Research Article Open Access
Efficacy and Bioremediation Enhancement Potential of Four Dispersants Approved For Oil Spill Control in Nigeria
| Temitope Olawunmi Sogbanmu* and Adebayo Akeem Otitoloju | |
| Ecotoxicology Unit, Department of Zoology, Faculty of Science, University of Lagos, Akoka, Lagos, Nigeria | |
| Corresponding Author : | Temitope Olawunmi Sogbanmu Ecotoxicology Unit, Department of Zoology Faculty of Science, University of Lagos, Akoka, Lagos, Nigeria Tel: +234(1)8985313 E-mail: topesho@gmail.com |
| Received: October 28, 2011; Accepted: February 03, 2012; Published: February 05, 2012 | |
| Citation: Sogbanmu TO, Otitoloju AA (2012) Efficacy and Bioremediation Enhancement Potential of Four Dispersants Approved For Oil Spill Control in Nigeria. J Bioremed Biodegrad 3:136. doi:10.4172/2155-6199.1000136 | |
| Copyright: © 2012 Sogbanmu TO, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The efficacy of four dispersants using the modified swirling flask method and their potential to support bacterial growth during simulated oil spill control were evaluated. On the basis of the derived efficacy ratios, OC/TT/OSI was the most effective dispersant tested followed by DS/TT/066, Biosolve and CCOD. The results of the bioremediation studies revealed that the dispersant, Biosolve supported the highest bacterial growth followed by CCOD, DS/TT/066 and OC/TT/OSI. Determination of left-over hydrocarbon content in dispersant-treated media showed that level of remediation was highest in media treated with DS/TT/066 followed by OC/TT/OSI, CCOD and Biosolve. Correlation analysis of bacterial growth with hydrocarbon content in assay media revealed that the dispersant, CCOD had the highest Bioremediation Enhancement Factor (-0.96) followed by Biosolve (-0.88), DS/TT/066 (-0.31) and OC/TT/OSI (0.15). The implications of these results in choosing dispersants that can enhance faster biodegradation of spilled crude oil in aquatic ecosystems were discussed.
| Keywords |
| Crude oil; Dispersants; Bioremediation; Oil spill control |
| Introduction |
| Crude oil spill in the environment has extensive harmful effects and requires the deployment of various control and/or clean-up strategies which include the use of dispersants that act by breaking up the oil slick into smaller droplets, thus ensuring easy dispersal and/or mixing in aquatic ecosystems. By decreasing the size of the oil droplets and dispersing the droplets in the water column, the oil surface area exposed to the water increases and natural breakdown of the oil is enhanced. Therefore these chemical dispersants have the potential to be effective tools in the management of crude oil spilled in the marine environment. However, several important factors must be considered in deciding which of the chemical dispersants to use. One of such factors is the toxicity of the dispersants on organisms in the environment. Fingas [1] reported that of the recent toxicity studies, most researchers (about 75%) found that chemically-dispersed oil was more toxic than physically-dispersed oil. Another factor is the physical effectiveness of the dispersant. Dispersant effectiveness is defined as the amount of oil that the dispersant puts into the water column compared to the amount of oil that remains on the surface. Investigations on the emulsification potential or efficacy of dispersants have shown that various dispersants vary in their efficacy [2]. While most test systems generally provide higher effectiveness data for easily dispersed oils, results can become highly disparate when testing more viscous products of heavy oils or weathered crudes or when attempting to correlate laboratory data with dispersant effectiveness observations from the field [3,4]. As a result, some regulatory bodies have introduced the Swirling Flask Test (SFT) as the standard method for evaluating the comparative effectiveness of oil spill clean-up agents on petroleum products. This implementation involved modifications undertaken to address problems encountered with the existing SFT procedure as specified by the United State Environmental Protection Agency (EPA) [5]. |
| Biodegradation is the major mechanism for removal of spilled petroleum hydrocarbons from the marine environment. For example, Wolfe et al. [6] estimated that from the time of the Exxon Valdez oil spill in 1989 until the fall of 1992, about 50% of the spilled oil was biodegraded either in the water column or in the intertidal sediments. Studies on the effect of dispersants on the fate of dispersed oil has often been conflicting with some workers proposal that dispersants had little effect on oil biodegradation, some suggested a positive effect and others noted a negative effect [7]. It has been suggested that dispersants tend to increase oil biodegradation by increasing the surface area for microbial attack and encouraging migration of the droplets through the water column making oxygen and nutrients more readily available [8]. Dispersants also may enhance biodegradation of bulk crude oil by increasing the bioavailable fraction of hydrocarbon. This is accomplished through mobilization of adsorbed hydrocarbon or by increasing its effective aqueous solubility [9]. However, studies on the effect of dispersants used in Nigeria and under Nigerian conditions on the natural biological remediation process initiated by microorganisms (hydrocarbon-utilizing) on crude oil has received little attention. The objectives of this study therefore are to determine the efficacy of four dispersants (DS/TT/066, Crystal Clear Oil Dispersant (CCOD), OC/ TT/OSI and Biosolve) available in the Nigerian market for oil spill control using the Modified Swirling Flask Method and to establish their effectiveness on the bioremediation of crude oil by indigenous bacteria species in order to achieve a possible integrated approach to crude oil spill control using both chemical and biological means. |
| Materials and Methods |
| Test flask |
| A sealed test flask made of glass was designed with stopcocks placed both in the applicator top and near the base of the flask for subsurface sampling according to Blondina et al. [5]. A Scientific Shaker Table (IKA HS 260 Basic model) was used to provide turbulence to the test samples. |
| Test chemicals |
| Crude oil: Forcados light crude oil used for this experiment was obtained from Shell (SPDC) production platform in Forcados, Burutu Local Government Area of Delta State, Nigeria. The physico-chemical properties of the crude oil include: sulphur content = 0.2%, API Gravity = 60/60F, rapid vapour pressure = 2.5 psi and pour point = 25. It was stored in a sealed plastic vessel in the laboratory at room temperature and used within a period of 30 days. |
| Dispersant I (DS/TT/066): Brown-coloured liquid containing surfactant mixed with a hydrocarbon solvent |
| Dispersant II (Crystal Clear Oil Dispersant (CCOD)): White milky liquid, non-ionic emulsifier, viscosity at 25°C = 2000 to 4000cP (Brookefield DV-II + Pro viscometer, spindle LV-4, Speed 10 RPM), pH at 1% solution = 6.6 to 8.4. |
| Dispersant III (OC/TT/OSI): Blue-coloured liquid, moderate alkaline (pH = 8.8), density = 0.9877 gcm-3. |
| Dispersant IV (Biosolve): This is a water-based, biodegradable hydrocarbon mitigation agent approved for oil spill control in Nigeria. The properties include: flash point = NA; water-based > 93.3°C; pour point = 0.5°C; viscosity = 490 cP; specific gravity = 1.025 at 15.5°C; pH = 9.37 ± 0.5; surface active agent, confidential; solvents, confidential; additives, confidential; and solubility in water, complete. |
| Efficacy studies |
| Into the flask was measured 120ml of fresh water. A ‘one-drop’ method was employed for preparing dispersed oil solutions [10] and test materials were delivered with a 1 ml syringe (improvised positive displacement pipette). After 90 μl of oil was delivered via the test flask’s upper stopcock, 10 μl of dispersant (Crude Oil: Dispersant Ratio = 9:1) was delivered in a single volume through the same stopcock. Swirling was then immediately initiated at a speed of 150 ± 5 rpm (revolutions per minute) for 10 minutes using the shaker table at laboratory temperature of 26 – 27°C. After swirling, a settling time of 10 minutes was allowed before extracting the sample to be analyzed [11]. From each sample mixture was obtained 100 ml by pouring through the top of the flask into a 120 ml bottle. This was stored in the refrigerator until analysis. The Total Hydrocarbon Concentration (THC) in the samples was analyzed by Gas Chromatography/Flame Ionization Detector (GC/FID). |
| Bioremediation studies |
| Mixtures containing crude oil and dispersants at ratio 9:1 of the test compounds were prepared and made up to 5 ml/l. For each mixture, the proportion of each constituent compound dictated by the ratio was computed and measured out. Crude oil was applied to the substrate before transferring the dispersant at the desired concentration into the bioassay tank. There were Two (2) replicates per mixture and these were exposed over a period of Thirty (30) days. |
| Isolation of hydrocarbon-utilizing bacteria: Into the test tube was pipetted 9 ml of distilled water and the tubes were covered with aluminium foil wrapped cotton wool and autoclaved at 121°C for 15 minutes. Minimal Salt Medium (MSM) was prepared using the concentrations stated above and to which was added Agar (solidifying agent). This was brought to boil in a water bath and autoclaved at 121°C for 15 minutes. The Agar was allowed to cool and then poured into sterile petri dishes. For isolation, 1 ml of sample mixture was taken with the aid of a syringe and transferred into a test tube containing 9 ml of distilled water (after cooling). Ten-fold serial dilution was done up to 7 times (that is, 107) and then 0.1 ml of sample (7th dilution) was injected onto sterile plates containing cooled agar. The sample was spread on the surface with the aid of a glass spreader for even distribution of colonies or cells. A sterile filter paper to which crude oil had been added and spread evenly was placed on the inside top cover of the petri dish before covering. These plates were stored in an incubator at room temperature for 5 to 10 days (optimal growth time for hydrocarbon utilizing Bacteria). |
| Total Hydrocarbon Concentration (THC) in samples: With the aid of a syringe, 100 ml of sample was obtained and transferred into a 120 ml bottle. The THC in the samples were determined by Gas Chromatographic (GC) analysis. Quantitative analysis was carried out with a HP3365 Chemstation software and utilized automated integration to obtain total area counts of chromatogram peaks. The data for Total Hydrocarbon Concentration (THC) measured in the samples by GC/FID were expressed in nanogram per microlitre (ng/μl). For data quality assurance, samples were run three times to obtain a mean value and a standard reference sample of known hydrocarbon content was analysed after every five measurements. The results indicated good correlations between the certified value for the reference sample and the analytical values obtained. Recovery of the certified standard reference samples was about 96% - 99%. |
| Statistical analysis |
| The Efficacy Ratio was calculated as follows: |
| Results of Total Hydrocarbon-Utilizing Bacteria (THUB) were expressed in colony forming units per ml (cfu/ml). The data for total hydrocarbon concentration (THC) in samples for the bioremediation studies were expressed in milligrams per litre (mg/l). Bioremediation Enhancement Factor (BEF) was calculated and expressed as Pearson correlation (r). The formula is as follows: |
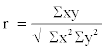 |
| Statistically significant BEF (P < 0.05) was determined using analysis of variance (ANOVA). All data were analyzed using GraphPad Prism 5 software. |
| Results and Discussion |
| The results of the efficacy studies revealed that dispersant OC/ TT/OSI treated medium contained the lowest surface concentration of hydrocarbon (8.96 ng/μl) followed by DS/TT/066 (10.25 ng/μl), Biosolve (19.73 ng/μl) and CCOD (674.43 ng/μl). Indicating that OC/ TT/OSI was the most effective test dispersant causing the dispersal of crude oil and transferring the spilled oil from the surface into the water column. On the basis of the derived efficacy ratios, OC/TT/OSI had the highest efficacy ratio of 146.31 followed by DS/TT/066 (127.90), Biosolve (66.44) and CCOD (1.94) (table 1). The chromatographs are shown in (Figures 1-5). This result is in consonance with the findings of Swannell et al. [12] which stated that the addition of dispersants greatly increased the level of oil dispersion in the experimental apparatus, encouraging the rapid formation of a much higher number of small droplets than was noted in microcosms subjected to physical forces only. |
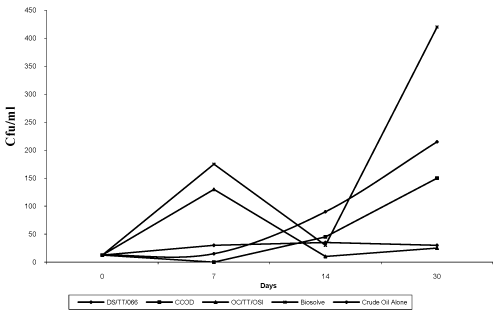
| The results of the bioremediation experiment showed Total Hydrocarbon-Utilizing Bacteria (THUB) values in cfu/ml (x107) ranging from 12.5 to 35 (CO : DI), 12.5 to 130 (CO : DIII), 12.5 to 150 (CO : DII), 12.5 to 215 (Crude Oil Alone) and 12.5 to 420 (CO : DIV) over the period of 30 days. The mixture containing Biosolve had the highest THUB after 30 days of observation followed by Crude Oil Alone, Crude Oil: CCOD, Crude Oil : DS/TT/066 and Crude Oil : OC/ TT/OSI had the least (Figure 6). The tested dispersants all supported the growth of indigenous bacteria, confirming that bacteria could utilize the nutrients available within the test media. This agrees with the report of Swannell et al. [12]. However, the pattern of growth of bacteria was slightly different depending on the dispersant. OC/TT/ OSI encouraged a normal sigmoidal growth curve as indicated by the maximal growth rate occurring shortly after the start of the experiment (Figure 6). |
| In contrast, for biosolve, a diauxic (biphasic) growth pattern was seen with an initial phase of growth followed by a temporary slow down and then a second phase of more rapid growth. As such the maximum growth rate was recorded later in the experiment (Figure 6). Diauxic growth often reflects the biodegradation of different substrates by bacteria and in this context, may reflect the decomposition of a component or components of the dispersant, which then facilitates additional growth. On the other hand, DS/TT/066 and CCOD showed progressive growth. |
| Results of the total hydrocarbon concentration in the test media showed values in mg/l ranging from 2018 to 0.02 (Crude oil : DS/ TT/066), 2018 to 0.11 (Crude oil : OC/TT/OSI), 2018 to 0.18 (Crude oil : CCOD), 2018 to 1.16mg/l (Crude Alone) and 2018 to 1.62 (Crude oil : Biosolve) over the period of 30 days. The mixture containing Biosolve had the highest THC left-over concentration (lowest effectiveness) followed by Crude Oil Alone, Crude Oil : CCOD, Crude Oil : OC/ TT/OSI and Crude Oil : DS/TT/066 with the least THC (highest effectiveness) by the 30th day. The THC results showed a decrease over the 30 day period in all the test media (Table 2). This reduction in the THC reflects that biodegradation occurred in the various media over the period of observation. Decrease in THC was more noticeable from the 14th to the 30th day in all the test media, indicating that the loss of THC increased with increasing period of exposure. This corroborates the report of Zahed et al. [13] that long exposure of oil to biodegradation processes was needed to maximize benefits of the breakdown process. |
| On the basis of the derived Bioremediation Enhancement Factor (BEF), the dispersant CCOD had the highest negative value of -0.96 followed by Biosolve (-0.88), DS/TT/066 (-0.31) and OC/TT/OSI (0.15) (Table 2). All the test mixtures except that containing the dispersant OC/ TT/OSI showed a negative correlation, that is, the THUB values were inversely proportional to the THC values. However, test of significance (ANOVA) showed that amongst the mixtures, there was no significant (P>0.05) differences in the BEF except for that containing dispersant, CCOD which showed a significant negative correlation (P < 0.05). This reveals that the CCOD was found to have the highest bioremediation enhancement ability by supporting the greatest bacterial growth with corresponding loss of THC in treated media. |
| This result is in consonance with the report of Varadaraj et al. [7] which stated that some dispersants had a positive effect on oil biodegradation. According to Mulyono et al. [8] the mechanism through which this increased oil degradation is achieved has been suggested to be the increasing of the surface area for microbial attack by the dispersant and encouraging migration of the droplets through the water column making oxygen and nutrients more readily available. Also, Venosa and Holder [14] reported that degradation was much more rapid for dispersed oil than for non-dispersed oil, because in the non-dispersed control, the microbial culture first had to generate its own biosurfactant to emulsify the oil before substantial degradation could occur. |
| Furthermore, the non-significant correlation (P > 0.05) that corresponds to no effect on bioremediation potential recorded for the other dispersants is consistent with the report of Fingas [1] which stated that some studies on biodegradation showed no observed effects with the addition of dispersants or surfactants. Also, Swannell et al. [12] reported that dispersants encouraging the formation of the largest numbers of small droplets did not necessarily promote the most rapid growth of hydrocarbon-degraders. This may be attributed to the toxicity of the dispersants to the microbial community. The toxic nature of some of the dispersants can be attributable to the base solvents and other additives. |
| In conclusion, the results, showed potentials in integrating both chemical, using dispersants and biological, utilizing hydrocarbondegrading bacteria means in speeding up the remediation of oil spills in aquatic ecosystems. However, there is need for further research on this integration in field studies in order to corroborate and ascertain the results of laboratory studies. |
| Acknowledgements |
| The authors are grateful to the Department of Petroleum Resources (DPR) in Nigeria and Mr. Kenneth Akpan for assistance in obtaining crude oxil and dispersants used for the study. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14845
- [From(publication date):
February-2012 - Nov 16, 2025] - Breakdown by view type
- HTML page views : 10157
- PDF downloads : 4688
