Special Issue Article Open Access
Efficacy of Artesunate Plus Amodiaquine for Treatment of Uncomplicated Clinical Falciparum Malaria in Severely Malnourished Children Aged 6?59 Months, Democratic Republic of Congo
P. Mitangala Ndeba1*, U. D’Alessandro2, P. Hennart3,5, P. Donnen3,5, D. Porignon4, G. Bisimwa Balaluka5,6, A. Bisimwa Nkemba6, N Cobohwa Mbiribindi6 and M. Dramaix Wilmet1,51Centre de recherche: épidémiologie, Biostatistique et recherche clinique, Ecole de Santé Publique, Université Libre de Bruxelles (Belgique)
2Department of Parasitology, Institute Tropical Medicine, Antwerp, Belgium
3Centre de recherche : épidémiologie et médecine préventive, Ecole de santé Publique, Université Libre de Bruxelles (Belgique)
4Unité de santé internationale, Département des Sciences de la Santé Publique, Université de Liège (Belgique).
5Centre Scientifique et Médical de l’Université Libre de Bruxelles pour ses Activités de Coopération (CEMUBAC)
6Département de nutrition, Centre de Recherche en Sciences Naturelles / Lwiro (République Démocratique du Congo)
- *Corresponding Author:
- Mitangala Ndeba Prudence
Centre de recherche: épidémiologie, biostatistique et recherche clinique
Ecole de Santé Publique, Université Libre de Bruxelles
CP 598, Route de Lennik 808, 1070 Bruxelles
Tel: +243 998 088 072
E-mail: prudendeb@yahoo.fr
Received Date: January 01, 2012; Accepted Date: April 21, 2012; Published Date: April 23, 2012
Citation: Ndeba PM, D’Alessandro U, Hennart P, Donnen P, Porignon D, et al. (2012) Efficacy of Artesunate Plus Amodiaquine for Treatment of Uncomplicated Clinical Falciparum Malaria in Severely Malnourished Children Aged 6–59 Months, Democratic Republic of Congo. J Clin Exp Pathol S3:005. doi: 10.4172/2161-0681.S3-005
Copyright: © 2012 Ndeba PM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Abstract
Background: Recent published studies on efficacy and safety of antimalarial treatment in children with Severe
Acute Malnutrition (SAM) suffering from uncomplicated malaria are not available.
Methods: Between March 2007 and December 2010 the efficacy of AS+AQ in treating uncomplicated malaria
children under five with SAM was carried out in Lwiro (Eastern Republic Democratic of Congo) according to the WHO
standard protocol. Among the 445 children included, 69 had SAM. AS+AQ was given according to national protocol.
Analysis was done using per protocol method. Odds ratio (OR) and their 95% confidence interval (95% CI) were
computed.
Results: The treatment failure rate was 24.4% of 414 infections included in the analysis. After adjustment for
malaria parasitemia, ACPR in children without SAM were 73.0% when it was 91.4% among those with SAM (OR
3.15 95%CI 1.19 – 8.30). Malaria parasitemia median at admission was statistically low among children who had
subsequently Adequate Clinical and Parasitological Response (ACPR).
Conclusion: AS+AQ has a good efficacy among children with both uncomplicated falciparum malaria and
malnutrition including severe acute form. AS+AQ dosing national strategy unmodified can be used, to treat under five
children with malnutrition including severe acute form suffering from uncomplicated malaria.
Keywords
Malaria; DRC; Efficacy treatment; Severe acute malnutrition
Introduction
In Sub-Saharian African, malaria and malnutrition often co-exist and represent an important public health burden [1,2]. Prompt treatment with an effective antimalarial treatment is one of the cornerstones of malaria control [3]. Because of unacceptable resistance, chloroquine (CQ), The most affordable and widely available antimalarial treatment in the past, has been replaced, according to the World Health Organization recommendations, by artemisinin-based combination treatments (ACT) [4,5]. This was followed by a strong advocating of the use of the ACT [6].
In Eastern Democratic Republic of Congo (DRC),resistance of Plasmodium falciparum to CQ has been documented since 1983 and was estimated at 80% in 2001 [7]. This prompted the National Malaria Control Program (NMCP) to change in 2005 the national antimalarial treatment policy and the first line treatment, from CQ to amodiaquine plus artesunate (AS+AQ). Nevertheless, since its implementation, few studies on AS+AQ efficacy have been carried out [8-10], and none of them took into account the patients’ nutritional state. Nevertheless it has been often reported that both malnutrition and malaria affect antimalarial disposition [11,12].
Malaria is endemic in areas where malnutrition is common. Among children suffering from uncomplicated malaria to treat using antimalarial for which efficacy tests are achieved, a good many of them are malnourished. Yet severely malnourished children are usually excluded from antimalarial efficacy studies. Consequently, to our knowledge, there are no recent published studies on efficacy and safety of antimalarial treatment in severely malnourished children with uncomplicated malaria. Therefore there is a need to further study the efficacy and the safety of artemisinin-based combination for uncomplicated malaria treatment in malnourished children although its efficacy and safety have been reported for use in children in Africa.
We compared the efficacy and safety of AS+AQ dosing DRC national strategy in malnourished vs non malnourished children aged 6–59 months with uncomplicated clinical falciparum malaria.
Methodology
The study was carried out between March 2007 and December 2010 in Lwiro pediatric hospital (LPH), in South Kivu province, Katana health district, at the eastern border of DRC. LPH is a unit of the nutrition department of Lwiro CRSN (“Centre de Recherche en Sciences Naturelles”), the oldest research center in the Kivu region for the last 60 years.
Patient recruitment
Children with suspected uncomplicated malaria attending LPH or its satellite nearest health centre were screened and enrolled in the study if they met the following inclusion criteria: age 6 -59 months old, fever (axillary temperature ≥37.5°C) or a history of fever in the previous 24 hours, Plasmodium falciparum mono-infection with density between 1,000 and 200,000/μl. Exclusion criteria were the following: cause for fever other than malaria; dangers signs (unable to sit or stand up, unable to drink or breastfeed, lethargy or unconsciousness, recent history of convulsions, persistent vomiting) or signs of severe malaria [13].
The aim and the procedures of the study were explained to the parent/guardian and an individual informed consent was obtained.
Treatment
Enrolled children were treated with AS+AQ (Falcimon®, Cipla ltd, Mumbai Central, Mumbai 400008, India) at the dose of 4 mg/kg for AS and 10 mg/kg for AQ given over 3 days (dosing DRC national strategy). AS+AQ was procured by Asrames (“Association régionale d’Aprovisionnement en Médicaments Essentiels”), the drug’s regional central distribution located in Goma, North Kivu province. Treatment was administered over 3 days (day 0-2) under the supervision of a nurse who kept the children at the clinic for about 1 hour post-treatment to check for possible vomiting. Treatment failures were given a full course of quinine according to the NMCP guidelines. Children with severe acute malnutrition were included in a nutritional rehabilitation program and managed according to the national nutritional therapeutic protocol.
Follow-up
After completion of the treatment, scheduled visits were at days 3, 7, 14 and 28. Between visits parents were encouraged to attend LPH whenever their child was sick. Community health workers did regular home visits to remind parents/guardians the next scheduled meeting.
Laboratory tests
Each visit a blood sample was collected by finger prick for thick and thin smears that were stained, with 3% Giemsa for 30 min (thin smears were first fixed with methanol). Parasite density was estimated by counting the number of asexual parasites against 200 White Blood Cells (WBC), assuming a WBC count of 8000/ μL. Serum albumin was measured using the bromocresol green assay on spectrophometer.
Anthropometric and clinical measurements
Weight, height and the middle upper arm circumference (MUAC) were collected at each follow-up visit by a skilled nurse from the HPL according to international recommendations [14]. Axillary temperature was measured with a digital thermometer.
Outcome measure
Outcomes were assessed after 28 days and were classified into four categories of therapeutic responses, i.e. Early Treatment Failure (ETF), Late Clinical Failure (LCF), Late Parasitological Failure (LPF), Adequate Clinical and Parasitological Response (ACPR) [13].
Statistical analysis
Children were not included in the analysis if (1) the parents/ guardians had administered another antimalarial during follow – up;(2) they withdrew consent; (3) the child had a concomitant disease that would interfere with the treatment outcome; (4) children were lost to follow up after two successive missing visits.
The z scores (ZS) height for age (HAZ), weight for age (WAZ) and weight for height (WHZ) were computed with the software WHO Anthro V2.0.4 using the reference population as defined by WHO in 2006 [15].
The HAZ, WHZ, WAZ, MUAC, albumin and the presence of edemas were used to quantify the degree of under nutrition. The cut offs were 115 and 125 mm for the MUAC and -3 and -2 for HAZ, WHZ and WAZ ([16,17]). Acute malnutrition was defined as a WHZ ≤ -2 while chronic malnutrition as a ZS for HAZ ≤ -2 [16]. Severe acute malnutrition (SAM) was defined by a WHZ < -3 and/or the presence of nutritional edema [17]. According to the previous experience on the prognostic indices for mortality in LPH, thresholds for albumin were 16 and 23 mg/L.
Analysis of treatment outcome was per protocol. Either the χ2 or the Fisher exact tests were used for proportions comparison, Mantel— Haenszel for adjustment and non parametric Kruskal-Wallis test for continuous variables with skewed distribution. Odds ratio (OR) for failure was computed with 95% confidence intervals (95%CI). All reported p-values were two-sided, considered statistically significant if less than 0.05.
Ethical considerations
The protocol was submitted to and approved by the Lwiro CRSN ethical committee. The procedure for obtaining consent was according to international guidelines and local habits for research on human subjects. Parents/guardians were informed they could withdraw at any time without compromising access to health care.
Results
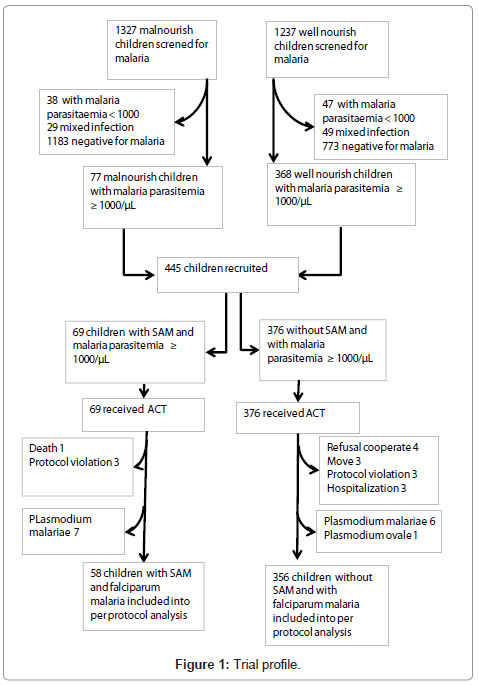
The figure 1 gives the trial profile. Baseline characteristics are reported in Table 1. The median age was 26.5 months (range: 6.0 – 57.97). SAM was found in 14.4% (62/431) children. Median age was 30.2 months (range: 7.3 – 56.9) in children with SAM vs 25.5 months (range: 6.0 – 57.97) in those without SAM (p=0.005). The risk for SAM increased with age, i.e. compared to <12 months old, odds ratio (OR): 1.66 (95% CI: 0.55 -5.01) in 12-23 months old, 3.59 (95%CI: 1.30 – 9.96) in 24 – 35 months old and 3.16 (1.14 – 8.74) in 36 – 59 months old. Nutritional status did not differ by gender.
| % (number) | fever % (number) | ||
|---|---|---|---|
| Age (in months) | |||
| < 12 | 16.2 (70) | 72.9 (51) | |
| 12 - < 24 | 26.9 (116) | 55.2 (64) | |
| 24 - < 36 | 26.0 (112) | 71.4 (80) | |
| 36 - 59 | 30.9 (133) | 57.1 (76) | |
| Gender | |||
| Female | 48.7 (210) | 63.8 (134) | |
| Edema | |||
| Yes | 13.0 (56) | 25.0 (14) | |
| WHZ | |||
| < -3 | 3.9 (17) | 17.6 (3) | |
| -3 - < -2 | 7.0 (30) | 43.3 (13) | |
| >= -2 | 89.1 (384) | 66.4 (255) | |
| HAZ | |||
| < -3 | 42.5 (183) | 50.3 (92) | |
| -3 - < -2 | 27.1 (117) | 70.9 (83) | |
| >= -2 | 30.4 (131) | 73.3 (96) | |
| MUAC (in mm) | |||
| < 115 | 7.4 (32) | 31.3 (10) | |
| 115 - < 125 | 10.4 (45) | 53.3 (24) | |
| >= 125 | 82.1 (354) | 66.9 (237) | |
| Serum albumin (g/L) | |||
| < 16 | 2.8* (12) | 8.3 (1) | |
| 16 - < 23 | 6.5* (28) | 35.7 (10) | |
| >= 23 | 90.7* (390) | 66.7 (260) | |
| * n=430 | |||
Table 1: Baseline characteristics under five children with uncomplicated falciparum malaria treated with AS+AQ in eastern DRC, from march 2007 to December 2010. (n=431).
Median parasite density was 26,600/μl (range: 1,000 – 198,000) and significantly lower in older children (Table 2) and in the undernourished one (Table 2). The body temperature was significantly lower in children suffering from SAM (n=62) [mean (standard deviation) 37.0°C (0.9)] compared to those without SAM (n=369) [mean (standard deviation) 38.3°C (1.3)] (p < 0.001).
| n | Median per µL (range) | p | ||
|---|---|---|---|---|
| Age (in months) | 0,033 | |||
| < 12 | 70 | 39 100 (1600 - 163420) | ||
| 12 - < 24 | 116 | 24 570 (1200 - 193600) | ||
| 24 - < 36 | 111 | 26 800 (1120 - 198000) | ||
| 36 - 59 | 133 | 19 280 (1000 - 170820) | ||
| Gender | 0,76 | |||
| Female | 210 | 26 480 (1000 - 181000) | ||
| Male | 220 | 26 900 (1040 - 198000) | ||
| SAM | < 0,001 | |||
| Yes | 61 | 7 600 (1040 - 162280) | ||
| No | 369 | 31 000 (1000 - 198000) | ||
| MUAC (mm) | 0,008 | |||
| < 115 | 32 | 10 550 (1460 - 110660) | ||
| 115 - < 125 | 45 | 19 840 (1120 - 198000) | ||
| ≥ 125 | 353 | 29 880 (1000 - 193600) | ||
| Albumin (g/dL)> | < 0,001 | |||
| < 16 | 12 | 2 330 (1040 - 32240) | ||
| 16 - < 23 | 28 | 10 040 (1680 - 88660) | ||
| ≥ 23 | 390 | 29 940 (1000 - 198000) | ||
| HAZ | 0,016 | |||
| < -2 | 299 | 24 390 (1000 - 198000) | ||
| ≥ -2 | 131 | 34 120 (1040 - 181000) | ||
Table 2: Malaria parasitemia by several features among under five children with uncomplicated falciparum malaria treated with AS+AQ in eastern DRC, from march 2007 to December 2010.
Overall, by day 28, the ACPR was 75.6% (313/414) and the total treatment failure was significantly lower in malnourished children after adjustment for malaria parasitemia (Table 3). Median parasitemia at admission was significant lower in children whose outcome was ACPR (21, 560/μL; range: 1,000 – 198,000) than in those with LCF (46,920/μL; range: 1,120 – 193,600) (n=69) and LPF (41,560/μL (2,020 – 140,000) (n=32) (p < 0.001).
| Nutritional indicator | % | (Number) | % | (Number) | OR* | (95% IC)* | p |
| Severe Acute malnutrition (SAM) | Yes (n=58) | NO (n=356) | 0.002 | ||||
| Late Clinical Failure (LCF) | 5.2 | (3) | 18.5 | (66) | 0.30 | (0.09 – 0.1.01) | |
| Late Parasitological Failure (LPF) | 3.4 | (2) | 8.4 | (30) | 0.47 | (0.11 – 2.08) | |
| Total Failure (TF) | 8.6 | (5) | 27.9 | (96) | 0.32 | (0.12 – 0.84) | |
| Adequate Clinical and Parasitological Response (ACPR) | 91.4 | (53) | 73.0 | (260) | 3.15 | (1.19– 8.30) | |
| MUAC | < 125 mm (n=74) | ≥ 125 mm (n=340) | 0.033 | ||||
| Late Clinical Failure (LCF) | 6.8 | (5) | 18.8 | (64) | 0.35 | (0.13 – 0.91) | |
| Late Parasitological Failure (LPF) | 6.8 | (5) | 7.9 | (27) | 0.94 | (0.35 – 2.55) | |
| Total Failure (TF) | 13.5 | (10) | 26.8 | (91) | 0.48 | (0.23 – 0.98) | |
| Adequate Clinical and Parasitological Response (ACPR) | 86.5 | (64) | 73.2 | (249) | 2.10 | (1.02 – 4.34) | |
| Serum albumin | <23 g/L (n=38) | ≥ 23 g/L (n=375) | 0.22 | ||||
| Late Clinical Failure (LCF) | 10.5 | (4) | 17.3 | (65) | 0.78 | (0.26 – 2.37) | |
| Late Parasitological Failure (LPF) | 5.3 | (2) | 8.0 | (30) | 0.84 | (0.19 – 3.76) | |
| Total Failure (TF) | 15.8 | (6) | 25.3 | (95) | 0.78 | (0.30 – 2.01) | |
| Adequate Clinical and Parasitological Response (ACPR) | 84.2 | (32) | 74.7 | (280) | 1.29 | (0.50 – 3.31) | |
| HAZ | < -2 (n=290) | >= -2 (n=124) | 0.78 | ||||
| Late Clinical Failure (LCF) | 17.2 | (50) | 15.3 | (19) | 1.25 | (0.70 – 2.24) | |
| Late Parasitological Failure (LPF) | 7.2 | (21) | 8.9 | (11) | 0.85 | (0.40 – 1.83) | |
| Total Failure (TF) | 24.5 | (71) | 24.2 | (30) | 1.11 | (0.67 – 1.82) | |
| Adequate Clinical and Parasitological Response (ACPR) | 75.5 | (219) | 75.8 | (94) | 0.90 | (0.55 – 1.49) | |
*Ajust for malaria parasitemia with 26 600/μL as threshold.
Table 3: Therapeutic responses to AS+AQ by nutritional indicators status in under five children with uncomplicated falciparum malaria in eastern DRC, between march 2007 to December 2010.
No child developed severe malaria after enrolment. One child with severe acute malnutrition died at day 2.
The Table 4 summarizes the adverse events (AEs) by nutritional status. Nutritional status did not have any effect on the occurrence of AEs. At least one adverse event concomitant with drug administration occurred in 9.7% (6/62) children with SAM as compared to 6.5% (24/369) without SAM (p=0.52). There were two severe adverse events among SAM children, both of them asthenia as the parent/guardian reported that the child could not participate to habitual activities.
| Severe acute malnutrition | ||
|---|---|---|
| Yes (n= 62) | No (n= 369) | |
| Adverse events | ||
| Anorexia | 2 | 8 |
| Asthenia | 2 | 1 |
| Diarrhoea | 0 | 4 |
| Vomiting | 2 | 5 |
| Nausea | 0 | 4 |
| Cough | 0 | 2 |
| Grade | ||
| Mild | 4 | 20 |
| Moderate | 0 | 4 |
| Severe | 2 | 0 |
*The only one predominant adverse events for the same children was considered.
Table 4: Adverse events* by nutritional status among under five children with uncomplicated falciparum malaria treated with AS+AQ in eastern DRC, from march 2007 to December 2010.
Discussion
After adjustment for malaria parasitemaia, more than a quarter of children (27.9%) without SAM experienced a treatment failure while this proportion was 8.6% among those with SAM. Children with SAM or MUAC < 125 mm had ACPR OR > 1 compared to those without SAM or with MUAC ≥ 125 mm. This can suggest that AS+AQ has a good efficacy among children with both uncomplicated falciparum malaria and malnutrition including a severe acute form in spite of their multiple deficiencies.
These results indicate also that the tolerance of AS+AQ dosing national strategy was globally good among children including those with SAM.
Overall, these results suggest that AS+AQ dosing national strategy unmodified can be used, to treat under five children with malnutrition including SAM suffering from uncomplicated falciparum malaria. Even if this study had not aimed to monitor AS+AQ efficacy, the total failure rates observed could be an alert suggesting that it could be indicated for DRC to think about possible actions for its malaria drug policy.
However, this study has important limitations requiring to be mentioned.
First, the study took place when malaria were hypoendemic all over Katana district. A large program of insecticide-treated bed nets (ITNs) distribution began when the study was carried out. A former study conducted in 1983 in the same region had shown plasmodia index variation between 25 and 44 percent indicating a mesoendemic malaria level [18]. When recruiting, it was difficult to include children suffering from malnutrition and uncomplicated malaria with parasitemia ≥ 1000 trophozoites/μL, one of major criteria inclusion in the study. The decrease of malaria endemicity during the study time add to the difficult to get malnourished children meeting inclusion criteria led to extend the recruitment period. During this period, Plasmodium species could change their sensitivity pattern to anti-malarial drugs.
Secondary, in this study we did not measure AS+AQ blood levels to demonstrate drug and metabolite concentration or its disposition in malnourished children. This may have led either to overestimations or to underestimation of total failure rates. Nevertheless, most of anti-malarial efficacy studies don’t include confirmation of drug metabolism; demonstration of the persistence of parasites in a patient receiving directly observed therapy is quite often considered sufficient.
Thirdly, in this study neither PCR (polymerase chain reaction) for confirmation of failure has been performed. This may also have led overestimations of failure instead of the re – infection. But the study has been carried out while a larger program of ITNs took place in the region. This could suggest that re-infection level could be low.
Lastly, these results may not be representative of the whole country. DRC is a huge country with multifaceted environmental factors including nutritional and malaria transmission patterns which may lead to regional variability in anti-malarial efficacy treatments.
In spite of these above limitations, this study has a public health strategy goal as a tool for uncomplicated malaria treatment in malnourished under five children using AS+AQ DRC national dosing.
Anti-malarial efficacy studies are scares in DRC although malaria is endemic in this huge country. More, there is no study which evaluated in the past anti-malaria efficacy in malnourished children in spite of malnutrition prevalence.
Since 2000, ACT has been recommended by WHO for the treatment of uncomplicated malaria [5]. By this way ACT has been adopted as a first-line treatment for uncomplicated malaria in several sub-Saharan countries among them the DRC. Unfortunately there are very limited pharmacokinetic studies of artemisinin, the main drug recommended for combination and derivate in African children in which chronically malnutrition is prevalent; therefore the relationship between plasma drug concentration and efficacy in these patients is unknown [19].
However, in a recent study, Verret and al. [12] concluded to an efficacy of artemisinin-based combination therapies in chronically malnourished children for repeated episodes of malaria in Ugandan children.
Looking at the way of malaria prevention in malnourished children, Danquah et al. [20] observed that the protective efficacies of intermittent preventive treatment with sulfadoxine-pyrimethamine in malnourished children in northern Ghana were roughly half or even less of those observed in non-malnourished children.
Our results by which children without SAM experienced a poor response to AS+AQ treatment than SAM children can be consistent with those done in studies aiming to identify pre-treatment risk factors of uncomplicated malaria treatment failure with CQ [21-23]. In those studies younger age, higher baseline temperature and higher initial parasitaemia were predictors of malaria treatment failure [21-23]. In our study, SAM children were significantly younger, had low body temperature and less malaria parasitemia density compared to those without SAM. Warsame et al. [24] observed also that patients who experienced clinical failure had significantly higher initial parasitaemia than those in whom there was an adequate clinical response.
Independently of the above mentioned factors predicting poor response to uncomplicated clinical malaria treatment, it was established that physiological changes in children with malnutrition were associated with abnormal disposition of drugs which could necessitate drug dosage modifications [25].
In human, Pussard and al observed that malaria and malnutrition increased plasma concentrations of quinine and reduced both the volume of distribution and the total plasma clearance [26]. Measuring the response to an acute uncomplicated Plasmodium falciparum malaria treatment with CQ, Olanrewaju WI and Johnson AW [27] observed that the proportion of children with persistence or recrudescence of parasitemia on days 4-7 and no significant reduction of parasitemia was higher among malnourished children compared to children with satisfactory nutritional status.
Because of the lack of sufficient published topical studies, the mechanism by which malnutrition can enhance or decrease antimalarial efficacy is unknown.
In a former study in an animal model, vitamin E deficiency enhanced the antimalarial action of qinghaosu against Plasmodium yoelii in young female mice, both in terms of decreased parasitemia and improved survival, but Selenium deficiency did not [28].
Not withstanding malnutrition, our results related to anti-malarial efficacy could be consistent with studies carried out after AS+AQ implementation in DRC for uncomplicated malaria treatment. In 2004, a study conducted in the south of the South Kivu province presented a day 28 PCR genotyping-adjusted failure rates of 6.7% using AQ+AS [8]. Between 2003 to 2004, in Boende in north-west, the unadjusted total failures on day 28 was 41.0%; it was 8.8% in Kabalo in southeastern of DRC [29]. These total rates failures were observed before the PNLP had introduced AQ + AS as the first-line regimen for uncomplicated malaria treatment through the whole country in 2005. More than five years later it can be understandable that the total failure rates could raise. By using, usually the drug efficacy steadily declined. In Rwanda, in the neighborhood of South Kivu province, using the combination amodiaquine + sulfadoxine/ pyrimethamine (AQ + SP), the total rates failures at day 28 was estimated as 17% in 2003 [24]. Two years after its adoption as the first-line antimalarial the total rates failures at day 28 raised up 25% [30].
Lastly, the most common adverse events observed were anorexia, vomiting, nausea and diarrhoea. These events observed are consistent with those in Rwanda using AS+AQ [31]. Given that drug retention explained by the pathophysiological changes occurring in malnutrition which could result in adverse events which may be masked by a constellation of signs malnutrition, in our study, the drug tolerance was globally good among children including those with SAM despite their weakness. All children with SAM received were admitted in an intensive phase of the nutritional rehabilitation program. This can suggest that administration of AS+AQ among children with respecting a regular rhythm of the daily diet can decrease the drug adverse events.
However, this study had not aimed to monitor AS+AQ efficacy, although these results cannot be representative for the whole huge country, they should alert the DRC national program to monitor the efficacy of its first-line anti-malarial for uncomplicated malaria treatment according to WHO malaria reports [32]. Actions including countrywide as ascertainment of treatment failure, assessment of other options available, and their cost and distribution, and reaching final consensus on the need to change should be carried out [3].
Conclusion
In Area where malaria and malnutrition co-exist, AS+AQ dosing recommended unmodified is an effective and safe drug which can be used to treat under five children with malnutrition including severe acute form suffering from uncomplicated falciparum malaria.
But the total failure rates observed in this study are an alert which could suggest that it could be indicated for DRC sanitary authorities to think about the possible actions of the malaria drug policy, even if this study had not aimed to monitor AS+AQ efficacy.
Acknowledgement
The authors would like thank the “CUD” (Belgian cooperation to the development) for its support in funding this study through a targeted inter-university project.
They also thank all children and their parents or guardians who have fully agreed to the total participation of their children in the study despite the climate of insecurity which prevailed in the region.
They thank the local and “CRSN” staff authorities for their good cooperation.
They thank Mrs. Mashukano Kikongo Alphonsine, Mrs Kapitula Kasereka and Paluku Katembo Jean Pierre for carrying out the parasitological diagnostic, Mrs. Nabintu and Mrs Balume, Kahunguzi, Karazo, Katoto, Mapendano, Nkemba and Simika for the quality of work in spite of the difficult working conditions characterized by insecurity prevailing in the Eastern part of DRC. Finally, they thank Mr. Alain Wodon who has prepared the data entry form controls and Miss Maisha for their encoding.
References
- World Health Organization (2009) Global estimates of malaria cases and deaths in 2008. World Malaria Report: 27.
- Nubé M, Sonneveld BG (2005) The geographical distribution of underweight children in Africa. Bull World Health Organ 83: 764-770.
- (1993) Implementation of the Global Malaria Control Strategy. Report of a WHO Study Group on the implementation of the global plan of action for malaria control, 1993-2000. World Health Organ Tech Rep Ser.
- White NJ (2004) Antimalarial drug resistance. J Clin Invest 113: 1084-1092.
- World Health Organization (2001) Antimalarial drug combination therapy: report of a WHO technical consultation. Document WHO/CDS/RBM/2001.35. World Health Organization, Geneva, Switzerland.
- Kremsner PG, Krishna S (2004) Antimalarial combinations. Lancet 364: 285-294.
- Kazadi WM, Vong S, Makina BN, Mantshumba JC, Kabuya W, et al. (2003) Assessing the efficacy of chloroquine and sulfadoxine-pyrimethamine for treatment of uncomplicated Plasmodium falciparum malaria in the Democratic Republic of Congo. Trop Med Int Health 8: 868-875.
- Swarthout TD, van den Broek IV, Kayembe G, Montgomery J, Pota H,et al. (2006) Artesunate + Amodiaquine and Artesunate + Sulphadoxine-Pyrimethamine for treatment of uncomplicated malaria in Democratic Republic of Congo: a clinical trial with determination of sulphadoxine and pyrimethamine resistant haplotypes. Trop Med Int Health 11: 1503-1511.
- van den Broek I, Kitz C, Al Attas S, Libama F, Balasegaram M, et al. (2006) Efficacy of three artemisinin combination therapies for the treatment of uncomplicated Plasmodium falciparum malaria in the Republic of Congo. Malar J 5: 113.
- Alker AP, Kazadi WM, Kutelemeni AK, Bloland PB, Tshefu AK, et al. (2008) dhfr and dhps genotype and sulfadoxine-pyrimethaminetreatment failure in children with falciparum malaria in the Democratic Republic of Congo. Trop Med Int Health 13: 1384-1391.
- Oshikoya KA, Sammons HM, Choonara I (2010) A systematic review of pharmacokinetics studies in children with protein-energy malnutrition. Eur J Clin Pharmacol 66: 1025-1035.
- Verret WJ, Arinaitwe E, Wanzira H, Bigira V, Kakuru A, et al. (2011) Effect of nutritional status on response to treatment with artemisinin-based combination therapy in young ugandan children with malaria. Antimicrob Agents Chemother 55: 2629-2635.
- OMS (2004) Evaluation et surveillance de l’efficacité des antipaludiques à plasmodium falciparum non compliqué. Organisation mondiale de la Santé. WHO/HTM/RBM/2003.50.
- OMS (1995) Rapport d’un comité OMS d’experts. Utilisation et interprétation de l’anthropométrie. Série de rapport techniques 854 : 473-475.
- WHO Multicentre Growth Reference Study Group (2006) WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length,weight- for-height and body mass index-for-age:Methods and development. Geneva, World Health Organization.
- WHO (1999) Management of severe malnutrition: a manual for physicians and other senior health workers. Geneva, World Health Organization.
- WHO, UNICEF (2009) Joint statement. WHO child growth and the identification of severe acute malnutrition in infants and children. Geneva, New York.
- Delacollette C, Van der Stuyft P, Molima K, Hendrix L, Wéry M (1990) Indices paludométriques selon l’âge et les saisons dans la zone de santé de Katana, au Kivu montagneux, Zaire. Ann Soc Belg Med Trop 70: 263-268. Kokwaro G, Mwai L, Nzila A (2007) Artemether/lumefantrine in the treatment of uncomplicated falciparum malaria. Expert Opin Pharmacother 8: 75-94.
- Danquah I, Dietz E, Zanger P, Reither K, Ziniel P, et al. (2009) Reduced efficacy of intermittent preventive treatment of malaria in malnourished children.Antimicrob Agents Chemother 53: 1753-1759.
- Ghalib HW, Al-Ghamdi S, Akood M, Haridi AE, Ageel AA, et al. (2001)Therapeutic efficacy of chloroquine against uncomplicated, Plasmodium falciparum malaria in south-western Saudi Arabia. Ann Trop Med Parasitol 95:773-779.
- Hamer DH, MacLeod WB, Addo-Yobo E, Duggan CP, Estrella B, et al. (2003) Age, temperature, and parasitaemia predict chloroquine treatment failure andanaemia in children with uncomplicated Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg 97: 422-428.
- Sowunmi A, Fateye BA, Adedeji AA, Fehintola FA, Gbotosho GO, et al. (2005) Predictors of the failure of treatment with chloroquine in children with acute,uncomplicated, Plasmodium falciparum malaria, in an area with high andincreasing incidences of chloroquine resistance. Ann Trop Med Parasitol 99535-544.
- Warsame M, Abdillahi A, Duale ON, Ismail AN, Hassan AM, et al. (2002) Therapeutic efficacy of chloroquine and sulfadoxine/pyrimethamine against Plasmodium falciparum infection in Somalia. Bull World Health Organ 80: 704-708.
- Oshikoya KA, Senbanjo IO (2009) Pathophysiological changes that affect drug disposition in protein-energy malnourished children. Nutr Metab (Lond) 6: 50.
- Pussard E, Barennes H, Daouda H, Clavier F, Sani AM, et al. (1999) Quinine disposition in globally malnourished children with cerebral malaria. Clin Pharmacol Ther 65: 500-510.
- Olanrewaju WI, Johnson AW (2001) Chloroquine-resistant Plasmodium falciparum malaria in Ilorin, Nigeria: prevalence and risk factors for treatment failure. Afr J Med Med Sci 30: 165-169.
- Levander OA, Ager AL Jr, Morris VC, May RG (1989) Qinghaosu, dietary vitamin E, selenium, and cod-liver oil: effect on the susceptibility of mice to the malarial parasite Plasmodium yoelii. Am J Clin Nutr 50: 346-352.
- Bonnet M, Broek I, van Herp M, Urrutia PP, van Overmeir C, et al. (2009) Varying efficacy of artesunate+amodiaquine and artesunate+sulphadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria in the Democratic Republic of Congo: a report of two in-vivo studies. Malar J 8: 192.
- Rwagacondo CE, Niyitegeka F, Sarushi J, Karema C, Mugisha V, et al. (2003) Efficacy of amodiaquine alone and combined with sulfadoxine—pyrimethamine and of sulfadoxine pyrimethamine combined with artesunate. Am J Trop Med Hyg 68: 743-747.
- Karema C, Fanello CI, van Overmeir C, van Geertruyden JP, van Doren W, et al. (2006) Safety and efficacy of dihydroartemisinin/ piperaquine (Artekin) for the treatment of uncomplicated Plasmodium falciparum malaria in Rwandan children. Trans R Soc Trop Med Hyg 100: 1105-1111.
- World Health Organization (2006) Guideline for the treatment of malaria. WHO Geneva; 2006. WHO/HTM/MAL/2006.110.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13841
- [From(publication date):
specialissue-2012 - Apr 21, 2025] - Breakdown by view type
- HTML page views : 9287
- PDF downloads : 4554