Effect of Persistent Organic Pollutants (Dioxins) on Rat Myocardium and Amelioration with Antioxidant Vitamins (Role of Aryl Hydrocarbon Receptors and Cytochrome P450)
Received: 10-Sep-2012 / Accepted Date: 24-Sep-2012 / Published Date: 26-Sep-2012 DOI: 10.4172/2161-0681.1000130
Abstract
Abstract
Epidemiologic studies have linked dioxins exposure to ischemic heart disease induced mortality. This study aimed
to investigate the effect of subchronic combined exposure to tetra chloro dibenzodioxin (TCDD) and dibenzofuran (DF)
on left ventricular myocardium of male rats; and the possible underlying mechanism. Moreover, this work aimed to
determine the ameliorative effect of antioxidant vitamins (A, E) supplementation. Fifty animals were classified into four
groups: control group (I); group (II) received vitamins only; group (III) included twenty animals received a dose of 1/10
of LD50 of combined TCDD/DF and group (IV) was included ten animals that received vitamins A and E in combination
with TCDD/DF. Left ventricular myocardium was examined using histological study, immunohistochemical detection of
Aryl Hydrocarbon Receptors (AHR) and mRNA expression of cytochrome P450 gene. Also, oxidant antioxidant markers
in tissue homogenate were detected. Progressive cardiomyopathy, myocardial necrosis, interstitial and perivascular
fibrosis were evident in a time related manner after dioxins treatment. This was associated with increased immunohistochemical
expression of AHR and mRNA expression of CYP1A1. Oxidative stress was evident in left ventricle of
dioxins treated group. These results suggested that combined TCDD/DF exposure has a potent cardio toxic effect
which could be ameliorated by simultaneous supplementation with vitamins A and E.
Keywords: Dioxins; Rat; Cardiomyocyte; Histology; Immunohistochemistry
308471Abbreviations
AH: Aryl Hydrocarbon; AHR: Aryl Hydrocarbon Receptors; CYP1A1: Cytochrome P450; DAB: Diamiobenzedine; DF: Dibenzofuran; GSH-PX: glutathione perioxidase; HW/BW: Heart Weight To Body Weight Ratio; LD50: Lethal Dose That Kill 50% of Animals; MSA: Myocyte Surface Area; PCDD: Polychlorinated Dibenzo Dioxin; PCB: Pentachlorobiphenyl; POPs: Persistant Organic Pollutants; SOD: Superoxide Dismutase; TBARS: Thiobarbituric Acid Reactive Substance; TCDD: Tetra Chloro Dibenzo Dioxin
Introduction
International attention is focusing on Persistent Organic Pollutants (POPs), some of the most dangerous chemicals in the world. POPs are released to the environment accidentally or as a result of uncontrolled human activities [1]. Among the list of the produced POPs there are Polychlorinated Dibenzo-P-Dioxins (PCDDs) and Dibenzofurans (DFs) [2]. They are long lasting and can travel great distances on air and water currents. These two substances are collectively known as dioxins [3].
The main source of Dioxins is uncontrolled burning processes of waste incineration and agricultural waste including rice straw burning [4,5] Other sources exist, such as emissions from electric steel making furnaces, cigarette smoke, automobile exhaust and combustion of fossil fuels [6] Burning of garbage generates dioxins in our communities, therefore they are found in the atmosphere and in the fallout in rain [7].
POPs toxicity builds up in the body when humans consume highfat foods, dairy products, fish, and other meats [2].
For over a decade, concerns have been raised about the impact of dioxins on our health with respect to heart [8] Kidney [9] and liver diseases [10] Most of the researches on POPs toxicity have focused primarily on an individual compound with limited value for predicting health hazards caused by exposure to complex mixtures of environmental contaminants [11,9]
Despite the evidence showing an association between human TCDD exposure and cardiovascular diseases, animal models elucidating the mechanisms underlying these effects are limited [12] found that Aryl Hydrocarbon Receptors (AHR) controls the adaptive up-regulation of xenobiotic metabolizing phase II enzymes that include cytochromes in response to POPs with concurrent oxidative stress [13]. Moreover, concomitant antioxidant supplementation has a protective role in TCDD-induced toxicity in mice [11].
The aim of this work was to determine the degree to which cardiac structure affection develops in an animal model exposed subchronically to combined TCDD and DF and the possible underlying mechanisms as regarding AHR and CYP1A1. Furthermore, we aimed to determine the possible protective effect of antioxidant vitamins A, E supplementation.
Materials and Methods
All experimental procedures were approved and carried out in accordance with the guidelines of the Faculty of Medicine, Zagazig University, Egypt.
Drugs and chemicals
(2,3,7,8)-Tetrachlorodibenzo-p-dioxin (TCDD>99% purity) was purchased from Supelco Analytical Bellefonte, PA (Cat No: 48599). The stock solutions of 10μg/ml were diluted with corn oil and acetone (99:1) to prepare the dosing solutions. Dibenzofuran (DF>98% purity, Case No: 132-64-9) was purchased from Sigma Aldrich. Vitamin A (Retinol>97% purity, 68-26-8) and Vitamin E (α tocopherol,>96%, 10191-4-0) were purchased from Sigma chemical Co, USA.
Animals
Adult Wistar male albino rats (weighing about 250 gm) were purchased from animal house of Faculty of Medicine, Zagazig University, Egypt. They were housed under standard laboratory conditions (Tm; 24 ± 2°C and a 12 h day/night cycles). The animals were allowed to acclimatize for 2 weeks before starting the experiment and observed for general health and suitability for inclusion in the study. They were given free access to a standard chow diet and drinking water ad libitum.
Experimental design
Fifty animals were classified into four groups: Group I was the control group included ten animals that further subdivided equally into two subgroups. Subgroup (Ia) received no treatment and (Ib) received solvent for dioxins (corn oil and acetone 99:1 ratio) for the same dose and duration as in other groups.
Group II: ten animals received orally a mixture of 2μg of vitamin A and 20μg vitamin E dissolved in corn oil daily for 60 days. The amounts of vitamins calculated by Radhey et al. [14] were selected on the basis of reported recommended daily allowances for human beings which are 1000μg vitamin A, 10mg vitamin E, for an average adult man [15].
Group III was the dioxins treated group that included twenty animals received 2.5 μg/kg TCDD and 89 mg/kg DF three times/week for 60 days by oral gavages. The dose selected in this study was 1/10 of LD50 concentration of TCDD [16] and DF [17]. Ten rats were sacrificed after 30 days and the rest after 60 days
Group IV was dioxins+vitamins supplemented group: included ten rats treated with antioxidant vitamins in combination with dioxins in the same dose and duration as in the other groups.
At time of sacrifice, rats were weighed then killed by an overdose of anesthesia. Subsequently, the heart was perfusion-fixed with 10% neutral buffered formalin. The hearts were excised and trimmed of excess adipose tissue. Hearts were weighed, coronal section of the ventricular portion dividing it into two halves was done, one half postfixed in 10% neutral buffered formalin and processed for preparation of paraffin sections for histological analysis. Part of other half of left ventricle of all groups were frozen in liquid nitrogen and processed for detection of oxidative stress markers and RNA extraction and reverse transcriptase–polymerase chain reaction for detection of cytochrome P450 (CYP1A1).
Histological analysis
The paraffin sections were stained according to Bancroft and Gamble [18] with Hematoxylin and Eosin (H&E) for examination of overall morphology and with van Gieson’s stain for examination of collagen and immunohistochemical detection of Aryl Hydrocarbon Receptors (AHR). Immunohistochemical reaction was carried out using avidin–biotin-complex immunoperoxidase system [19]. Serial sections of paraffin embedded sections were deparaffinized on charged slides. The sections were incubated in hydrogen peroxide 0.1% for 30 min to block the endogenous peroxidase then incubated with the primary antibody. The primary antibody used was AHR (US Biological-A1059- 72R (1-100)). Then, the slides were incubated with the secondary antirabbit antibodies versal kits (Zymed laboratories), diluted 1:200 for 30 min. Staining was completed by incubation with substrate Chromogen Called Diamiobenzidine (DAB) which resulted in brown-colored precipitate at the antigen sites and Mayer’s hematoxylin was used as a counter stain. For negative control, the primary antibody was replaced by phosphate buffer solution. Finally, the AHR cytoplasmic and to a less extent nuclear site of reaction were stained brown and nuclei stained blue.
Morphometric analysis
Myocyte Surface Area (MSA) was measured in sections stained with H&E and suitable cross sections were defined as having nearly circular capillary profiles and nuclei [20]. Each field was scanned together with a microscale by a “Leica Quin” image analyser computer system (Leica Imaging System Ltd., Cambridge, England). The measuring frame of a standard area is equal to 7286,78 μm². The same method was applied for measuring area percentage of the red colored areas for collagen fibers in Van Geison stained sections but the measuring frame was of area 118476,6 and an objective lens 100 in 10 fields for each specimen. The average of 20 regions was used for measuring MSA and 10 regions for area% of collagen fibers in each specimen of all groups.
Biochemical analysis in heart tissue
Preparation of tissue homogenates: Tissue samples were homogenized in 0.1 M ice-cold phosphate buffer (pH 7.4) and the homogenate was centrifuged at 10,000 g at 4°C for 5 minutes. The supernatant was used for the estimation of TBARS, superoxide dismutase and glutathione peroxidase activities.
Determination of thiobarbituric acid reactive substances (TBARS): 0.5 ml homogenate mixture was mixed with 0.5 ml normal saline (0.9 g % NaCl) and 2 ml of TBA-TCA mixture (0.392 g thiobarbituric acid in 75 ml of 0.25 N HCl with 15 g trichloroacetic acid, volume up to 100 ml by 95 % ethanol) and boiled at 100°C for 10 min. This mixture was then cooled at room temperature and centrifuged at 4000 g for 10 min. The whole supernatant was taken in spectrophotometer cuvette and read at 535 nm. 1,1,3,3-Tetramethoxypropane was employed as a standard [21].
Determination of superoxide dismutase activity (SOD): Myocardial tissue SOD activity was determined using the nitroblue tetrazolium (NBT) method described by Sun et al. [22]. In this method, NBT is reduced to blue formazan by superoxide (O2-) which has a strong absorbance at 560 nm.
Determination of glutathione peroxidase activity (Gsh-Px): Myocardial tissue glutathione peroxidase activity was measured by monitoring the continuous decrease in NADPH concentration using H2O2 as a substrate [23]. In a cuvette, potassium phosphate buffer (500 mM), EDTA (50 mM), sodium azide (20 mM), glutathione reductase (15 U/mL), NADPH (1.5 mM), reduced glutathione (250 mM), tissue homogenate (0.01 ml) and H2O2 (10 mM) were mixed; the absorbance was followed at 340 nm, and the change in absorbance per minute was calculated.
Molecular biology analysis in heart tissue
RNA extraction and reverse transcriptase–polymerase chain reaction of CYP1A1 Gene: All enzymes and cofactors used for reverse transcription and polymerase chain reaction ((RT-PCR) were purchased from iNtRON BIOTECHNOLOGY, INC (Gyeonggido, 462-120, Korea). Sequences of PCR primers for amplification of CYP1A1, and B-actin are summarized in (Table 1).
| Primer | Forward | Reverse |
|---|---|---|
| CYP1A1 | 5'-CCATGACCAGGAACTATGGG-3' | 5'-TCTGGTGAGCATCCAGGACA-3' |
| B-actin | 5'-CCTCTATGCCAACACAGT-3' | 5'-AGCCACCAATCCACACAG-3' |
Table 1: Sequences of PCR primers for amplification of CYP1A1, and B-actin.
Total RNA was isolated from left ventricular tissue using RNA–spin Total RNA Extraction Kit (Gyeonggi-do, Korea) and then, RT-PCR reaction was performed using Maxime RT-PCR premix kit (Korea) in a 20 μL reaction mixture containing OptiScriptTM RT System, RT-PCR buffer (10x), dNTPs, i-Star TaqTM DNA polymerase, 500 ng of total RNA and 10-20 pmole of each forward and reverse primer (1 cycle of Reverse transcription reaction 45°C for 30 min then inactivation of RTase at 94°C for 5 min). Then 25 cycles for CYP1A1, and 23 cycles for B-actin by denaturing at 94°C for 45 sec, annealing for 58°C for 45 sec, and extending at 72°C for 45 sec followed by final extension at 72°C for 5 min RT-PCR products were detected as a single band on a 1.5% agarose gel stained with ethidium bromide. Band intensity was quantified by using the gel documentation system (BioDO, Analyser) supplied by Biometra according to the following amplification procedure: Relative expression of each studied gene (R) was calculated following the formula: R=Densitometrical units of each studied gene/ Densitometrical units of B-actin.
Statistical Analysis
Data were presented as Mean ± SD. Differences between groups were assessed by one-way Analysis of Variance (ANOVA) followed by Post Hoc multiple comparison tests (least significant different test; LSD) to compare changes among individual groups. The significance level was established at P<0.05.
Results
Histological results
Histological examination of both control subgroups Ia, Ib and group II showed similar structure. Figures for subgroup Ia were used to differentiate with other groups.
H&E stained sections of control left ventricle revealed normal structural architecture of cardiomyocytes. They appeared as long parallel, striated, branched muscle fibers. The acidophilic cytoplasm contained centrally located oval nuclei. The intercellular spaces were narrow and contained blood capillaries and few connective tissue cells (Figure 1A). Myocardial arterioles appeared with thin intima lined by endothelial cells and tunica media comprising few layers of muscles and thin adventitia (Figure 1B).
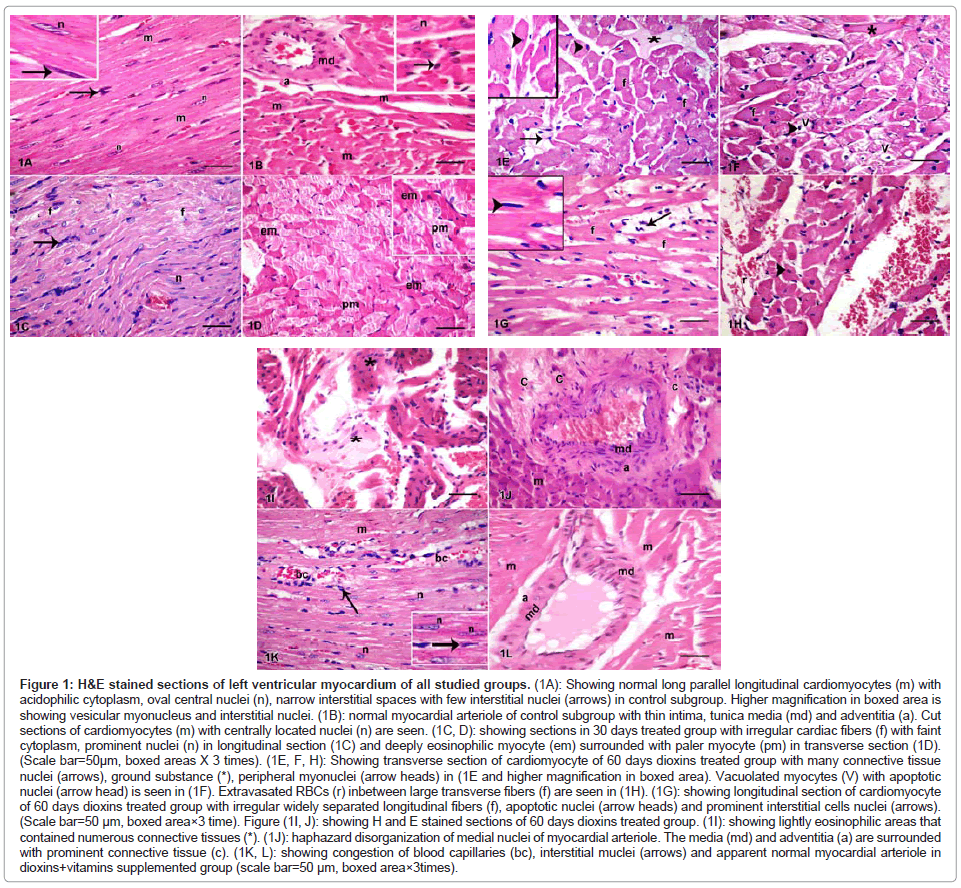
Figure 1: H&E stained sections of left ventricular myocardium of all studied groups. (1A): Showing normal long parallel longitudinal cardiomyocytes (m) with acidophilic cytoplasm, oval central nuclei (n), narrow interstitial spaces with few interstitial nuclei (arrows) in control subgroup. Higher magnification in boxed area is showing vesicular myonucleus and interstitial nuclei. (1B): normal myocardial arteriole of control subgroup with thin intima, tunica media (md) and adventitia (a). Cut sections of cardiomyocytes (m) with centrally located nuclei (n) are seen. (1C, D): showing sections in 30 days treated group with irregular cardiac fibers (f) with faint cytoplasm, prominent nuclei (n) in longitudinal section (1C) and deeply eosinophilic myocyte (em) surrounded with paler myocyte (pm) in transverse section (1D). (Scale bar=50μm, boxed areas X 3 times). (1E, F, H): Showing transverse section of cardiomyocyte of 60 days dioxins treated group with many connective tissue nuclei (arrows), ground substance (*), peripheral myonuclei (arrow heads) in (1E and higher magnification in boxed area). Vacuolated myocytes (V) with apoptotic nuclei (arrow head) is seen in (1F). Extravasated RBCs (r) inbetween large transverse fibers (f) are seen in (1H). (1G): showing longitudinal section of cardiomyocyte of 60 days dioxins treated group with irregular widely separated longitudinal fibers (f), apoptotic nuclei (arrow heads) and prominent interstitial cells nuclei (arrows). (Scale bar=50 μm, boxed area×3 time). Figure (1I, J): showing H and E stained sections of 60 days dioxins treated group. (1I): showing lightly eosinophilic areas that contained numerous connective tissues (*). (1J): haphazard disorganization of medial nuclei of myocardial arteriole. The media (md) and adventitia (a) are surrounded with prominent connective tissue (c). (1K, L): showing congestion of blood capillaries (bc), interstitial muclei (arrows) and apparent normal myocardial arteriole in dioxins+vitamins supplemented group (scale bar=50 μm, boxed area×3times).
Examination of H&E stained sections of dioxins treated group revealed structural alteration after 30 days of treatment. Irregular fibers with faint cytoplasm were commonly seen (Figure 1C). The prominent changes consisted of small foci of myocardial fiber changes, characterized by deeply eosinophilic cardiomyocytes surrounded with paler stained myocytes (Figure 1D).
At the end of the study after 6o days, irregular larger sized fibers with small darkly stained apoptotic nuclei and vacuolated cytoplasm were commonly seen. In addition, areas of interstitial accumulation of small to moderate amounts of lightly basophilic, homogeneous material-presumably ground substance- were also commonly seen (Figure 1E,F,G). In some animals, hemorrhage and extravasation of RBCs were seen inbetween myocytes ( Figure1H). Abundant interstitial connective tissues were replaced the affected cardiomyocytes (Figure 1I).
Coronary arterioles with perivascular fibrosis were observed in H&E stained sections of dioxins treated group. Haphazard disorganization of tunica media nuclei and proliferation of adventitial connective tissue was detected 60 days after dioxins treatment (Figure 1J).
Examination of H&E stained sections of dioxins+vitamins supplemented group showed that cellular infiltration and congestion of myocardial blood capillaries were seen in some regions. Myocardial arteriolar wall appeared formed of intima, media and adventitia (Figure 1K, L).
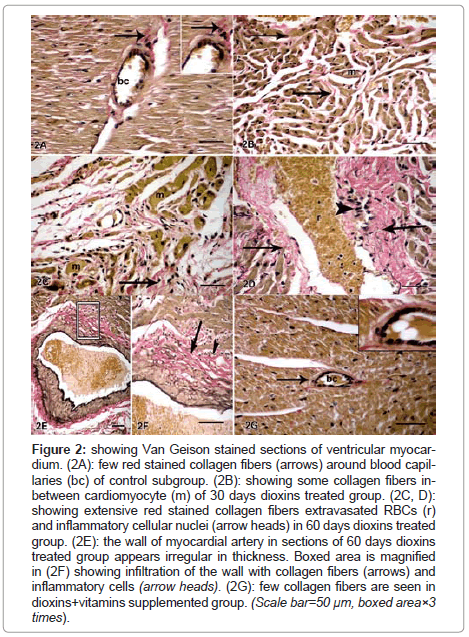
Van Geison stained sections showed minimal interstitial and perivascular red stained collagen fibers between cardiomyocyte and around blood capillary of control subgroup (Figure 2A) and dioxins+vitamins supplemented group (Figure 2G). Interstitial fibrosis was detected 30 days after dioxins treatment (Figure 2B) and progressively increased after 60 days (Figure 2C,D). The wall of myocardial arteries was irregular and infiltrated with collagen fibers and inflammatory cells (Figure 2E,F,G). The results were confirmed by statistical analysis of area % of collagen fibers in all studied groups (Table 2).
| Control N=10 |
Vitamins N=10 |
30days dioxins N=10 |
60days dioxins N=10 |
dioxins+vitamins N=10 | |
|---|---|---|---|---|---|
| HW | 0.721 ± 0.1 | 0.710 ± 0.01 | 1.078 ± 0.12a | 1.123 ± 0.13a | 1.11 ± 0.1b |
| HW/BW(X10-3) | 3.81 ± 0.123 | 3.7 ± 0.14 | 5.03 ± 0.049a | 5.14 ± 0.012a | 3.4 ± 0.017b |
| MSA (µm2) | 203.3 ± 13.4 | 199 ± 12.9 | 338.9 ± 23.3a | 634.9 ± 31.6a | 226.5 ± 6.03b |
| Collagen% | 2.2 ± 0.035 | 2.1 ± 0.055 | 5.06 ± 0.087a | 8.16 ± 0.988a | 2.97 ± 0.106b |
bp<0.001significant relative to dioxins treated group
Table 2: HW, HW/BW ratio, myocyte surface area (MSA) and area % of collagen fibers in all studied groups. All results are expressed as mean ± SD.
Figure 2: showing Van Geison stained sections of ventricular myocardium. (2A): few red stained collagen fibers (arrows) around blood capillaries (bc) of control subgroup. (2B): showing some collagen fibers inbetween cardiomyocyte (m) of 30 days dioxins treated group. (2C, D): showing extensive red stained collagen fibers extravasated RBCs (r) and inflammatory cellular nuclei (arrow heads) in 60 days dioxins treated group. (2E): the wall of myocardial artery in sections of 60 days dioxins treated group appears irregular in thickness. Boxed area is magnified in (2F) showing infiltration of the wall with collagen fibers (arrows) and inflammatory cells (arrow heads). (2G): few collagen fibers are seen in dioxins+vitamins supplemented group. (Scale bar=50 μm, boxed area×3 times).
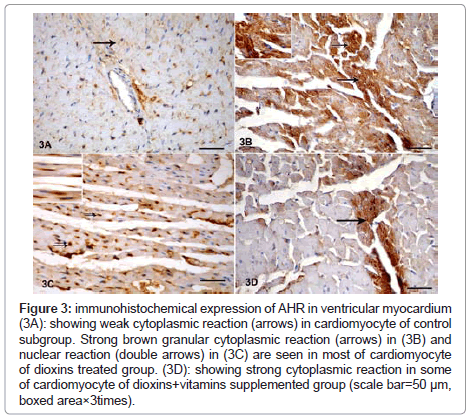
Immunohistochemical staining of AHR revealed weak brown reaction in control subgroup (Figure 3A). Strong reaction in most of cardiomyocyte of dioxins treated group both after 30 and 60 days of dioxins exposure was observed. The reaction appeared mainly cytoplasmic (Figure 3B) and nuclear in some specimens (Figure 3C). Strong granular cytoplasmic AHR immunoreaction was detected in some cardiomyocyte of dioxins+vitamins supplemented group (Figure 3D).
Figure 3: immunohistochemical expression of AHR in ventricular myocardium (3A): showing weak cytoplasmic reaction (arrows) in cardiomyocyte of control subgroup. Strong brown granular cytoplasmic reaction (arrows) in (3B) and nuclear reaction (double arrows) in (3C) are seen in most of cardiomyocyte of dioxins treated group. (3D): showing strong cytoplasmic reaction in some of cardiomyocyte of dioxins+vitamins supplemented group (scale bar=50 μm, boxed area×3times).
Morphometrical results
The presence of cardiomyocyte hypertrophy was evaluated by calculating the ratio of heart weight to body weight (HW/BW ratio) and by measurement of the surface area of cardiomyocytes (MSA). Significant increase in HW/BW ratio and in MSA was detected in dioxins treated group especially after 60 days as compared with control group. However, the group treated with both vitamins+dioxins showed significant decrease in both parameters as compared with dioxins treated group (Table 2).
Biochemical results
Effect of exposure to dioxins on tbars and antioxidant enzyme levels in rat heart tissue (Table 3):Significant increase in TBARS level compared to control were observed following 30 days and 60 days of dioxins exposure (P<0.001 in both groups). TBARS level was significantly decreased in the dioxins+vitamins supplemented group compared to dioxins-treated groups (P<0.001).
| Control n=10 |
Vitamins n=10 |
30days dioxins n=10 |
60days dioxins n=10 |
dioxins+vitamins n=10 | |
|---|---|---|---|---|---|
| TBARS(nmol/g wet tissue) | 9.1 ± 1.6 | 8.9 ± 0.9 | 15.2 ± 2.7a | 17.2 ± 2.8a | 10.8 ± 1.2ab |
| SOD (U/g wet tissue) | 24.3 ± 5.5 | 24.5 ± 5.6 | 15.6 ± 2.2a | 15.7 ± 2.2a | 23.9 ± 2.7b |
| GSH-px(U/g wet tissue) | 46.9 ± 0.5 | 47.1 ± 0.7 | 35 ± 2.1a | 29.6 ± 0.3a | 45.7 ± 1.5ab |
ap<0.001significant relative to control
bp<0.001significant relative to dioxins treated group
Table 3: Effect of exposure to dioxins on TBARS and antioxidant enzyme levels in rat heart tissue. All results are expressed as mean ± SD.
SOD and GSH-Px activities were significantly decreased in the 30 days and 60 days dioxins–treated group compared to control (P<0.002, P<0.001 respectively in both groups). SOD and GSH-Px activities were significantly increased in the dioxins+vitamins supplemented group compared to dioxins treated groups (P<0.004, P<0.001respectively in both groups).
No significant differences were observed in the SOD activity in the dioxins+vitamins supplemented group compared to control. However, there was a significant decrease in GSH-Px activity in the dioxins+vitamins supplemented group compared to control (P<0.01).
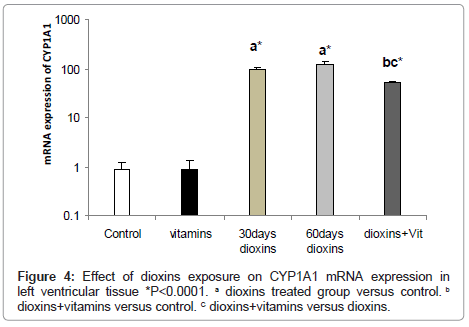
Cyp1a1 Mrna expression: mRNA expression of the CYP1A1 was examined in the left ventricle after 30 days of exposure to dioxins to investigate AHR stimulation. CYP1A1 mRNA expression was significantly up regulated in heart tissues of dioxins-exposed rat, mostly in the left ventricle (99.5 ± 8.4 fold).
To confirm sustained induction of CYP1A1, mRNA expression was also examined after 60 days of dioxins exposure. Induction of CYP1A1 mRNA expression of dioxins-exposed rat was greater after 60 days of exposure (120 ± 15.4 fold) in left ventricular tissue.
In the dioxins+vitamins supplemented group, mRNA expression of the CYP1A1 was significantly reduced to (50.9 ± 4.2 fold) in left ventricular tissue (Figure 4).
Discussion
In this study, time related progressive myocardial degeneration was observed in the left ventricle of dioxins treated group. The histological changes in this study were associated with increased expression of AHR and mRNA of CYP1A1 together with oxidative stress and vascular changes.
The detected multiple foci of myocardial degeneration were typical for the appearance of cardiomyopathy in rat heart described by Mac Kenzie and Alison [24] and Jokinen et al. [25]. The prominent changes, seen after 30 days, consisted of small foci of myocardial fiber necrosis, characterized by deeply eosinophilic myocardial fibers surrounded with paler stained myocytes. As lesion development progressed at the end of the study, the necrotic myocardial fibers were replaced by proliferating interstitial connective tissue. This was associated with interstitial and myocardial fibrosis together with myocardial arteritis. Some cardiomyocytes appeared with peripherally located pyknotic myonuclei and dark esinophilic cytoplasm which indicated apoptosis [26]. Similar histological findings of progressive cardiomyopathy, myocardial necrosis and fibrosis were detected in rats following chronic treatment with TCDD and pentachlorobiphenyl (PCB) for two year [25].
The present results demonstrated that combined exposure to TCDD/DF induce cardiac hypertrophy indicated by increase in relative heart weight (HW/BW) and myocyte surface area [27,28] detected increase in heart weight in mice with active AHR which was related to increase in left ventricular weight.
Haphazard disorganization of tunica media together with infiltration and fibrosis were observed in the myocardial vessels of 60 days dioxins treated group of this study. Also, the arterial wall appeared not uniform in thickness as compared to control group. Similar vascular lesion described as arteritis was observed [25] in mesenteric and pancreatic arteries following chronic treatment with TCDD/PCB. They suggested that this arteritis were caused by the vasoactive effects of dioxin compounds. Moreover, TCDD through the activation of AHR, may be able to influence blood pressure in mice [29] In addition, TCDD has been shown to cause contractile defects and impairment of the capacity of the avian heart sarcoplasmic reticulum to sequester Ca2+ [30] Exposure of isolated rat ventricular myocytes to TCDD prolonged the action-potential duration and lead to abnormal triggered-after depolarization [31].
Cardiomyopathy could result from anoxia that is secondary to vascular changes or from direct toxicity to myocardial cells [24]. After loss of myocardial tissue, thicker and longer cardiomyocytes with large nuclei may be observed in the intact myocardial zone. Hypertrophy is associated with hemodynamic changes brought about by disordered contractility. Persistent myocardial ischemia (a major factor for chronic ischemic heart disease) usually occurs when the blood flow through coronary arteries with stenosis does not meet oxygen demand of cardiomyocytes [32]. According to Greaves [33] the presence of fibrosis is a common end-stage finding with myocardial degeneration and results from different etiologies and pathogenesis (i.e., initial, direct, chemically induced insult to the myocardium).
Strong immunohistochemical reaction for AHR was observed in this study especially after subchronic exposure in 60 days dioxins treated group as compared to control. Both cytoplasmic and nuclear AHR reaction was demonstrated in dioxins treated group with only weak cytoplasmic reaction in control group. AHR can act on many types of cells and mediate a series of biological processes [34] AHR was expressed normally in the heart but to a less extend than that in liver [35].
Peters et al. [36] illustrated that dioxin toxicity was mediated by its interaction with AHR. Mice in which the AHR has been genetically deleted fail to exhibit any overt toxic responses typically observed after TCDD exposure, demonstrating that AHR activation is required to mediate TCDD toxicity. The AHR is a cytosolic receptor bound to a chaperone complex. In this form, AHR is predominately localized in the cytoplasm [37] upon binding of a ligand such as dioxin, AHR translocates into the nucleus and dissociates from its chaperone complex into the nucleus [38]. The AHR subsequently dimerizes with its DNA binding partner leading to increased transcription of AHR and their bind to specific DNA response elements, resulting in transcription of certain genes, such as mammalian cytochrome (CYP1A1 and CYP1A2) [39].
Oxidative stress has been proposed as a mechanism of the toxicity of TCDD which appears to be AHR mediated. CYP1A1 as a member in the AH gene battery, have been suggested to mediate this TCDDinduced oxidative stress [40]. Induction of CYP1A1 is associated with the production of hydrogen peroxide during cytochrome P450 metabolic cycling [41]. Thus large induction of CYP1A1 by TCDD toxicity results in free radical formation and lipid peroxidation.
The current data displayed dioxins-induced reduction in the activities of antioxidants enzymes SOD, and GSH-PX associated with increased TBARS in the heart tissue of dioxins treated group. Thus, the increased level of lipid peroxides in dioxins treated rats might be due to free radical mediated membrane damage which together with decreased antioxidants defense systems results in oxidative stress. The observed decline in the activity of GSH-PX in dioxins treated group may be ascribed to the reduction in reduced glutathione and an increase in the level of peroxides caused by dioxins [42]. This pro-oxidant shift in the intracellular redox state may induce cell death by either direct cell membrane damage by lipid peroxidation [43] or apoptosis through activation of transcription factors or DNA damage [44]. So, the observed structural changes including both forms of necrosis and apoptosis in cardiomyocytes after dioxins treatment in this study was related to oxidative stress.
Simultaneous supplementation of dioxins treated group with antioxidant vitamins (A, E) in the present study ameliorated the left ventricular myocardium damage by histological examination. Decreased immunohistochemical reaction of AHR associated with decreased expression of CYP1A1 gene were detected in dioxins+vitamins supplemented group compared to dioxins treated group. These synergistic effects of vitamin A and vitamin E may help explain the greater antioxidant effects of the vitamin mixture compared with that produced by alpha-tocopherol alone [45,46] or retinol alone [47].
We chose the two commonly used antioxidant vitamins, not only because of their efficacy as antioxidants but also because of their clinical relevance and minimal toxicity [48]. Alpha-tocopherol is a potent lipophilic antioxidant [49]. It suppresses the lipid peroxidation by inhibiting TBARS formation [50] and reversing the action of dioxins on GPX and SOD levels by regenerating glutathione and direct scavenging reaction of α tocopherol with superoxide radicals [11].
Hakansson et al. [51] indicated that some of the toxic effects of TCDD resembled retinoid deficiency. Moreover, vitamin A decrease the TCDD-increased CYP1A1 expression in liver through the reduction in AHR mRNA expression which has an ameliorative effect on liver damage caused by TCDD in a CYP1A1-dependent manner [47].
Together, the structural and molecular data of this study indicated that the left ventricle is a direct target for dioxins, and there were complicated interactions between the two pollutants TCDD/DF on the cardiotoxicity through activation of AHR signaling pathway. Activation of AHR resulted in increased cytochrome P450 that caused oxidative stress. Also, there is a possible role for ischemia induced by myocardial arteritis on myocardial structure. Moreover, the scavenging effect of vitamins E and A was associated with inhibition of AHR and CYP1A1 related oxidative stress resulted in decreased TCDD/DF induced cardiotoxicity.
So, highly exposed population for dioxins may be at special risk for cardiac disease. Nutritional awareness for exposed population is critical and antioxidant vitamins A&E are recommended as a cardioprotective agent for at risk population.
Acknowledgements
This work was funded by support of academic research in Zagazig University Projects, Zagazig University Postgraduate & Research Affairs.
References
- Neumeister L (2001) Beyond POPs: Evaluation of the UNEP Chemical Substitutes of the POPs Pesticides Regarding Their Human and Environmental Toxicity PAN Germany Nernstweg Hamburg, Germany.
- Fiedler H (2003) Dioxins and Furans. In: The Handbook of Environmental Chemistry, Part O, Persistent Organic Pollutants, Springer Verlag, Berlin, Germany 3: 125-201.
- Letcher RJ, Bustnes JO, Dietz R, Jenssen BM, Jorgensen EH, et al. (2010). Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci Total Environ. 408: 2995-3043
- Kao JH, Chen KS, Tsai CH, Li HW, Chang-Chien GP (2007) Effects of burnings of wax apple stubble and rice straw on polychlorinated dibenzo-p-dioxin and dibenzofuran concentrations in air and soil. J Air Waste Manag Assoc 57: 457-464.
- Minomo K, Ohtsuka N, Nojiri K, Hosono S, Kawamura K (2011) Polychlorinated dibenzo-p-dioxins, dibenzofurans, and dioxin-like polychlorinated biphenyls in rice straw smoke and their origins in Japan. Chemosphere 84: 950-956.
- SÃdlová T, Novák J, Janosek J, Andel P, Giesy JP, et al. (2009) Dioxin-like and endocrine disruptive activity of traffic-contaminated soil samples. Arch Environ Contam Toxicol 57: 639-650.
- Deng YY, Jia LJ, Li K, Rong ZY, Yin HW (2011) Levels of PCDD/Fs in agricultural soils near two municipal waste incinerators in Shanghai, China. Bull Environ Contam Toxicol 86: 65-70.
- Deng YY, Jia LJ, Li K, Rong ZY, Yin HW (2011) Levels of PCDD/Fs in agricultural soils near two municipal waste incinerators in Shanghai, China. Bull Environ Contam Toxicol 86: 65-70.
- Ha MH, Lee DH, Son HK, Park SK., Jacobs DR Jr (2009). Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999-2002. J Hum Hypertens 23: 274-286.
- Lu CF, Wang YM, Peng SQ, Zou LB, Tan DH, et al. (2009) Combined effects of repeated administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin and polychlorinated biphenyls on kidneys of male rats. Arch Environ Contam Toxicol 57: 767-776.
- Chen HL, Su HJ, Wang YJ, Guo YL, Liao PC, et al. (2006) Interactive effects between CYP1A1 genotypes and environmental polychlorinated dibenzo-p-dioxins and dibenzofurans exposures on liver function profile. J Toxicol Environ Health A 69: 269-281.
- Alsharif NZ, Hassoun EA (2004) Protective effects of vitamin A and vitamin E succinate against 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced body wasting, hepatomegaly, thymic atrophy, production of reactive oxygen species and DNA damage in C57BL/6J mice. Basic Clin Pharmacol Toxicol. 95: 131–138
- Slezak BP, Hatch GE, DeVito MJ, Diliberto JJ, Slade R, et al. (2000) Oxidative stress in female B6C3F1 mice following acute and subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol Sci 54: 390-398.
- Radhey SV, Anugya M, Nalini S (2007) In vivo chlorpyrifos induced oxidative stress: Attenuation by antioxidant vitamins. Pesticide Biochemistry and Physiology. 88: 191–196.
- Suiter CJW, Crowley MF (1984) Nutrition: Principles and Application in Health Promotion. (2nd edn), J.B. Lippincott Company, Philadelphia, USA.
- Ye L, Leung LK (2008) Effect of dioxin exposure on aromatase expression in ovariectomized rats. Toxicol Appl Pharmacol 229: 102-108.
- Brewster DW, Uraih LC, Birnbaum LS (1988) The acute toxicity of 2,3,4,7,8-pentachlorodibenzofuran (4PeCDF) in the male Fischer rat. Fundam Appl Toxicol 11: 236-249.
- Bancroft J, Gamble A (2002) Theory and Practise of Histological Techniques. (5th edn), Churchil, Livingstone, New York, USA.
- Kiernan JA (1999) Histological and histochemical methods : theory and practice (3rd edn), Butterworth-Heinemann, Oxford , UK.
- Harada K, Komuro I, Shiojima I, Hayashi D, Kudoh S, et al. (1998) Pressure overload induces cardiac hypertrophy in angiotensin II type 1A receptor knockout mice. Circulation 97: 1952-1959.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34: 497-500.
- Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105: 114-121.
- MacKenzie WF, Alison RH (1990) Heart, in Pathology of the Fischer Rat. Academic Press, San Diego, CA, USA.
- Jokinen MP, Walker NJ, Brix AE, Sells DM, Haseman JK, et al. (2003) Increase in cardiovascular pathology in female Sprague-Dawley rats following chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3,3',4,4',5-pentachlorobiphenyl. Cardiovasc Toxicol 3: 299-310.
- Saraste A, Pulkki K (2000) Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res 45: 528-537.
- Brunnberg S, Andersson P, Lindstam M, Paulson I, Poellinger L, et al (2006) The constitutively active Ah receptor (CA-Ahr) mouse as a potential model for dioxin exposure effects in vital organs. Toxicology 224 191–201.
- Kopf PG, Huwe JK, Walker MK (2008) Hypertension, cardiac hypertrophy, and impaired vascular relaxation induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin are associated with increased superoxide. Cardiovasc Toxicol 8: 181-193.
- Flesch-Janys D, Berger J, Gurn P, Manz A, Nagel S, et al. (1995) Exposure to polychlorinated dioxins and furans (PCDD/F) and mortality in a cohort of workers from a herbicide-producing plant in Hamburg, Federal Republic of Germany. Am. J. Epidemiol 142: 1165–1175.
- Canga L, Paroli L, Blanck TJ, Silver RB, Rifkind AB (1993) 2,3,7,8-tetrachlorodibenzo-p-dioxin increases cardiac myocyte intracellular calcium and progressively impairs ventricular contractile responses to isoproterenol and to calcium in chick embryo hearts. Mol Pharmacol 44: 1142-1151.
- Xie A, Walker NJ, Wang D (2006) Dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin) enhances triggered afterdepolarizations in rat ventricular myocytes. Cardiovasc Toxicol 6: 99-110.
- Pangonyte D, Stalioraityte E, Ziuraitiene R, Kazlauskaite D, Palubinskiene J, et al. (2008) Cardiomyocyte remodeling in ischemic heart disease. Medicina (Kaunas) 44: 848-854.
- Greaves P (2000) Cardiovascular system, In Histopathology of Preclinical Toxicity Studies Elsivier, Amsterdam, 254–311.
- Puga A, Ma C, Marlowe JL (2009) The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol 77: 713-722.
- Thum T, Borlak J (2000) Gene expression in distinct regions of the heart. Lancet 355: 979-983.
- Peters JM, Narotsky MG, Elizondo G, Fernandez-Salguero PM, Gonzalez FJ, et al. (1999) Amelioration of TCDD-induced teratogenesis in aryl hydrocarbon receptor (AhR)-null mice. Toxicol Sci 47: 86-92.
- Mehrabi MR, Steiner GE, Dellinger C, Kofler A, Schaufler K, et al. (2002) The arylhydrocarbon receptor (AhR), but not the AhR-nuclear translocator (ARNT), is increased in hearts of patients with cardiomyopathy. Virchows Arch 441: 481-489.
- Heid SE, Pollenz RS, Swanson HI (2000) Role of heat shock protein 90 dissociation in mediating agonist-induced activation of the aryl hydrocarbon receptor. Mol Pharmacol 57: 82-92.
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, et al. (2000) Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol 59: 65-85.
- Nebert DW, Puga A, Vasiliou V (1993) Role of the Ah receptor and the dioxin-inducible [Ah] gene battery in toxicity, cancer, and signal transduction. Ann N Y Acad Sci 685: 624-640.
- Poulos TL, Raag R (1992) Cytochrome P450cam: crystallography, oxygen activation, and electron transfer. FASEB J 6: 674-679.
- Banudevi S, Krishnamoorthy G, Venkataraman P, Vignesh C, Aruldhas MM, et al. (2006) Role of alpha-tocopherol on antioxidant status in liver, lung and kidney of PCB exposed male albino rats. Food Chem Toxicol 44: 2040-2046.
- Slater TF (1984) Free-radical mechanisms in tissue injury. Biochem J 222: 1-15.
- Buttke TM, Sandstrom PA (1994) Oxidative stress as a mediator of apoptosis. Immunol Today 15: 7-10.
- Latchoumycandane C, Mathur PP (2002) Effects of vitamin E on reactive oxygen species-mediated 2,3,7,8-tetrachlorodi-benzo-p-dioxin toxicity in rat testis. J Appl Toxicol 22: 345-351.
- Manson JE, Bassuk SS, Stampfer MJ (2003) Does vitamin E supplementation prevent cardiovascular events? J Womens Health (Larchmt) 12: 123-136.
- Yang YM, Huang DY, Liu GF, Zhong JC, Du K, et al. (2005) Inhibitory effects of vitamin A on TCDD-induced cytochrome P-450 1A1 enzyme activity and expression. Toxicol Sci 85: 727-734.
- Sies H, Stahl W (1995) Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr 62: 1315S-1321S.
- Gomez-Fernandez JC, Villalain J, Aranda FJ, Ortiz A, Micol V, et al. (1989) Localization of alpha-tocopherol in membranes. Ann N Y Acad Sci 570: 109-120.
- Oda H, Yamashita K, Sasaki S, Horio F, Yoshida A (1987) Long-term effects of dietary polychlorinated biphenyl and high level of vitamin E on ascorbic acid and lipid metabolism in rats. J Nutr 117: 1217-1223.
- Hakansson H, Manzoor E, Ahlborg UG (1991) Interaction between dietary vitamin A and single oral doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on the TCDD-induced toxicity and on the vitamin A status in the rat. J Nutr Sci Vitaminol (Tokyo) 37: 239-255.
Citation: Wahba NS, Amer MG, Karam RA, Mohamed RH (2012) Effect of Persistent Organic Pollutants (Dioxins) on Rat Myocardium and Amelioration with Antioxidant Vitamins (Role of Aryl Hydrocarbon Receptors and Cytochrome P450). J Clin Exp Pathol 2:130. DOI: 10.4172/2161-0681.1000130
Copyright: © 2012 Wahba NS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16226
- [From(publication date): 11-2012 - Nov 08, 2025]
- Breakdown by view type
- HTML page views: 11266
- PDF downloads: 4960