant view and to maintain tension during mucosal incision and submucosal trimming using a needle-knife procedure. C-EMR was performed with the patient under sedation by periodic intravenous administration of midazolam (0.05-0.1 mg/kg/h) and propofol (2 mg/kg/h), with continuous monitoring of blood pressure, heart rate, and blood oxygen saturation levels. For submucosal injection, hyaluronic acid or a mixed solution of glycerin, epinephrine, and indigo carmine dye was used. After submucosal injection, a needle knife (KD-10Q-1; Olympus) with the electrosurgical generator (VIO300D; ERBE Elektromedizin GmbH, Tübingen, Germany) in dry-cut mode was used to make an incision around the entire circumference of the lesion, approximately 5-10 mm outside the tumor. In order to remove sufficient lateral and vertical margins in this method, it is essential to make a mucosal incision remotely situated from the tumor. Subsequently, a submucosal injection was administered to elevate the lesion. A 27-mm or 33-mm snare wire (Captivator® I or II; Boston Scientific Japan, Tokyo, Japan) with the electrosurgical generator in Endocut Q mode was used to resect the lesion. Finally, hemostatic forceps (FD-411QR; Olympus) was used in the soft coagulation mode to restrain hemorrhage or prophylactically ablate the exposed vessels. |
| Pathological assessments |
| All specimens were cut into 2-mm slices for evaluation. The resected samples were diagnosed by hematoxylin-eosin staining. An electron microscope was used by a pathologist at our hospital to evaluate all lesions. Microscopic evaluation included the following examination: histological type, depth of invasion, lateral and vertical resection margins, and level of lymphovascular involvement. |
| Follow-up colonoscopy |
| The cases that were histopathologically diagnosed as having neither lymphovascular involvement nor positive resection margins after C-EMR were observed during follow-up. After resection, followup colonoscopy was performed at 3 months. Cases without local recurrence on initial follow-up endoscopy were followed up once every year. Local recurrence was defined as detection of residual carcinoid on pathological examination of the biopsied tissues. |
| Ethics |
| Because EMR for rectal carcinoids <10 mm is used worldwide as a standard therapy, C-EMR, which has same procedural steps such as mucosal incision in ESD and snaring in EMR, was not a special procedure; therefore, the data were prospectively evaluated, and all endoscopic procedures were performed without the approval of our hospital’s ethics committee. However, written informed consent was obtained from all patients for colonoscopic treatment as well as all the scheduled follow-up examinations. |
| Results |
| The characteristics of the 6 patients who underwent C-EMR in this study are presented in table 1. None of the patients had symptoms of carcinoid syndrome, and all lesions were incidentally observed in the rectum (upper rectum, 3; lower rectum, 3) on screening endoscopy. The mean age of the patients was 54.2 ± 13.6 years (range, 33-72 years), and the male-to-female ratio was 4:2. There were no cases that demonstrated depression of the tumor surface. All 6 cases were diagnosed as carcinoid tumors because of location, yellow color, and submucosal tumor-like morphology. In addition, the depths of the tumors were diagnosed as submucosal invasion; this phenomenon was recognized by thinning of the third layer corresponding to the submucosal layer on endoscopic ultrasound in all 6 cases. Furthermore, 4 of the 6 cases were histopathologically diagnosed as carcinoid tumor by forceps biopsy. (Table 2) |
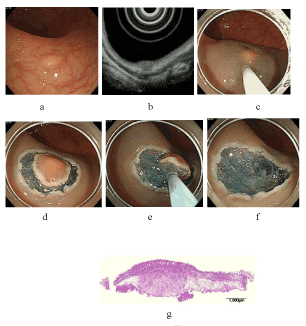
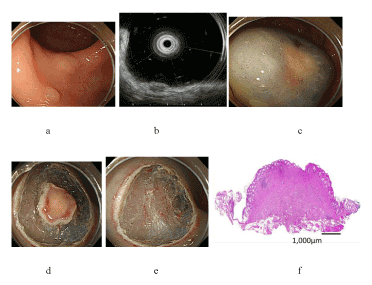
| The mean tumor size was 6.8 ± 1.8 mm (range, 4-9 mm). The mean procedure time was 19.7 ± 5.1 min (range, 12-26 min). The en bloc resection rate was 100% (6/6), and the complete resection rate was 50% (3/6). The depth of the tumors remained within the submucosa, and clear lateral and vertical resection margins were pathologically confirmed in all 6 patients. However, 3 lesions resulted in unsatisfactory complete resection because of lymphovascular involvement (both lymphatic and vascular involvement in 1 case, lymphatic involvement only in 2 cases); moreover, radical surgical therapy was also performed in these 3 cases, and there were no cases with lymph node metastases or residual tumors in the resected specimens. There were no cases with complications such as delayed bleeding and perforation. In complete resection cases, follow-up colonoscopy was performed at 3 months after C-EMR, and no distant or local recurrence was observed during the follow-up period (median, 4 months; range, 4-26 months). The typical procedures in C-EMR for rectal carcinoid tumor are presented in (Figure 1). A case of rectal carcinoid resected by C-EMR with mucosal incision made in the vicinity of the tumor is displayed in figure 2. |
| Discussion |
| There have been some reports on factors related to the metastatic rate of carcinoid tumors, and some have stated that the rate depended on tumor size [19]. Other reports have stated that the tendency for metastatic spread was associated with tumor size, histopathological differentiation, muscular invasion, and lymphovascular invasion [7,20]. Among these factors, tumor size was a simple and reliable indicator for risk of metastatic involvement, which occurred in 3% of tumors with <10 mm diameter [7,20]. Konishi et al. have reported prognosis and risk factors of metastasis in colorectal carcinoids [21]. Al Natour et al. also stated that tumor size and depth of invasion were predictors of lymph node metastasis [22]. Recently, a consensus has developed that small rectal carcinoids without muscular invasion, lymphovascular invasion, and lymph node metastasis should be resected radically by endoscopic therapy. Moreover, Soga et al. reported that tumors ≤ 10 mm in diameter were radically resected by endoscopic technique because of its lower rate of metastasis [7]. On the other hand, 86% of rectal carcinoids are ≤ 10 mm [4]. Furthermore, 75% of rectal carcinoid tumors have submucosal tumor depth [23]. Considering these reasons, most rectal carcinoid tumors detected on screening colonoscopy are treated by endoscopic therapy. Nevertheless, because it is often difficult to accurately measure tumor size and to diagnose the presence of muscular invasion by conventional endoscopy, endoscopic ultrasound (EUS) is performed to determine whether endoscopic resection is indicated. There are some reports documenting that EUS was useful in diagnosing the depth of tumor invasion [24-26]. In the present study, before endoscopic therapy, the depths of the tumors were diagnosed as involving the submucosal layer by thinning of the third layer corresponding to the submucosal layer using EUS in all 6 cases. |
| Conventionally, EMR has been performed for endoscopic treatment of superficial colorectal tumors [8-11]. However, incomplete resection of carcinoid tumors is not rare because most of the tumor occupies the submucosal layer [7,23]. Some investigators have reported that conventional snare polypectomy or EMR resulted in unsatisfactory complete resection of colonic carcinoid tumors [27-29]. Lee et al. stated that the positive resection vertical margin rate was 28.6% by EMR [15]. Concurrently, ESD has recently been reported to be useful for treating colorectal superficial tumors [12,13]. Moreover, ESD has frequently been reported to be useful for treatment of rectal carcinoid [15-18]. However, ESD has the disadvantage of a high frequency of complications, including bleeding and perforation, which is a particularly difficult challenge [30]. Moreover, because ESD requires advanced technical skills, longer procedure time, and specific devices, this technique is not yet a standard treatment. |
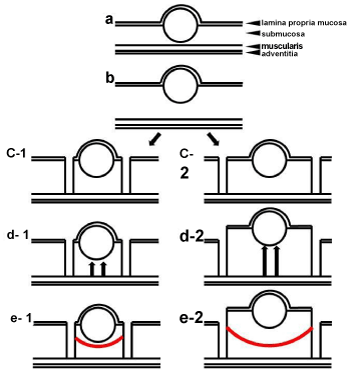
| For submucosal lesions such as rectal carcinoid tumors, endoscopic treatment with a special technique for deeper resection to achieve clear margins has been reported: endoscopic submucosal resection with a ligation device (ESMR-L) or EMR with a ligation device (EMR-L), for which ligation devices were used before resection, enables deeper vertical resection margins to be achieved [31-34]. EMR with a cap (EMR-C) uses a transparent cap and suction to excise the submucosal layer to ensure a safe vertical margin [35-37]. A two-channel endoscope and a holding device with which the submucosal injected portion beneath the tumor is held and lifted are used to perform two-channel EMR [27]. These methods have considerable en bloc resection rates when compared with that of ESD. Instead of these techniques, we perform C-EMR to treat rectal carcinoids. Conventional EMR has a risk in which slipping of the snare results in a positive lateral resection margin, and grasping the tumor itself or shallow submucosal layer results in a positive vertical resection margin. However, C-EMR ensures not only clear lateral margins but also clear vertical margins as sufficient circumferential mucosal incision and submucosal trimming allows gripping of the submucosal layer under the tumor directly during the snaring procedure. To excise a sufficient lateral and vertical margin in this method, it is important to make a mucosal incision away from the tumor by at least 5-10 mm. The reason for this is that setting aside a space for submucosal injection enables to achieve sufficient elevation of the lesion after re-injection to the submucosal layer before snaring, and this also ensures an adequate vertical resection margin. Figure 3 elucidates the relationship between the distance taken from the tumor in the mucosal incision and the depth of the snaring. |
| As presented in figure 2, when mucosal incision is made in the vicinity of the tumor, the quality of lifting after re-injection into the submucosal layer may be poor due to a narrow space under the lesion. In this situation, the level of snaring to the submucosal layer may be shallow, and this may result in positive or unclear vertical margins. In this case, the actual measured distance from the vertical margin to the lower edge of the tumor was 30 μm, which was microscopically determined. In contrast, as displayed in figure 1, when mucosal incision is made away from the tumor, the quality of lifting after re-injection into the submucosal layer may be good, and the lift time may be longer by virtue of a broad space under the lesion. In this situation, the level of snaring to the submucosal layer may be deep, and this may result in clear vertical margins. Furthermore, in this case, the actual measured distance from the vertical margin to the lower edge of the tumor was determined microscopically as 150 μm. These procedures resulted in histopathologically clear lateral and vertical resection margins around all 6 lesions in this study. The use of a needle knife for mucosal incision requires some experience to gain familiarity and proficiency, but it is easily learned and applied by endoscopists with experience in performing ESD. In case of less-experienced endoscopists, the use of a transparent cap enables to achieve stable mucosal incisions. In 3 of the 6 cases in the present study, C-EMR was performed by an endoscopist with 2 years of experience in endoscopy, and en bloc resections were achieved in all 3 lesions without complications. Lee et al. reported that the mean ESD procedure time for treating small rectal carcinoid tumors was 28.4 ± 17.2 min, which was longer than the mean time of 19.7 ± 5.1 min in our study [30]. The procedure time for resection using C-EMR may be shorter than that using ESD. Toyonaga et al. stated that C-EMR, which they defined simplified ESD, was a welcome addition to ESD for inexperienced endoscopists because it required less technical proficiency than did ESD [38]. The differences in the cost among ESD, EMR-L or ESMR-L, EMR-C, two-channel EMR, and C-EMR depend on the device used for the endoscopic procedures. For example, the cost in the case of ESD using only 1 device is about the same as the cost for EMR-L or ESMR-L. However, the cost in the case of ESD using more than 2 devices is higher than those of the other methods. C-EMR is less expensive than ESD or ESMR-L because the needle knife used for mucosal incision is reusable, and the main device is only a snare. Similarly, EMR-C only needs a tip attachment, and two-channel EMR takes advantage of reusable grasping forceps. From the viewpoint of medical treatment fees in Japan, ESD (183700 JPY) costs about 3.7 times as much as EMR (50000 JPY). Considering this reason, if rectal carcinoid is curatively treated by C-EMR, the economic benefits would be higher. Thus, C-EMR is a more useful endoscopic treatment modality than ESD with regard to operation time, expertise, experience, and cost-effectiveness. |
| Limitations |
| The limitations of this study include its single-center retrospective nature and the relatively small number of cases. Moreover, variations in the lesion size, endoscopes, skill level of the operators, and shorter follow-up periods were other limitations. |
| Conclusions |
| Our results revealed that C-EMR is a feasible and effective option for treating rectal carcinoids of <10 mm in diameter. Further prospective studies comparing other methods with C-EMR for rectal carcinoid tumors are warranted. |
| Acknowledgements |
| We thank our colleagues in the Shiga University of Medical Science hospital for assistance in writing this paper. |
References
- Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, et al. (2008) Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 9: 61-72.
- Scherübl H (2008) Options for gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 9: 203.
- Soga J (1994) Carcinoid tumors: A statistical analysis of a Japanese series of 3216 reported and 1180 aoutopsy cases. Acuta Medica et Biologica 42:87-102.
- Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD (2005) Current status of gastrointestinal carcinoids. Gastroenterology 128: 1717-1751.
- Soga J (1997) Carcinoids of the rectum: an evaluation of 1271 reported cases. Surg Today 27: 112-119.
- Ishikawa H, Imanishi K, Otani T, Okuda S, Tatsuta M, et al. (1989) Effectiveness of endoscopic treatment of carcinoid tumors of the rectum. Endoscopy 21: 133-135.
- Soga J (2005) Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer 103: 1587-1595.
- Conio M, Repici A, Demarquay JF, Blanchi S, Dumas R, et al. (2004) EMR of large sessile colorectal polyps. Gastrointest Endosc 60: 234-241.
- Kiesslich R, Neurath MF (2004) Endoscopic mucosal resection: an evolving therapeutic strategy for non-polypoid colorectal neoplasia. Gut 53: 1222-1224.
- Yokota T, Sugihara K, Yoshida S (1994) Endoscopic mucosal resection for colorectal neoplastic lesions. Dis Colon Rectum 37: 1108-1111.
- Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG (2002) Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc 55: 390-396.
- Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, et al. (2010) Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc 24: 343-352.
- Tamegai Y, Saito Y, Masaki N, Hinohara C, Oshima T, et al. (2007) Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy 39: 418-422.
- Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, et al. (2007) Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc 66: 966-973.
- Lee DS, Jeon SW, Park SY, Jung MK, Cho CM, et al. (2010) The feasibility of endoscopic submucosal dissection for rectal carcinoid tumors: comparison with endoscopic mucosal resection. Endoscopy 42: 647-651.
- Hamada Y, Tanaka K, Tano S, Katsurahara M, Kosaka R, et al. (2012) Usefulness of endoscopic submucosal dissection for the treatment of rectal carcinoid tumors. Eur J Gastroenterol Hepatol 24: 770-774.
- Ishii N, Horiki N, Itoh T, Maruyama M, Matsuda M, et al. (2010) Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc 24: 1413-1419.
- Park HW, Byeon JS, Park YS, Yang DH, Yoon SM, et al. (2010) Endoscopic submucosal dissection for treatment of rectal carcinoid tumors. Gastrointest Endosc 72: 143-149.
- Federspiel BH, Burke AP, Sobin LH, Shekitka KM (1990) Rectal and colonic carcinoids. A clinicopathologic study of 84 cases. Cancer 65: 135-140.
- Shim KN, Yang SK, Myung SJ, Chang HS, Jung SA, et al. (2004) Atypical endoscopic features of rectal carcinoids. Endoscopy 36: 313-316.
- Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, et al. (2007) Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut 56: 863-868.
- Al Natour RH, Saund MS, Sanchez VM, Whang EE, Sharma AM, et al. (2012) Tumor size and depth predict rate of lymph node metastasis in colon carcinoids and can be used to select patients for endoscopic resection. J Gastrointest Surg 16: 595-602.
- Matsumoto T, Iida M, Suekane H, Tominaga M, Yao T, et al. (1991) Endoscopic ultrasonography in rectal carcinoid tumors: contribution to selection of therapy. Gastrointest Endosc 37: 539-542.
- Kobayashi K, Katsumata T, Yoshizawa S, Sada M, Igarashi M, et al. (2005) Indications of endoscopic polypectomy for rectal carcinoid tumors and clinical usefulness of endoscopic ultrasonography. Dis Colon Rectum 48: 285-291.
- Martínez-Ares D, Souto-Ruzo J, Varas Lorenzo MJ, Espinós Pérez JC, Yáñez López J, et al. (2004) Endoscopic ultrasound-assisted endoscopic resection of carcinoid tumors of the gastrointestinal tract. Rev Esp Enferm Dig 96: 847-855.
- Hokama A, Oshiro J, Kinjo F, Saito A (1996) Utility of endoscopic ultrasonography in rectal carcinoid tumors. Am J Gastroenterol 91: 1289-1290.
- Onozato Y, Kakizaki S, Iizuka H, Sohara N, Mori M, et al. (2010) Endoscopic treatment of rectal carcinoid tumors. Dis Colon Rectum 53: 169-176.
- Zhou PH, Yao LQ, Qin XY, Xu MD, Zhong YS, et al. (2010) Advantages of endoscopic submucosal dissection with needle-knife over endoscopic mucosal resection for small rectal carcinoid tumors: a retrospective study. Surg Endosc 24: 2607-2612.
- Kim KM, Eo SJ, Shim SG, Choi JH, Min BH, et al. (2012) Treatment outcomes according to endoscopic treatment modalities for rectal carcinoid tumors. Clin Res Hepatol Gastroenterol.
- Lee EJ, Lee JB, Choi YS, Lee SH, Lee DH, et al. (2012) Clinical risk factors for perforation during endoscopic submucosal dissection (ESD) for large-sized, nonpedunculated colorectal tumors. Surg Endosc 26: 1587-1594.
- Niimi K, Goto O, Fujishiro M, Kodashima S, Ono S, et al. (2012) Endoscopic mucosal resection with a ligation device or endoscopic submucosal dissection for rectal carcinoid tumors: an analysis of 24 consecutive cases. Dig Endosc 24: 443-447.
- Kim HH, Park SJ, Lee SH, Park HU, Song CS, et al. (2012) Efficacy of endoscopic submucosal resection with a ligation device for removing small rectal carcinoid tumor compared with endoscopic mucosal resection: analysis of 100 cases. Dig Endosc 24: 159-163.
- Abe T, Kakemura T, Fujinuma S, Maetani I (2008) Successful outcomes of EMR-L with 3D-EUS for rectal carcinoids compared with historical controls. World J Gastroenterol 14: 4054-4058.
- Moon JH, Kim JH, Park CH, Jung JO, Shin WG, et al. (2006) Endoscopic submucosal resection with double ligation technique for treatment of small rectal carcinoid tumors. Endoscopy 38: 511-514.
- Sohn DK, Han KS, Hong CW, Chang HJ, Jeong SY, et al. (2008) Selection of cap size in endoscopic submucosal resection with cap aspiration for rectal carcinoid tumors. J Laparoendosc Adv Surg Tech A 18: 815-818.
- Oshitani N, Hamasaki N, Sawa Y, Hara J, Nakamura S, et al. (2000) Endoscopic resection of small rectal carcinoid tumours using an aspiration method with a transparent overcap. J Int Med Res 28: 241-246.
- Celebi Kobak A, Zeybel M, Ayhan S, Kara E, Ellidokuz E (2006) Endoscopic submucosal resection of a rectal carcinoid tumor by cap aspiration - snare resection method. Turk J Gastroenterol 17: 313-315.
- Toyonaga T, Man-I M, Morita Y, Sanuki T, Yoshida M, et al. (2009) The new resources of treatment for early stage colorectal tumors: EMR with small incision and simplified endoscopic submucosal dissection. Dig Endosc 21: S31-37.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14290
- [From(publication date):
specialissue-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9654
- PDF downloads : 4636
