Effects of Propofol on Epileptiform Activity and Hippocampal Morphology in Febrile Convulsions and Pilocarpine Induced Seizures
Received: 05-Jun-2012 / Accepted Date: 21-Aug-2012 / Published Date: 23-Aug-2012 DOI: 10.4172/2161-0681.1000123
Abstract
Abstract
Epidemiological and recent prospective analyses of long Febrile Seizures (FS) support the idea that such seizures
can provoke Temporal Lobe Epilepsy (TLE) in some children. Because of the high prevalence of these seizures, if
epilepsy was to arise as their direct consequence, this would constitute a significant clinical problem. Animal studies
have revealed that exposure of hippocampal neurons to FS early in life, particularly prolonged or frequently repetitive
FS, or together with brain malformation, may lead to sustained dysfunction of these cells including long-term memory
impairment or epileptogenesis, in spite of the absence of neuronal damage. We established a hyperthermia model
of febrile convulsions in young adult rats, and studied the effects of propofol treatment general anesthetic acting via
GABA-A receptor on epileptiform activity and the morphological features of medial temporal lobe.
We found statistically significant neuronal losses in the CA1, CA3 and dentate gyrus regions of hyperthermiaapplied
rats as compared to the control rats. We also observed that propofol administration suppressed epileptic
discharges in EEG and prevented clinical seizures behaviors. It took a median of 11 (range, 6-40) minutes for propofol
to stop seizures. We conclude that prevention of epilepsy-related damage can be achieved via epileptogenesis-based
clinical approaches, and that propofol is an effective agent in the hyperthermia-induced status epilepticus model.
Keywords: Epileptogenesis; Febrile convulsions; Pilocarpine; Hippocampus; Propofol
312009Introduction
Temporal Lobe Epilepsies (TLE), which represent approximately 60% of all partial epilepsies, are a group of medical disorders in which patients experience recurrent epileptic seizures arising from temporal lobes of the brain. It has been shown that most of these patients undergo an initial precipitating injury occurred early in life, usually before the age of 4 years. In addition to trauma, birth injuries and encephalitis, Febrile Seizures (FS), which develop in 2–5% of children with fever, have been regarded as a leading initial precipitating injury that provoke seizure in the absence of intracranial infection or any other well-defined causes, and thus has been accepted as a potential etiologic factor of TLE in human [1,2]. Although simple FS are thought to be benign, experimental and clinical evidence support that the risk of developing epilepsy after FS increases, if it is prolonged, multiple and lateralized [3].
Analyses of adults with TLE also demonstrate a high prevalence (30-50%) of a history of FS during early childhood [4]. Therefore, models of FS in the immature rat have been developed [5,6] and enhanced susceptibility to pilocarpine-induced seizures has been reported in these models [7]. Although pathophysiology of TLE is still unclear, one speculated mechanism is mutations of the genes that lead to defective sodium channels which stay open for too long, thus making the neurons hyper-excitable. Large amounts of glutamate released from these neurons triggers excessive calcium release from the postsynaptic glutamatergic neurons, and excessive calcium release can be neurotoxic to the affected cell. The hippocampus, which contains a large volume of glutamatergic neurons, is especially vulnerable to epileptic seizure, subsequent spread of excitation, and possible neuronal death [8]. The most susceptible neurons in hippocampus are reported to be those in the hilus of the Dentate Gyrus (DG) [9]. Another possible mechanism of TLE involves mutations leading to a highly localized failure of γ-aminobutyric acid (GABA)-mediated inhibition. There is compelling evidence to suggest that GABA neuron dysfunction plays a causal role in seizures and status epilepticus since baby food deficient in GABAsynthesizing enzyme cofactor pyridoxine caused seizures which can be terminated by pyridoxine replacement, compounds that interfered with GABA synthesis produced status epilepticus in normal animals [10], and status epilepticus initiated by excitatory chemoconvulsants and electrical stimulation decreased dentate granule cell inhibition and overcame paired pulse inhibition just before epileptiform activity emerged [9,11].
Propofol is an intravenous alkyl-phenol anesthetic that functions through potentiation of GABA-A receptor activity, thereby slowing the channel-closing time [12,13] and acting as a sodium channel blocker [14]. A retrospective study of 33 children with refractory status epilepticus indicated that propofol was more effective than thiopental in terminating seizures [15] and studies in adults indicated that propofol terminated seizures in 67% of patients [16]. With this information in mind, we therefore aimed at investigating the effects of propofol on epileptiform activity and hippocampal morphology in rat models of seizure induced by hyperthermia and/or pilocarpine induced Status Epilepticus (SE), alone or in combination.
Materials and Methods
Study population
All experimental protocols were approved according the guidelines of the Animal Care, Use and Ethics Committee of Uludag University and carried out in accordance with the European Communities Council Directive. The rats were housed one per cage at 20-24°C under a 12:12-h light: dark regime and received Standard laboratory chow and tap water ad libitum. Rats were randomly assigned into control (n = 28) and treatment groups (n = 21). Rats in both control and treatment groups were then divided into 4 (groups C1-C2-C3-C4) and 3 (groups T1-T2-T3) sub-groups, respectively, of which details are given in (Table 1).
| Procedures/ Solutions Applied | Seizure Severity (mean) | SE Behavioral Score (mean) | SE Latency (min) | Survival Rate (%) | Propofol Dose Response Latency (min.) | ||
|---|---|---|---|---|---|---|---|
| Control Groups | C1 (n=7) | Saline Only | 100% | ||||
| C2 (n=7) | Hipertermia + Saline | 2 | 100% | ||||
| C3 (n=7) | Pilocarpine + Saline | 5 | 29 | 100% | |||
| C4 (n=7) | Hipertermia + Pilocarpine + Saline | 2,14 | 5 | 26 | 100% | ||
| Treatment Groups | T1 (n=7) | Hipertermia + Propofol | 1,85 | 100% | Applied* | ||
| T2 (n=7) | Pilocarpine + Propofol | 5 | 28 | 100% | 8,6 | ||
| T3 (n=7) | Hipertermia + Pilocarpine + Propofol | 1,85 | 5 | 25 | 100% | 13,4 |
Table 1: Comparative results are applied to groups of transactions.
Hyperthermia protocol
Rats subjected to hyperthermia were exposed to 10 recurring FS every two days starting from day 21st following their birth. Experimental febrile seizures were induced by using a hot-water bath with the dimensions of 30x30x60 cm. The hot water bath was filled with water ensuring that the heads of the immature rats stay out of the water when they stand up; the water was heated up to 45°C and the temperature was kept constant throughout the experiments [17]. The rats were kept in water without movement restriction until the first sign of a seizure was observed (app. 2-4 min). As soon as the first signs of seizure were observed, the rats were taken immediately out of the water and placed in a plexiglas observation cage. Seizure phenomenon was observed, including the latency, the duration and severity of seizures and the temperature of rats. The seizure latency was the time segment recorded from submergence of rats to the onset of seizure signs while seizure duration was the recorded time period starting from the onset of seizure signs to the phase which the rats recover normal, free and conscious movements. Seizure severity grade was scored with regard to the animals’ involuntary muscular contractions and behavioral response (Grade 0, no convulsive response; Grade 1, facial clonic jerks; Grade 2, upper extremities clonic jerks; Grade 3, rearing; Grade 4, rearing and falling). Body temperatures of the rats were also recorded using a rectal probe (SKT 100B, Biopac Systems Inc, CA) before exposure to hot water and at seizure onset. Hyperthermia-applied immature rats were then observed under standard laboratory care conditions until they reach 250-350 grams of weight.
Pilocarpine induced status epilepticus protocol
A total of 14 rats from both control and treatment groups (hyperthermia applied and non-applied groups, n=7 for each) were injected with 380 mg/kg pilocarpine HCI in saline intraperitoneally (Sigma-Chemical Co., St. Louis) [8,18]. ECoG waves were continuously recorded starting from 15 minutes before the pilocarpine administration until the detection of SE onset and SE was accepted as the epileptiform activity that lasted for a minimum of 30 minutes.
ECoG recordings for a period of 15 minutes were also obtained at 90 minutes and 24 hours (prior to sacrifice) after pilocarpine injection. All the rats in the four groups were monitored with regard to the (1) SE development, (2) SE latency, (3) behavioral SE severity and finally (4) 24-hour survival. The behavioral severity was determined according to the scoring system for focal seizures with secondary generalization for pilocarpine-induced seizures as described previously [17,19]. Score 0: no convulsive response; score 1: staring with mouth clonus; score 2: automatisms; score 3: unilateral forelimb clonus; score 4: bilateral forelimb clonus; score 5: bilateral forelimb clonus with rearing and falling; score 6: tonic–clonic seizure (Table 2). Seizures were further classified into 2 categories: (1) partial seizures (scores 1–3) or (2) secondarily generalized seizures (scores 4–6).
| Groups | Degeneration | CA1 | CA3 | CA4 | DG | p (Comparing to control G1) |
|---|---|---|---|---|---|---|
| Group 2 | Grade 0 | 10.58 ± 0.03 | 10.86 ± 0.09 | 11.97 ± 0.09 | 14.64 ± 0.05 | |
| Grade 1 | 1.92 ± 0.06 | 4.43 ± 0.07 | 4.32 ± 0.80 | 3.83 ± 0.03 | p<0.05 | |
| Grade 2 | 1.56 ± 0.02 | 2.75 ± 0.01 | 4.58 ± 0.62 | 4.91 ± 0.91 | ||
| Group 3 | Grade 0 | 10.52 ± 0.01 | 10.65 ± 0.01 | 11.82 ± 0.01 | 14.57 ± 0.01 | |
| Grade 1 | 1.77 ± 0.01 | 4.27 ± 0.01 | 4.43 ± 0.01 | 3.82 ± 0.03 | p<0.05 | |
| Grade 2 | 1.51 ± 0.01 | 2.74 ± 0.01 | 6.08 ± 0.02 | 7.03 ± 0.02 | ||
| Group 4 | Grade 0 | 10.59 ± 0.05 | 10.65 ± 0.01 | 12.00 ± 0.12 | 14.63 ± 0.02 | |
| Grade 1 | 1.81 ± 0.03 | 4.33 ± 0.04 | 4.37 ± 0.02 | 3.80 ± 0.04 | p<0.05 | |
| Grade 2 | 1.53 ± 0.01 | 2.79 ± 0.02 | 5.35 ± 0.51 | 5.92 ± 0.74 | ||
| Group 5 | Grade 0 | 10.69 ± 0.24 | 10.40 ± 0.15 | 12.07 ± 0.18 | 14.79 ± 0.16 | |
| Grade 1 | 1.85 ± 0.06 | 4.35 ± 0.04 | 4.24 ± 0.09 | 3.77 ± 0.09 | p<0.05 | |
| Grade 2 | 1.49 ± 0.01 | 2.95 ± 0.17 | 4.83 ± 0.60 | 5.43 ± 0.77 | ||
| Group 6 | Grade 0 | 10.57 ± 0.02 | 10.64 ± 0.10 | 12.61 ± 0.29 | 14.39 ± 0.07 | |
| Grade 1 | 2.01 ± 0.08 | 4.40 ± 0.19 | 4.83 ± 0.19 | 3.86 ± 0.06 | p<0.05 | |
| Grade 2 | 1.68 ± 0.07 | 2.67 ± 0.02 | 4.90 ± 0.43 | 4.59 ± 0.85 | ||
| Group 7 | Grade 0 | 10.40 ± 0.07 | 11.48 ± 0.49 | 12.04 ± 0.14 | 14.29 ± 0.13 | |
| Grade 1 | 1.85 ± 0.03 | 4.30 ± 0.02 | 4.28 ± 0.07 | 3.89 ± 0.04 | p<0.05 | |
| Grade 2 | 1.63 ± 0.06 | 2.39 ± 0.20 | 5.22 ± 0.36 | 6.78 ± 0.17 | ||
| All groups | p (Between sides) | |||||
| Right hemisphere | Grade 0 | 10.47 ± 0.11 | 10.87 ± 0.08 | 9.93 ± 0.42 | 14.82 ± 0.17 | p>0.05 |
| Grade 1 | 1.40 ± 0.04 | 1.26 ± 0.09 | 1.29 ± 0.06 | 1.44 ± 0.09 | ||
| Grade 2 | 1.06 ± 0.06 | 1.24 ± 0.12 | 1.24 ± 0.04 | 1.10 ± 0.10 | ||
| Left hemisphere | Grade 0 | 10.47 ± 0.10 | 10.86 ± 0.08 | 10.10 ± 0.40 | 15.52 ± 0.27 | |
| Grade 1 | 1.34 ± 0.03 | 1.10 ± 0.06 | 1.26 ± 0.06 | 1.40 ± 0.06 | ||
| Grade 2 | 1.04 ± 0.06 | 1.60 ± 0.06 | 1.13 ± 0.02 | 1.12 ± 0.01 |
Table 2: Histopathologic evaluation; means and SEMs of the neuron numbers in unit area (Grade 0, normal appearance; Grade 1, moderately degenerated; Grade 2, highly degenerated neurons with pyknotic nuclei and dark appearances).
Administration of propofol
All the rats in treatment groups, which were subjected to (1) hyperthermia induced FS, (2) pilocarpin induced SE and (3) a combination of hyperthermia induced FE and pilocarpin induced SE, respectively were injected with i.p. propofol (Propofol %1 Fresenius, 200 mg amp, Fresenius Kabi, Sweden). In the two pilocarpin induced SE groups, 1mg/kg propofol was initially administrated i.p. as post-status loading dose 5 minutes after the onset of SE. 2 mg/kg boluses, repeated every following 5 minutes, were then given until the seizures were discontinued or a total maximal dose of 10 mg/kg was achieved. After the seizures were terminated, maintenance doses started from 2 mg/kg and gradually decreased by 0.5 mg/kg every 6 hours were administered to conclude in 24 hours [20]. In the hyperthermia induced FS group, propofol was given in a similar approach although SE was not observed in this group.
Electrocorticography (ECoG)
For electrophysiological recordings, under sterile surgical conditions and the sodium thiopental anesthesia (50 mg/kg, ip), three 0.6 mm stainless steel screws (Plastics One Inc, Roanoke, VA) were implanted as epidural electrodes in the undersized holes drilled in the skull as previously described in detail [21] seven days prior to surgical intervention. Briefly, one of the electrodes was placed on primary somatosensory (S1) cortex (2.5 mm posterior to bregma and 2.5 mm laterally from the sagittal suture on the right side). Remainder two of the electrodes were installed bilaterally above the frontal sinus (10 mm anterior to bregma and 1 mm lateral to each side of midsagital plane); the one on the left side serving as the ground. Electrodes were wired to a receptacle (MS333/2A, Plastics One, USA), fixed to the skull using dental cement and the skin was sutured. ECoG recordings were taken in a special plastic and transparent cage (30x30x35 cm) and were obtained using S1 electrode referred to ipsilateral frontal electrode. The signals amplified by an amplifier (EEG100C, Biopac Systems Inc, CA, USA) and recorded (band pass 0.5–130 Hz, 120 second) by a data acquisition system (Mp150 Data Acquisition system, Biopac Systems Inc, CA, USA) and processed with Acknowledge 3.7 software (Biopac Systems Inc, CA, USA). The power spectra obtained by use of Fast Fourier transform (FFT) were divided into 0.5-3 Hz (delta), 4-8 Hz (theta), 8-13 Hz (alpha), and 13-30 Hz (beta) frequency bands and Median Power Frequency (MF=the frequency below which 50% of the power) were also calculated using data acquisition software.
Histopathological Evaluation
After the last monitoring procedure was completed at 24th h, the rats were given a lethal dose of anesthetic and perfused through the left cardiac ventricle with 0.9% NaCl followed by a fixative solution containing 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were then removed and immersed in the same fixative for 48 hours. Each slice was then processed into paraffin wax, cut coronally into 7 μm thick sections with reference to Bregma - 2.8 [22]. Every fifth section was stained using the cresyl violet staining protocol. Sections were examined for pyramidal neuronal damage at cornu ammonis areas (CA); CA1, CA3, and dentate gyrus regions. Hippocampal cell loss was scored in a total of 49 rats that could survive for 24 hours as previously described [18].
Statistical Analysis
Categorical variables were given in numbers and percentages and analyses of the study were made in SPSS (ver. 13.0, Chicago, IL). Kruskal Wallis test was used to compare the effect of SE and propofol treatment on seizure severity grades, behavioral SE severity and hippocampal cell numbers among groups. Post-hoc comparisons were made using the Mann-Whitney U test. In each test, the data are expressed as the mean ± S.E.M. and p<0.05 is accepted as statistically significant.
Results
In the first stage of the study, we observed the characteristic behaviors of febrile convulsions induced by hyperthermia made by hotwater bath immersion method in all rats. The average seizure severity of the 28 hyperthermia applied young adult rats in both control (C2 and C4) and treatment groups (T1 and T3) was estimated as 1.96. Seizure severity scores, estimated as described earlier, ranged between 1 and 3, and the average seizure severity of the hyperthermia applied young adult rats in both control and treatment groups are presented in (Table 1).
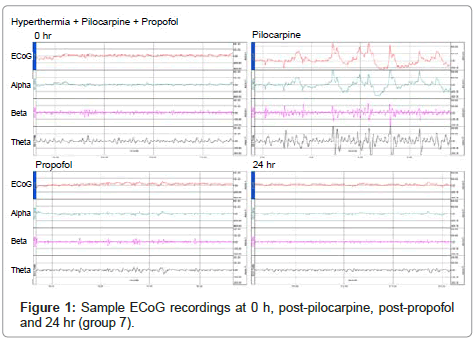
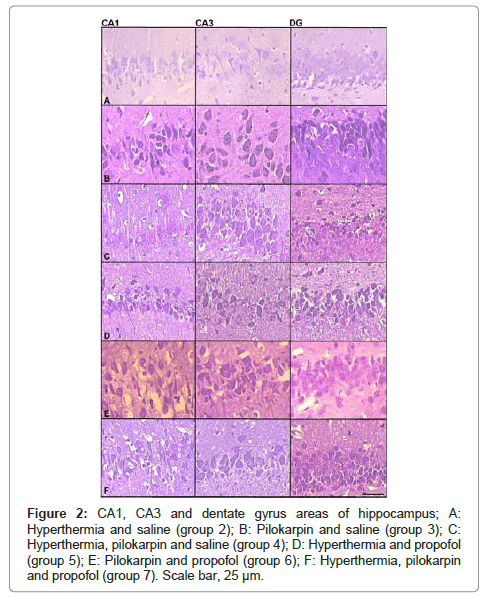
When convulsing doses of pilocarpine (380 mg/kg) were administered to rats in groups C3, C4, T2 and T3, all rats developed SE with a mean latency of 27.25 minutes (ranging from 16 to 41 minutes) and all rats survived the 24 hour follow-up period following the pilocarpine administration. Average behavioral SE score of these rats was 5 and isolated spikes on ECoG recordings were encountered in all rats that developed SE (Figure 1). The behavioral SE score, SE latency, survival rates observed in both control and treatment groups are also presented in Table 1. When post-status propofol responses in a total of 14 rats from T2 and T3 groups were evaluated, an average propofol dose response was observed at 11 (6–40) minutes with an average of 2.4 doses administrated. Average propofol dose response latency in groups T2 and T3 were, 8.6 and 13.4 minutes, respectively (Table 1) and suppression of seizure activity was evidenced by the suppression burst pattern observed in ECoG recordings (Figure 1). There were no animals that did not respond to propofol and none of them displayed seizure activity in ECoG recordings obtained at 24th hour, although seizure was terminated following administration of 8 doses in a single rat in T2 group. Propofol was also applied to the rats in the T1 group in order to investigate the possible effect of propofol onto hippocampal formation, although experimental SE was not induced in this group. In groups C3 and C4, which pilocarpine was administered but not treated with propofol, SE continued unabated and persisted during the entire 24 hr follow-up period. Histopathological evaluations revealed significantly increased neuronal degeneration in all groups compared to naive controls (group C1) in all hippocampal regions investigated (p<0.05, for all comparisons). However, no significant difference was observed between the groups C2, C3, C4, T1, T2 and T3. Neuron numbers subdivided according to their degeneration grade and examples of micrographs from which histopathologic evaluation is performed, are presented in (Table 2) and (Figure 2), respectively.
Figure 2: CA1, CA3 and dentate gyrus areas of hippocampus; A: Hyperthermia and saline (group 2); B: Pilokarpin and saline (group 3); C: Hyperthermia, pilokarpin and saline (group 4); D: Hyperthermia and propofol (group 5); E: Pilokarpin and propofol (group 6); F: Hyperthermia, pilokarpin and propofol (group 7). Scale bar, 25 μm.
Discussion and Conclusion
Epileptogenesis can be described as an increase of excitability due to cellular and molecular changes in the brain following a brain damage and emergence of recurrent spontaneous seizures. Currently, answers to important questions the spectrum of changes in the neuronal network during epileptogenesis is a result of different etiologies are sought [23]. The high incidence of epilepsy development in humans– due to the impact of the seizures in the immature brain–results in the fact that while the brain completes its normal development, the damage caused by epilepsy maintains epileptogenesis [24].
Existence of FS in histories of patients with TLE indicates a possible disturbance in neuronal migration. When TLE tissue samples were examined, it was observed that the granular cell layer was more than normal and that a significant portion of these neurons acquired an extended bipolar form. As such, disturbed circuits occur in hippocampal formation in TLE [25]. In addition to cell damage and excessive branching increase in hippocampuses of TLE patients, lessening in GABA-A receptors and GABA-A subunits has been observed [26].
Seizures that occur in the early stages of life have been shown to cause Hippocampal Sclerosis (HS) and consequently TLE in a variety of studies. Prolonged FS have been observed to cause abnormal growth of neuron axon terminals and by creating sprouting in Mossy fibers, to formation of new synapses and recurrent circuits in the hippocampus [27].
Certain FS types and features are asserted to be associated with the type of the epilepsy that develops [28]. When they examined treatment resistant temporal lobe epilepsy surgery series, by the authors reported that the most common pathology is HS and they have encountered prolonged FS stories that can be held responsible for the etiology of epilepsy in 30% of this group. Experimental studies in rats show that hyperthermia stimulates epileptiform activity in hippocampus and that hyperthermia also stimulates seizures in rats, on which neuronal migration defect has been generated. Similar to the results obtained from animal experiments, evidence proving the presence of pre-seizure neuro-developmental hippocampal pathology in some cases with FS and it is asserted that FS occurs as this hippocampal pathology leads to febrile sensitivity [29]. We have applied the hyperthermia model developed by the authors for rats in our study as well [17].
Every other day, the rats were kept at 45°C for a maximum of 4 minutes and complex febrile seizures were created as the model. The average seizure severity result of the hyperthermia-applied animals in both the control and treatment groups was 1.96 (1-3). This result of ours has been found consistent with the study conducted by Klauenberg BJ and Sparber SB [5]. The EEG changes in pilocarpine-induced SE are defined as significant increase in theta rhythm and isolated horns in hippocampus, synchronization of hippocampal and cortical activities, and isolated electrographic seizures [30].
In our epilepsy model created via administration of 380 mg pilocarpine, SE accompanied by 100% EEG change occurred in the pilocarpine-applied 3rd, 4,th, 6th, and 7th groups. Injection of 300–400 mg/kg pilocarpine is reported to cause generalized convulsive SE at a rate of 83-100% in various studies. The SE latency in the study groups was found to be 27.25 (16–41) minutes. These findings of ours are consistent with those in the literature [8,18]. In the postmortem study conducted on 20 patients who died during or a short time after SE, the authors report that the hippocampus was apparently affected, and that, particularly in CA1, acute cerebral changes characterized by total neuronal loss were observed. Other studies demonstrated destructions in the CA1 and CA3 pyramidal cell layers in the hippocampus; in amygdala, thalamus, Purkinje cells in the cerebellum; and in the nerve cells in the corpus striatum and the middle cerebral cortical layers. In addition, the longer the duration of the seizure, the higher is the level of brain damage [8,31].
It was observed in studies made with Li-pilocarpine in young rats that while certain cells such as CA1-CA3 pyramidal and dentate hilar were sensitive, CA2 neurons and certain cells such as the dentate granular cells were resistant [31].
In the SE model we have constructed with pilocarpine, we have found statistical differences as compared with the control group in the morphological examination of CA1, CA3 and dentate gyrus. Via examining a TLE model of SE constructed by pilocarpine in our study, we have concluded that it is significant in terms of Mesial Temporal Sclerosis (MTS)-HS. When we examined the CA1, CA3 and dentate gyrus regions of hyperthermia-applied rats, we have encountered statistically significant neuronal losses as compared to the control rats, on which no procedures were conducted. This suggests that the complicated febrile convulsion created as a hyperthermia model can be a cause with regards to MTS and HS. The effect of the major inhibitor neurotransmitter GABA plays an important role in the termination of epileptic seizures [31]. Heat dependency of the anticonvulsant action that emerges as a response of GABA-A receptors to benzodiazepines and the fact that functional disorders in GABA-A receptors play a role in hyperthermic seizures experimentally created in rats are significant findings [32]. Although the use of many agents such as midazolam, propofol, high-dose thiopental or pentobarbital, IV valproate, topiramate, tiagabine, ketamine, isoflurane, and IV lidocaine for Resistant Status Epilepticus (RSE) treatment is suggested, construction of treatment protocols is quite difficult as there is a lack of prospective studies on this matter [33]. Nevertheless, midazolam or propofol infusions accompanied with Continuous EEG monitoring are among the preferred treatment methods. Both agents have been shown to be effective in the control of seizures in cases with RSE [20].
Ohmori et al. [34], have shown that propofol, with its anticonvulsant effect, inhibits epileptiform activity originating from the hippocampal region in their study on rats. In a study conducted on rabbits, propofol has been demonstrated to suppress epileptic discharges and it has clinically been shown to end seizures in seizure behaviors. In the mentioned study, the time to stop seizures by propofol is identified as 6 minutes and it is reported that seizures did not continue in the follow ups [35]. In another study that compares propofol with other benzodiazepines, it is reported that propofol acts fast, however, it causes relapses at a rate of 19-33%, and therefore, recurring doses might be useful regarding treatment [36].
In our study, we have observed that administration of propofol suppresses epileptic discharges in EEG and stops clinical seizures behaviors. It took 11 (6–40) minutes for propofol to stop seizures. This result is longer than what was reported in literature. When the seizures recurred in 2 lab animals after 4 hours, 1 mg/kg propofol was administered intraperitoneally as a load dose and 5 minutes later, a onetime repetitive 2 mg/kg bolus was administered and the seizure was stopped. Our study had a relapse rate of 14.3%, which made us consider that this is less than what was reported in the current literature and that this could be attributed to the administration of repetitive doses in our study.
There are a lot of evidences showing the close relation between epileptic seizures and body temperature. For example, high fever often provokes seizures in children. Some studies in recent years have revealed that excitotoxic brain damage and hypoxic hippocampal damage are associated with changes in brain temperature [37]. Experimental studies have demonstrated that electrophysiological epileptiform activity increases as temperature rises, whereas hypothermia reduces ictal discharges. Although most febrile convulsions are generally assumed to be of benign character, some authors currently argue that prolonged febrile seizures may cause brain damage or neurological sequela [38].
In another study, Lundgren et al. [39], investigated the effects of hypothermia and hyperthermia on brain damage in SE induced by flurothyl in rats. They showed that following SE lasting for 45 minutes, hypothermia (32.5°C) healed epileptic neuronal damage in the neocortex and globus pallidus, whereas hyperthermia (41°C) increased damage.
In our study, the high-dose (380 mg/kg) pilocarpine model is preferred due to its similarity to human TLE in terms of neuropathological damage and as they are resistant to various anticonvulsants. In the high-dose pilocarpine model, the fact that SE causes serious brain damage and high mortality rates within 24 hours has been shown in previous studies [40]. The seizures possibly start due to the deterioration of the balance between the excitatory and inhibitory neurotransmitters. This leads to the emergence of abnormal neural impulses. The hypothesis related to the degradation of the balance between excitation and inhibition in epileptogenesis is diminished GABA-ergic functions. In epileptic animal and human, blocking GABA-ergic neuronal transmission opens the way to increased excitation. The same findings are also true for seizures and SE. Studies on electrophysiological properties of GABA-A receptors on dentate granule cells show that as the duration of seizures prolongs, a significant change is observed in the sensitivity of receptors to diazepam. Postmortem studies in SE have proved the existence of severe neuronal damage in the temporal lobes [38].
The comparison of neuronal damage in the CA1, CA3 and dentate gyrus regions of the hyperthermia-applied rats, which were untreated and treated with propofol, yielded statistically significant results as compared with brain damage caused by SE induced with flurothyl which could be reduced through mild hypothermia (32.5°C) [39]. These results show that body temperature plays a significant role in the occurrence of epileptic seizures and associated neuronal damage. Although the responsible mechanisms are not fully understood, it can be seen that febrile seizures may lead to hippocampal neuronal damage.
Consequently, in our experimental animal model, in which hyperthermia was created and status epilepticus was induced with pilocarpine, the result that hyperthermia caused cell damage particularly in the hippocampus and dentate gyrus was obtained through comparisons with the control groups. We have concluded that propofol is effective in the status epilepticus model and that it is also efficient in our experimental model that constitutes grounds to epilepsy as a result of hyperthermia. As there is only a few studies in this field, administration of propofol in the treatment of status epilepticus that develops due to hyperthermia seems to be an approach that should be focused on. Prevention of damages associated with epileptic seizures may be improved through clinical approaches towards the root causes of epileptogenesis.
References
- Mathern, G. Babb T. and Armstrong , D. (1997) Mesial temporal lobe epilepsy. In: J. Engel, Jr and T. Pedley, Editors, Epilepsy: A Comprehensive Textbook, Lippincott-Raven, New York. pp. 133–155.
- Scantlebury MH, Heida JG (2010) Febrile seizures and temporal lobe epileptogenesis. Epilepsy Res 89: 27-33.
- Andermann F (1992) International symposium on benign partial and generalized epilepsies of childhood. Summation. Epilepsy Res Suppl 6: 215-222.
- Cendes F, Andermann F, Dubeau F, Gloor P, Evans A, et al. (1993) Early childhood prolonged febrile convulsions, atrophy and sfclerosis of mesial structures, and temporal lobe epilepsy: an MRI volumetric study. Neurology 43: 1083-1087.
- Klauenberg BJ, Sparber SB (1984) A kindling-like effect induced by repeated exposure to heated water in rats. Epilepsia 25: 292-301.
- Morimoto T, Nagao H, Yoshimatsu M, Yoshida K, Matsuda H (1993) Pathogenic role of glutamate in hyperthermia-induced seizures. Epilepsia 34: 447-452.
- Gulec G, Noyan B (2001) Do recurrent febrile convulsions decrease the threshold for pilocarpine-induced seizures? Effects of nitric oxide. Brain Res Dev Brain Res 126: 223-228.
- Mello LE, Cavalheiro EA, Tan AM, Kupfer WR, Pretorius JK, et al. (1993) Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia 34: 985-995.
- Sloviter RS (1987) Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science 235: 73-76.
- Bankier A, Turner M, Hopkins IJ (1983) Pyridoxine dependent seizures--a wider clinical spectrum. Arch Dis Child 58: 415-418.
- Lerma J (2003) Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci 4: 481-495.
- Kotani Y, Shimazawa M, Yoshimura S, Iwama T, Hara H (2008) The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther 14: 95-106.
- Vanlersberghe C., Camu F. (2008) Propofol. Handbook of Experimental Pharmacology; 182 :227252.
- Haeseler G, Karst M, Foadi N, Gudehus S, Roeder A, et al. (2008) High-affinity blockade of voltage-operated skeletal muscle and neuronal sodium channels by halogenated propofol analogues. Br J Pharmacol 155: 265-275.
- van Gestel JP, Blussé van Oud-Alblas HJ, Malingré M, Ververs FF, Braun KP, et al. (2005) Propofol and thiopental for refractory status epilepticus in children. Neurology 65: 591-592.
- Rossetti AO, Reichhart MD, Schaller MD, Despland PA, Bogousslavsky J (2004) Propofol treatment of refractory status epilepticus: a study of 31 episodes. Epilepsia 45: 757-763.
- Jiang W, Duong TM, de Lanerolle NC (1999) The neuropathology of hyperthermic seizures in the rat. Epilepsia 40: 5-19.
- Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, et al. (1984) Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res 321: 237-253.
- Veliskova, J. Behavioral characterization of seizures in rats. In: Pitkänen, A., Schwartzkroin, P.A., Moshé, S.L. (eds) (2006) Models of seizures and epilepsy. Elsevier Academic Press, Burlington. pp. 601-611.
- Prasad A, Worrall BB, Bertram EH, Bleck TP (2001) Propofol and midazolam in the treatment of refractory status epilepticus. Epilepsia 42: 380-386.
- Haberham ZL, van den Brom WE, Venker-van Haagen AJ, Baumans V, de Groot HN, et al. (1999) EEG evaluation of reflex testing as assessment of depth of pentobarbital anaesthesia in the rat. Lab Anim 33: 47-57.
- Paxinos, G., Watson, C. (1998) The Rat Brain in Stereotactic Coordinates. Academic Pres, Sydney; Figure: 32.
- Pitkanen, A., Kharatishvilli, I., Karhunen, H., Lukasiuk, K., Immonen, R., Nairismägi, J., Gröhn, O., Nissinen, J. ( 2007) Epileptogenesis in experimental models. Epilepsia; 48: 13-20.
- Lado FA, Sankar R, Lowenstein D, Moshé SL (2000) Age-dependent consequences of seizures: relationship to seizure frequency, brain damage, and circuitry reorganization. Ment Retard Dev Disabil Res Rev 6: 242-252.
- Blümcke I, Thom M, Wiestler OD (2002) Ammon's horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol 12: 199-211.
- McDonald JW, Garofalo EA, Hood T, Sackellares JC, Gilman S, et al. (1991) Altered excitatory and inhibitory amino acid receptor binding in hippocampus of patients with temporal lobe epilepsy. Ann Neurol 29: 529-541.
- Huang CC, Chang YC (2009) The long-term effects of febrile seizures on the hippocampal neuronal plasticity-clinical and experimental evidence. Brain Dev 31: 383-387.
- Saltik S, Angay A, Ozkara C, Demirbilek V, Dervant A (2003) A retrospective analysis of patients with febrile seizures followed by epilepsy. Seizure 12: 211-216.
- Kobayashi E, Lopes-Cendes I, Guerreiro CA, Sousa SC, Guerreiro MM, et al. (2001) Seizure outcome and hippocampal atrophy in familial mesial temporal lobe epilepsy. Neurology 56: 166-172.
- Cavalheiro EA, Santos NF, Priel MR (1996) The pilocarpine model of epilepsy in mice. Epilepsia 37: 1015-1019.
- Wasterlain CG, Fujikawa DG, Penix L, Sankar R (1993) Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia 34: S37-S53.
- Fukuda M, Morimoto T, Nagao H, Kida K (1997) The effect of GABAergic system activity on hyperthermia-induced seizures in rats. Brain Res Dev Brain Res 104: 197-199.
- Claassen J, Hirsch LJ, Emerson RG, Mayer SA (2002) Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 43: 146-153.
- Ohmori H, Sato Y, Namiki A (2004) The anticonvulsant action of propofol on epileptiform activity in rat hippocampal slices. Anesth Analg 99: 1095-110.
- De Riu PL, Petruzzi V, Testa C, Mulas M, Melis F, et al. (1992) Propofol anticonvulsant activity in experimental epileptic status. Br J Anaesth 69: 177-181.
- Parviainen I, Kälviäinen R, Ruokonen E (2007) Propofol and barbiturates for the anesthesia of refractory convulsive status epilepticus: pros and cons. Neurol Res 29: 667-671.
- van Gassen KL, Hessel EV, Ramakers GM, Notenboom RG, Wolterink-Donselaar IG, et al. (2008) Characterization of febrile seizures and febrile seizure susceptibility in mouse inbred strains. Genes Brain Behav 7: 578-586.
- Tancredi V, D'Arcangelo G, Zona C, Siniscalchi A, Avoli M (1992) Induction of epileptiform activity by temperature elevation in hippocampal slices from young rats: an in vitro model for febrile seizures? Epilepsia 33: 228-234.
- Lundgren J, Smith ML, Blennow G, Siesjö BK (1994) Hyperthermia aggravates and hypothermia ameliorates epileptic brain damage. Exp Brain Res 99: 43-55.
- Morrisett RA, Jope RS, Snead OC 3rd (1987) Effects of drugs on the initiation and maintenance of status epilepticus induced by administration of pilocarpine to lithium-pretreated rats. Exp Neurol 97: 193-200.
Citation: Bican A, Bora I, Kafa IM, Kurt MA (2012) Effects of Propofol on Epileptiform Activity and Hippocampal Morphology in Febrile Convulsions and Pilocarpine Induced Seizures. J Clin Exp Pathol 2:123. DOI: 10.4172/2161-0681.1000123
Copyright: © 2012 Bican A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15441
- [From(publication date): 9-2012 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 10758
- PDF downloads: 4683