Research Article Open Access
Effect of Temperature, Wavelength, pH, Ion Pair Reagents and Organic Modifi ers’ Concentration on the Elution of Cystatin C. Stability of Mobile Phase
Manar Khalid Fayyad1, Adel Khalil Misha2and Othman Ibrahem Yousef Al-Musaimi3*1Department of Chemistry, University of Jordan, Amman-Jordan
2Analytical Research Department, Hikma Pharmaceuticals- Amman
3Department of Chemistry, University of Ha'il, Ha'il –Saudia Arabia
- *Corresponding Author:
- Othman Ibrahem Yousef Al-Musaimi
Department of Chemistry
University of Ha'il
Ha'il –Saudia Arabia
Tel: 962 78 5782259
E-mail: Musamiau@Gmail.com
Received date: September 20, 2010; Accepted date: September 29, 2010; Published date: October 01, 2010
Citation: Fayyad MK, Misha AK, Yousef Al-Musaimi OI (2010) Effect of Temperature, Wavelength, pH, Ion Pair Reagents and Organic Modifiers' Concentration on the Elution of Cystatin C. Stability of Mobile Phase. J Anal Bioanal Tech 1:103. doi: 10.4172/2155-9872.1000103
Copyright: © 2010 Fayyad MK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Robustness of an analytical chromatographic method for separation of cystatin c has been veri fi ed. Changes in many parameters were carried out, such as, wavelength, column oven, mobile phase composition, chromatographic column.
Imperative changes have altered the ef fi ciency of the chromatographic separation; such changes include pH alteration of the mobile phase as well as alkyl sulfonate molarity changing.
All robustness conditions showed no major effect on the chromatographic separation of the analyte except with the changes related to TFA and alkyl sulfonate ion pair reagents. Peak area RSD, asymmetry and No. of theoretical plates were < 0.7%, < 1.2 and > 10,000, respectively. Results obtained using mobile phase after 6 months of storage have proven its stability and possibility of use. Gradient elution mode was utilized to elute cystatin c with a UV detection of 224 nm. Ace and Waters C8 (150 x 4.6 mm i.d., 5 μ m) as chromatographic columns were used.
Keywords
Cystatin C; Robustness; TFA; Stability of mobile phase
Introduction
Cystatin C (CC) is composed of 120 amino acid residues with a molecular weight of about 14 KDa and two intra-chain disulfide bonds. CC has an isoelectric point (PI) equal to 9.2; accordingly, and due to protonation of the acid group it has a positive charge at physiological pH. [1-5].
Ion pair liquid chromatography technique (Cation analysis) has been used for separation and quantification of CC, incorporating trifluoroacetic acid (TFA) and 1-hexane sulfonic acid sodium salt in the mobile phase.
Two ion pair reagents were included in the mobile phase. CC has a leader hydrophobic sequence which enhances the binding with the hydrophobic surface of the stationary phase. 1-hexane sulfonic acid sodium salt reagent was used to bind with the stationary phase instead of the analyte [5]. To accomplish an efficient separation, using of both ion pair reagents is deemed necessary. Typical working concentrations of both reagents were incorporated in the mobile phases [6].
Different factors render the use of TFA, it has low volatility at low pH values (in the present work pH 2.4), it sharpens the peak shape where the ionic interaction chance between the silanol groups of the stationary phase and CC is reduced and finally it has a low absorption at the working wavelength.
Organic modifiers can also compete with the ion pair for the stationary phase and then decreasing the effective capacity of the column. [7-9].
Experimental
Chemicals
CC protein was purchased from Scipac (UK), HPLC grade acetonitrile, methanol, 1-hexane sulfonic acid sodium salt, trifluoroacetic acid (TFA) and acetone were purchased from Merck (Germany).
Chromatographic conditions
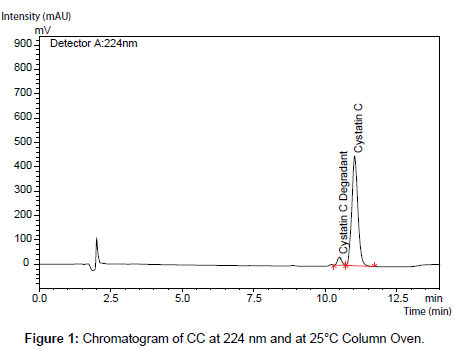
Shimadzu HPLC system (Shimadzu, Japan) with a degasser, low pressure gradient pump, column oven, an autosampler, a UV detector was used. Data acquisition was performed with LC-Solution 1.21 software. A reversed phase Ace C8 (150 × 4.6 mm i.d., 5 μm) column was placed in the column oven at 25°C.
Mobile phase A of 0.01M 1-hexane sulfonic acid sodium salt plus 0.05% TFA, pH 2.4 (filtered through 0.45 μm Teflon filter) and mobile phase B (acetonitrile: methanol: mobile phase A) (300: 300: 225, v/v/v), pH 2.5) [10].
Method Development and Validation
Stability of mobile phase solutions
Mobile phase has been stored at 25°C for six months. RSD were determined from 5 consecutive injections of calibration urine containing CC at its pathological concentration
Robustness
It is defined as per the ICH Guideline Q2 (R1) [11], a measure of the method to remain unaffected by deliberate changes in the parameters of the method.
Eight chromatography parameters were altered; these parameters were found to contribute significantly to the method: acetonitrile and methanol content in the mobile phases, column oven temperature, wavelength, TFA and 1-hexan sulfonic acid sodium salt content in the mobile phases which corresponds to the pH value and the buffer molarity, respectively. Chromatographic column from another supplier with the same specification, in addition, to another alkyl sulfonic acid sodium salt ion pair reagent were also used. Analysis was carried out using a calibration urine containing CC at its pathological concentration (Table 1).
| Parameter | - | 0 | + |
|---|---|---|---|
| Methanol content in mobile phase, constant Acetonitrile (mL) | 285 | 300 | 315 |
| Acetonitrile content in mobile phase, constant Methanol (mL) | 285 | 300 | 315 |
| Mobile Phase A (mL) | 210 | 225 | 240 |
| Wavelength (nm) | 229 | 224 | 221 |
| Column Oven Temperature (°C) | 20 | 25 | 30 |
| TFA Content (mL) | 0 | 0.5 | 1.0 |
| Alkyl Sulfonic Acid Sodium Salt (M) | 0 | 0.01 | NP |
Table 1: Selected parameters and their variabilities*. Selected parameters and their variabilities*.
Results and Discussion
Stability of mobile phase
Close retention time of CC peak, RSD < 0.7%, No. of theoretical plates was > 10,000 and the asymmetry factor was < 1.2, all prove the stability of mobile phase after six months of room temperature storage. (Table 2).
| Injection # | Peak Area Stability Mobile Phase (X 10-6) |
Peak Area Fresh Mobile Phase (X 10-6) |
|---|---|---|
| 1 | 6.24 | 6.65 |
| 2 | 6.24 | 6.64 |
| 3 | 6.26 | 6.63 |
| 4 | 6.31 | 6.60 |
| 5 | 6.27 | 6.54 |
| Average | 6.27 | 6.61 |
| RSD % | 0.47 | 0.67 |
| No. of Theoretical Plates | 10407 | 12726 |
| Asymmetry | 1.12 | 1.14 |
| Retention time (tr) | 10.4 | 11.0 |
Table 2: CC Using Stability Mobile Phase.
Robustness
No. of theoretical plates, asymmetry factors, RSD of peak areas and retention times were used to evaluate the effects on the qualitative responses (Table 3-6).
| Injection # | Peak Area (219 nm) (X 10-6) |
Peak Area (229 nm) (X 10-6) |
|---|---|---|
| 1 | 6.54 | 3.17 |
| 2 | 6.55 | 3.18 |
| 3 | 6.54 | 3.18 |
| 4 | 6.54 | 3.18 |
| 5 | 6.52 | 3.17 |
| Average | 6.54 | 3.18 |
| RSD % | 0.17 | 0.17 |
Table 3: CC at Different Wavelengths (± 5 nm).
| Injection # | Peak Area (+ 5 °C) (X 10-6) |
Peak Area (- 5 °C) (X 10-6) |
|---|---|---|
| 1 | 4.97 | 4.80 |
| 2 | 5.00 | 4.84 |
| 3 | 5.01 | 4.84 |
| 4 | 5.01 | 4.85 |
| 5 | 5.01 | 4.85 |
| Average | 5.00 | 4.84 |
| RSD % | 0.35 | 0.43 |
Table 4: CC at Different Column Oven Temperatures (± 5°C).
| Injection # | Peak Area Using1-Pentane ion pair Reagent (X 10-6) |
Peak Area Using1-Hexan ion pair Reagent (X 10-6) |
|---|---|---|
| 1 | 6.23 | 6.65 |
| 2 | 6.26 | 6.64 |
| 3 | 6.29 | 6.63 |
| 4 | 6.27 | 6.60 |
| 5 | 6.25 | 6.54 |
| Average | 6.26 | 6.61 |
| RSD % | 0.36 | 0.67 |
Table 5: Using of 1-Pentane Sulfonic Acid Sodium Salt in the Mobile Phase.
| Injection # | Peak Area Using Ace Column (X 10-6) |
Peak Area Using Waters Column (X 10-6) |
|---|---|---|
| 1 | 5.76 | 6.65 |
| 2 | 5.82 | 6.64 |
| 3 | 5.86 | 6.63 |
| 4 | 5.87 | 6.60 |
| 5 | 5.87 | 6.54 |
| Average | 5.84 | 6.61 |
| RSD % | 0.81 | 0.67 |
| No. of Theoretical Plates | 13982 | 12726 |
| Asymmetry | 1.10 | 1.14 |
| Retention time (tR) | 10.8 | 11.0 |
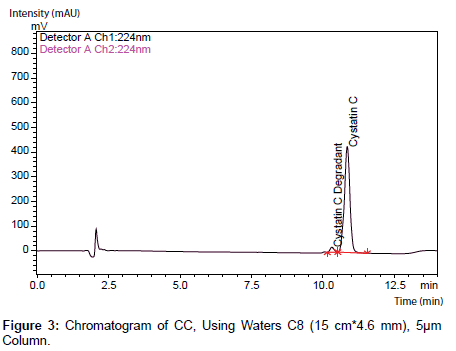
Table 6: CC Using Waters Column.
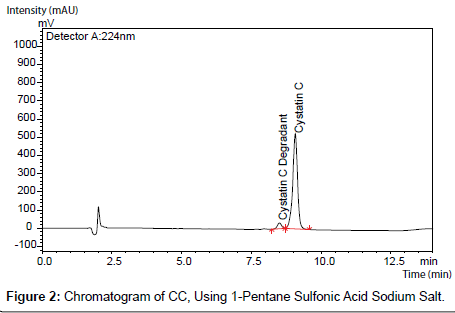
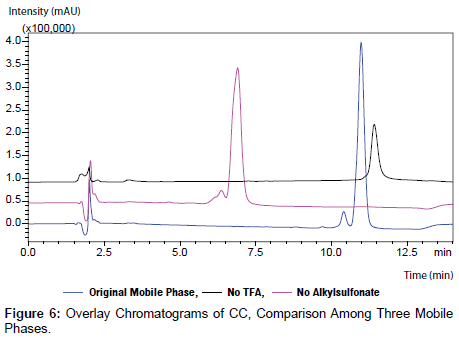
No major changes were observed as a result of the alterations made except when TFA and alkyl sulfonate ion pair reagents were eliminated from the mobile phases (Figure 1-3).
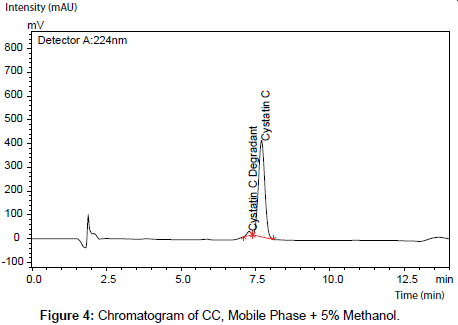
Alteration of organic modifiers content (acetonitrile and methanol) has affected only the retention time of CC peak. Nevertheless, the obtained RSD values for CC peak area did not exceed 0.6% (Figure 4,5) (Table 7,8).
| Injection # | Peak Area (+ 5 %) (X 10-6) |
Peak Area (- 5 %) (X 10-6) |
|---|---|---|
| 1 | 6.15 | 6.08 |
| 2 | 6.21 | 6.14 |
| 3 | 6.22 | 6.16 |
| 4 | 6.20 | 6.14 |
| 5 | 6.18 | 6.14 |
| Average | 6.19 | 6.13 |
| RSD % | 0.45 | 0.49 |
Table 7: ± 5 % Acetonitrile Added to Mobile Phase, Constant Methanol.
| Injection # | Peak Area (+ 5%) (X 10-6) |
Peak Area (- 5 %) (X 10-6) |
|---|---|---|
| 1 | 6.00 | 6.10 |
| 2 | 6.08 | 6.18 |
| 3 | 6.06 | 6.19 |
| 4 | 6.06 | 6.18 |
| 5 | 6.07 | 6.18 |
| Average | 6.05 | 6.17 |
| RSD % | 0.52 | 0.60 |
Table 8: ± 5 % Methanol Added to Mobile Phase, Constant Acetonitrile.
Interestingly, CC peak was distorted when no TFA is added to the mobile phase and the peak area of CC was reduced by about 67%. This phenomenon can be attributed to the silanol groups in the stationary phase that have been activated as a result of high pH value (7.0). Moreover, CC protein might dissociate at high pH values due to denaturation process. [6,12].
CC peak was distorted when no alkyl sulfonates reagent is added to the mobile phase in addition to retention time shifting was also observed. The interaction between the hydrophobic leader of CC sequence and the hydrophobic stationary phase is considered as a reason behind the observed distortion, whereas, in case of alkyl sulfonates-containing mobile such interaction occurs with the alkyl sulfonate reagent. The reduction in the retention time (6.9 minutes instead of 10 minutes) can be also attributed to the low concentration of the alkyl sulfonate in the mobile phase (Figure 6).
Conclusion
The present study has investigated the effect of varying some of the chromatography parameters of the previously developed and validated HPLC method. The obtained results proved the robustness of the method in addition to the stability of the mobile phase. According to the obtained results, the presence of the both ion pair reagents, TFA and the alkyl sulfonate, is extremely important to achieve the maximum separation and sensitivity.
Acknowledgement
Financial support has been furnished by Hikma Pharmaceuticals-Amman.
References
- Bobek LA, Levine MJ (1992) Cystatins--inhibitors of cysteine proteinases. Crit Rev Oral Biol Med 3: 307-332.
- Berti PJ, Storer AC (1994) Local pH-dependent conformational changes leading to proteolytic susceptibility of cystatin C. Biochem J 302: 411-416.
- Mussap M, Vestra MD, Fioretto P, Saller A, Varagnolo M, et al. (2002) Cystatin C is a more sensitive marker than creatinine for the estimation of GFR in type 2 diabetic patients. Kidney Int 61: 1453-1461.
- Hoek FJ, Kemperman FA, Krediet RT (2003) A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant 18: 2024-2031.
- Filler G, Bokenkamp A, Hofmann AW, Le Bricon T, Martinez-Bru C, et al. (2005) Cystatin C as a marker of GFR—history, indications, and future research. Clin Biochem 38: 1-8.
- Chen Y, Mehok AR, Mant CT, Hodges RS (2004) Optimum concentration of trifl uoroacetic acid for reversed-phase liquid chromatography of peptides revisited. J Chromatogr A 1043: 9-18.
- Snyder LR, Kirklend JJ, Glajch JL (1997) Practical HPLC Method Development (2nd ed). USA: Library of Congress.
- Yasuda M, Sonda T, Hiraoka T, Horita A, tabata M (2003) Effects of the Molecular Properties of Mixed Solvents on the Elution of Alkyl Benzoates in RPLC. Anal Sci 19: 1637-1641.
- Yuen PST, Dunn SR, Miyaji T, Yasuda TH, Sharma K, et al. (2004) A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol renal Physiol 286: F1116-F1119.
- Al-Musaimi OIY,Fayyad MK, Mishal AK (2009) Novel liquid chromatographic determination of cystatin C in human urine. J Chromatogr B 877: 747-750.
- Guidance for Industry: ICH Q2 (R1), Validation of Analytical Procedures: Text and Methodology 1994.
- Nawrocki J (1997) The silanol group and its role in liquid chromatography. J Chromatogr A 779: 29-71.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17887
- [From(publication date):
October-2010 - Nov 17, 2025] - Breakdown by view type
- HTML page views : 12937
- PDF downloads : 4950