Research Article Open Access
Effect of Full-Dose Ondansetron on Tobacco Use among Male Alcohol- Dependent Outpatients - A Preliminary Study
Joao Maria Corrêa Filho1 and Danilo Antonio Baltieri1,2*1Department of Psychiatry, University of São Paulo, Brazil
2Department of Psychiatry of ABC Medical School, Santo Andre, Sao Paulo, Brazil
- *Corresponding Author:
- Danilo Antonio Baltieri
Avenida Angélica, nº 2100
conjunto 13. CEP: 01228-200, São Paulo, S.P. Brazil
Tel: +5511-3120-6896
E-mail: dbaltieri@uol.com.br
Received date: April 27, 2013; Accepted date: May 13, 2013; Published date: May 24, 2013
Citation: Filho JMC, Baltieri DA (2013) Effect of Full-Dose Ondansetron on Tobacco Use among Male Alcohol-Dependent Outpatients - A Preliminary Study. J Addict Res Ther 4:150. doi:10.4172/2155-6105.1000150
Copyright: © 2013 Filho JMC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Background: Alcohol and nicotine act synergistically so that individuals dependent on alcohol have higher rates of nicotine dependence, and smokers tend to consume more ethanol than non-smoking alcohol users. A type of pharmacological therapy which deals with both conditions simultaneously can be extremely welcome. This study aims to evaluate if ondansetron influences the number of cigarettes smoked and the quantity of ethanol consumed among alcoholic smokers who completed the treatment.
Method: This research primarily evaluated the efficacy and safety of ondansetron at a 16 mg/day dosage to treat alcohol- and nicotine-dependent outpatients. A double-blind, placebo-controlled, 12-week study was carried out at the University of São Paulo, Brazil. The total sample comprised 65 men, 18-60 years of age, with International Classification of Diseases (ICD-10) diagnoses of alcohol and nicotine dependencies.
Results: Thirty-eight subjects (58.5%) completed this study. Ondansetron at a 16 mg/day dosage was no more effective than placebo in decreasing the mean number of cigarettes smoked and increasing the proportion of alcohol abstinence throughout this study among alcoholic smokers who completed the treatment.
Conclusions: Our study is in line with other trials that did not prove efficacy of ondansetron for smoking cessation.
Keywords
Ondansetron; Alcohol dependence; Alcoholic smokers; Pharmacological trial
Introduction
Daily smoking prevalence in Brazil is around 15% and about 30% of Brazilian smokers consume more than 20 cigarettes per day. This fact suggests that there is a relevant proportion of tobacco users who require therapeutic support based on pharmacological and behavioral approaches [1].
Alcohol-dependent individuals are a group at high risk of frequent and heavy smoking. In fact, the prevalence of smoking among treatmentseeking alcoholics is around 80%. Furthermore, most alcoholics who smoke continue to report a high level of cigarettes smoked during the withdrawal from alcohol as a way to cope with unpleasant symptoms [2].
Alcohol and nicotine act synergistically so that patients that drink and smoke drink more than non-smokers, and drinkers smoke more than non-drinkers. Behavioral studies suggest a role for nicotinic acetylcholine receptors (nACh) in the mediation of alcohol sensitivity [3]. Ethanol may excite Ventral Tegmental Area (VTA) neurons through nACh receptors, facilitating the dopaminergic activity and consequently improving rewarding effects [4]. There seems to be an up-regulation of nACh receptors in situations where there is chronic nicotine intake, enhancing the pleasurable effects of alcohol consumption. In addition, alcohol use may potentiate the activity of certain subunits of acetylcholine receptors and this can cancel out nicotine’s ability to desensitize them, increasing the smoking behavior [5]. Also, studies have provided evidence that a shared genetic basis may underlie the tendency to use both alcohol and nicotine [6].
These neurobiological findings may have important implications for the treatment of alcohol and nicotine dependence. The selection of the most appropriate treatment approach may depend on whether or not the patient is dependent on both drugs [7], and a type of pharmacological therapy which deals with both conditions simultaneously should be welcome.
To date, Food and Drug Administration approved the following medications for the treatment of alcohol dependence - dissulfiram, acamprosate, oral naltrexone, and extended-release injectable naltrexone, and 3 therapies for smoking cessation - nicotine replacement, bupropion, and varenicline. Several other medications are under active study and are sometimes prescribed for alcoholism or nicotine dependence treatment on an off-label basis, such as ondansetron, topiramate, baclofen, pregabalin, and some antidepressants [8,9]. However, few medications, such as topiramate, have been tested to treat both dependencies simultaneously [10,11].
Among these non-approved drugs, ondansetron has emerged as a promising medication for the treatment of alcohol dependence, mainly among early-onset alcoholics [12]. Ondansetron has been also tested for smoking cessation but two short-term trials found no effect on abstinence or withdrawal symptoms [13,14]. Despite the discouraging effects of ondansetron on reinforcing properties of nicotine, there are no good long-term studies, and the oral dosage tested has been commonly low [15].
5-HT3 receptors are densely distributed in the mesocorticolimbic neuronal terminals, regulating dopamine release. Ondansetron blocks the peripheral and central 5-HT3 receptors, and through central antagonism, it inhibits dopamine-release cell firing in the nucleus accumbens15. In addition, ondansetron appears to have activity at the 5-HT1, 5-HT1B, α1-adrenergic, μ-opioid receptors, and α7 and α4β2 nicotinic receptors [16]. This means that this medication may have anxiolytic, antidepressant, and anti-nociceptive properties [17]; furthermore, it can cause release of acetylcholine [18]. Another postulated mechanism for the anxiolytic and antidepressant activities is that the antagonism at 5-HT3 receptor increases the synaptic concentration of the neurotransmitter that is made available to bind elsewhere [19]. Antidepressant and anxiolytic effects of ondansetron seem to be dose-dependent [20].
Considering the high safety and low toxicity of ondansetron in the treatment of different medical problems [15], as well as its presumed efficacy at doses above 12 mg per day for other neuropsychiatric disorders [21-23], we decided to test this drug among alcoholic smokers at a dosage usually prescribed to manage chemotherapy-induced nausea and vomiting, in the same way as has been carried out in studies on its efficacy for treating neuropsychiatric illnesses. In addition, although ondansetron at dosages of 16 and 32 mg daily has shown efficacy and safety as rescue anti-emetic treatment, comparison between both doses has not shown significant difference [24], suggesting that 16 mg of this medication can be a possible ceiling dose. Furthermore, the Brazilian drug information leaflet recommends a maintenance dose of 8 mg twice a day for anti-emetic treatment. Also, according to McFee and McGuigan [25], ondansetron may be given as an oral 16- to 24-mg single dose or 8 mg twice daily for adults.
The aim of this study was to evaluate if full-dose ondansetron influences the number of cigarettes smoked and alcohol consumed throughout an outpatient treatment.
Methods
Participants
Male patients, aged 18-60 years, and with diagnoses of alcohol and nicotine dependencies (based on the International Classification of Diseases - ICD-10) that enrolled as outpatients in the Assistance Sector of the Interdisciplinary Group of Studies on Alcohol and Drugs at the University of São Paulo (PROGREA) were assessed for trial. This service (PROGREA) is exclusively dedicated to the treatment of males with alcohol and/or any other type of drug abuse or dependence.
Exclusion criteria were: (a) <18 years or ≥ 60 years of age; (b) clinically significant medical disease that might have interfered with the evaluation of the study medication, or presence of a safety concern (e.g., cirrhosis, kidney impairment, unstable hypertension, diabetes mellitus, seizure disorder, cardiac failure); (c) a current diagnosis of dependence or abuse of other substances, verified through the application of the DAST (Drug Abuse Screening Test, when cut-off ≥ 5) [26], and interview with participants’ family; (d) clinically significant psychiatric illness, including any psychotic disorder, bipolar disorder, or severe depression; (e) previous treatment with ondansetron within 6 months of randomization; (f) current use of disulfiram, naltrexone or acamprosate; (g) current use of any psychotropic medication including antidepressants, mood stabilizers, antipsychotics, anxiolytics, stimulants, or hypnotics; (h) inability to give full informed consent, and (i) clinical history of mental retardation as it would reduce the accuracy of the information given.
Procedure
Before initiating the double-blind treatment, participants underwent 2-week detoxification period. This pre-study phase was conducted on an outpatient basis, and the participants were given medications with reference to their CIWA-AR (Revised Clinical Institute Withdrawal Assessment for Alcohol) scores [27], such as up to 5 mg/day lorazepam and 300 mg/day vitamin B1, in case they manifested withdrawal symptoms. Mean cellular volume (MCV), laboratory tests to screen for liver disorders- γ-glutamyl-transpeptidase (GGT), Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), and a clinical evaluation of heart function- Electrocardiography (ECG), observation, palpation, and auscultation - were performed in this pre-study phase. Participants included in this study manifested from minimal to moderate withdrawal symptoms, which allowed them to be treated on an outpatient basis.
Following this detoxification period, the participants were randomly divided into two groups through computer-generated random numbers. All participants were instructed to take 1 capsule in the morning and one at night. Once a week, the participants received an envelope with 2 packages containing 7 capsules. One package was designated for morning dosing and the other for night-time; participants were instructed to take two capsules per day.
One group received 2 capsules each containing 8 mg ondansetron, and the other group received 2 placebo capsules every day during the 12 weeks. All capsules in each treatment group were identical in appearance and size and had been manufactured and distributed by the Pharmacy Sector at the Psychiatric Institute of the Clinical Hospital of São Paulo University. This study was not sponsored by a pharmaceutical company.
At each appointment, participants received standardized brief cognitive behavioral interventions. The overall goal of these interventions was to increase the person’s ability to cope with high-risk situations that could precipitate relapses. The drinking and smoking behaviors of the participants were reviewed in each visit, and the medication compliance and motivation for change were improved using motivational interviewing strategies. It was recommended that participants monitor good and bad daily situations during all treatment, and this was discussed with their doctors and, when possible, related to drinking and smoking behaviors. The following topics were standardized and applied to each patient during this treatment: management of negative mood, assertiveness, drug refusal skills, enhancement of social support networks, and relapse prevention.
The codes referent to the medications used were only revealed to the researchers only after all participants had completed the study. Only 2 pharmacists from the Pharmacy Sector at the Psychiatric Institute of the Clinical Hospital of the University of São Paulo knew what medication corresponded to which specific code. The packages containing the capsules were distributed to participants by 2 trained research assistants, who had also been blinded to the study and who assessed the outcome of each participant throughout the study period.
All participants provided written informed consent. They were informed about the study objectives, the nature of the treatment offered, the profile of medications tested, and that the medications they would receive would be chosen at random. The participants were assured of the confidentiality of the data, and were informed that they were free to withdraw their consent and discontinue participation in the study at any time without prejudice to their continued medical care. The Ethics Committee of the Clinical Hospital of the University of São Paulo approved this study.
Measures
After a full history and clinical examination, participants who fulfilled the inclusion criteria initiated this study. Socio-demographic data and lifetime drinking and smoking histories were obtained in a standardized, semi-structured interview commonly used in the therapeutic setting of the PROGREA.
Before initiating this double-blind treatment, all participants were evaluated with the revised Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) [27], the Short Alcohol Dependence Data (SADD) [28], and underwent two-week detoxification period. We also assessed subjects on safety measures including vital signs, weight, adverse effects, and concomitant medication use.
Following the detoxification period, the Timeline Follow Back (TLFB) method [29] was used to quantify self-reported drinking and smoking. The participants were assessed 6 times for smoking behavior, at weeks 2, 4, 6, 8, 10 and 12 after the baseline. We could assess the number of cigarettes smoked as well as the quantity of alcohol used by each patient.
In addition, the Hamilton Depression Rating Scale (HDRS) [30], and the Obsessive-Compulsive Drinking Scale (OCDS) [31] were applied at weeks 1, 6, and 12 of the study. The UKU Side Effect Rating Scale (UKU) [32] was applied at each visit.
For all participants, drinking and smoking behavior was evaluated based on the patient’s self-report at each appointment, and by interviewing a family member at each patient’s appointment. Alcohol abuse hepatic indices, such as Gamma Glutamyl-Transpeptidase (GGT), Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), and Mean Cellular Volume (MCV) were also measured. Cardiac exam (observation, palpation and auscultation), and abstinence symptoms of all participants were evaluated at each appointment.
In addition, medication compliance was evaluated at each appointment by asking participants the following questions: (a) Have you already forgotten to take your medications? (b) Are you sometimes neglectful in regard to your medicine time? (c) Do you skip your medicine time when you are feeling well? (d) When you feel bad (sick, feeling side effects) due to the medicine, do you skip it? Only participants who answered in the negative to the 4 questions were considered adherent to this study. Those that answered affirmatively to one or more than these four questions were considered as protocol violators. Furthermore, the capsules in the returned packages were counted (capsules taken subtracted from capsules given) at every appointment. We coded an individual as adherent if they took 80% or more of the total prescribed pills on a particular week.
In this research, three reasons for dropping out were investigated, such as “refusal to continue” (the patient affirmed that he wanted to stop this type of treatment and try others), “protocol violation” (the patient answered affirmatively to one or more than four questions above), and “premature discontinuation of the follow-up” (the patient gave up following the study and did not manifest any desire to be treated differently).
Outcome criteria
For the proposals of this study, we have firstly verified baseline differences between the ondansetron and placebo groups in terms of sociodemographic features, severity of alcohol dependence, alcohol abuse hepatic indices, and adherence to the treatment provided.
Next, only the alcoholic smokers who completed the study were investigated regarding smoking reduction because they adhered to all requirements of this study, including the correct usage of the medication prescribed.
Statistical analysis
Baseline differences between the two groups were determined using the parametric t test for continuous variables, except when the variances between two variables were unequal, according to Levene’s criteria. Categorical variables were compared by using the χ2 test or the Fisher’s exact test, following Monte Carlo’s method.
We used the Generalized Estimating Equation approach (GEE) [33], which accommodated the repeated measures data and accounted for within-subject correlations. Non-normally distributed variables were log-transformed. The influence of medication groups on the cigarettes smoked and on the proportion of abstinent days throughout the treatment was analyzed by using the GEE model with an autoregressive correlation structure. For all statistical tests performed, a significance threshold of 0.05 was adopted. Data were analyzed using SPSS 20.
Results
Sample characteristics
Sixty-five alcoholic smokers entered the treatment. Participants were encouraged to participate in AA groups, but this was not an obligatory condition of participation in this study. As illustrated in Table 1 and 2, there were no significant differences between both medication groups in terms of socio-demographic data, psychometric measures, and biochemical tests.
Dropouts
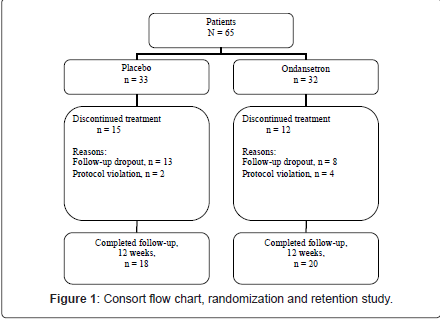
As illustrated in Figure 1, twenty-seven (41.54%) participants dropped out of the trial. Fifteen (57.7%) participants randomized to placebo, and 12 (42%) subjects randomized to ondansetron dropped out from this study and this difference was not significant (χ2 (1) = 0.42, p = 0.51). As illustrated in Table 3 and 4, there were no significant differences between completers and non-completers in terms of sociodemographic data, psychometric measures, and biochemical tests.
| Characteristic | Ondansetron (n = 32) | Placebo (n = 33) | p |
|---|---|---|---|
| Age, mean (SD) | 43.44 (9.53) | 42.67 (8.67) | t = 0.34, 63 df, p = 0.73 |
| Marital status, n (%) Married Single Separated / Widowed |
17 (53.12) 3 (9.38) 12 (37.50) |
24 (72.73) 3 (9.09) 6 (18.18) |
χ2 = 3.20, 2 df, p = 0.21 |
| Education, n (%)
12th grade or less High school or more |
16 (50) 16 (50) |
18 (54.55) 15 (45.45) |
χ2 = 0.13, 1 df, p = 0.81 |
| Quantity of ethanol per day (in grams), mean (SD)a |
328.20 (218.26) |
295.26 (167.94) |
t = 0.68, 63 df, p = 0.49 |
| Years since alcohol-related problems occurred, mean (SD) | 12.94 (11.21) | 12.94 (8.15) | t < 0.01, 63 df, p > 0.99 |
| Early Onset Alcoholics, n (%) | 14 (43.75) | 14 (42.42) | χ2 = 0.01, 1 df, p = 0.91 |
| Family history of alcoholism, n (%) | 25 (78.12) | 27 (81.81) | χ2 = 0.14, 1 df, p = 0.76 |
| Previous treatments for alcoholism, n (%) | 21 (65.62) | 22 (66.67) | χ2 < 0.99, 1 df, p > 0.99 |
| Monthly income (in R$, the Brazilian currency) mean (SD) |
1537.97 (1470.56) |
1499.09 (880.48) |
t = 0.13, 63 df, p = 0.90 |
| Cigarettes smoked/day mean (SD) |
17.34 (8.37) | 15 (12.78) | t = 0.87, 63 df, p = 0.39 |
aIndicates alcohol usage during the last three months preceding the study.
Table 1: Baseline psychosocial characteristics and alcohol use-related aspects between ondansetron and placebo groups.
Cigarettes smoked and Percent Abstinent Days throughout the treatment among alcoholic smokers who adhered to the treatment
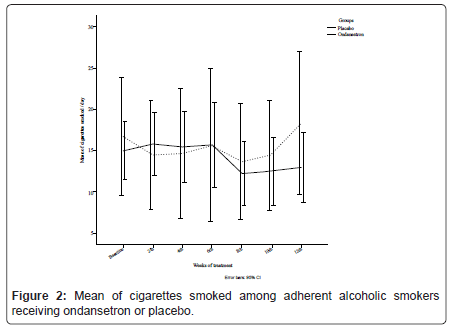
Thirty-eight alcoholic smokers (58.5%) completed this treatment. GEE analysis did not indicate a significant difference between ondansetron and placebo for the mean number of cigarettes smoked [χ2 (6) = 7.05, p = 0.32], where on-average, ondansetron group smoked 14.14 (SD = 1.67) cigarettes per day compared with 15.36 (SD = 3.30) for placebo, as it is illustrated in Figure 2.
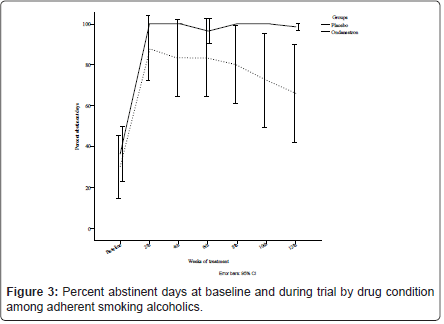
Similarly, the GEE analysis did not demonstrate a significant effect of ondansetron over placebo for the proportion of abstinent days [χ2 (6) = 8.15, p = 0.23], where on-average, there was 85.91% (SD = 2.05) occurrence of abstinence in the ondansetron group compared with 68.47% (SD = 6.86) for placebo throughout the medication period, illustrated in Figure 3.
Safety
The profile of side effects by medication group is shown in Table 5. Although participants in the ondansetron group more frequently reported constipation, it was not significantly different from the placebo group.
| Characteristic | Ondansetron (n = 32) | Placebo (n = 33) | p |
|---|---|---|---|
| Plasma GGT, U/L; mean (SD) (reference range 8-61) |
109.66 (89.71) |
232.39 (491.06) | t = -1.39, 63 df, p = 0.17 |
| Plasma ALT, U/L; mean (SD) (reference range < 41) |
35.37 (26.24) |
36.06 (29.98) | t = -0.09, 63 df, p = 0.92 |
| Plasma AST, U/L; mean (SD) (reference range < 37) |
33.97 (23.97) |
47.58 (45.64) | t = -1.50, 63 df, p = 0.14 |
| Plasma MCV, f/L; mean (SD) (reference range 80-100) |
95.72 (8.60) |
94.74 (6.43) | t = 0.51, 63 df, p = 0.61 |
| CIWA-AR, mean (SD) | 13.09 (8.87) | 14.24 (9.54) | t = -0.50, 63 df, p = 0.62 |
| SADD, mean (SD) | 28.41 (8.61) | 24.73 (6.94) | t = 1.90, 63 df, p = 0.06 |
| OCDS, mean (SD) | 48.47 (8.89) | 44.40 (10.09) | t = 1.72, 63 df, p = 0.09 |
| HDRS, mean (SD) | 9.01 (5.07) | 10.15 (5.57) | t = -0.87, 63 df, p = 0.39 |
Biological markers were measured and instruments were applied over two weeks
before the beginning of double-blind study.
GGT: Gamma-Glutamyl Transferase; ALT: Alanine Aminotransferase; AST:
Aspartate Aminotransferase; MCV: Mean Cellular Volume; CIWA-AR: Clinical
Institute Withdrawal Assessment; SADD: Short Alcohol Dependence Data; OCDS:
Obsessive-Compulsive Drinking Scale; HDRS: Hamilton Depression Rating Scale.
Table 2: Baseline hepatic indices and psychometric measures between ondansetron and placebo groups.
Sample Power
G* Power statistical program was used in a MANOVA for repeated measures with tests of between-within interaction effects. The significance criterion was set at 0.05, and the test used was 2-tailed.
With regards to the variable ‘mean number of cigarettes smoked per day’ evaluated across the study, the sample comprising 38 subjects achieved 14% power to detect differences between the 2 treatment groups versus the hypothesis of equality between both conditions. Theoretically, a sample of 225 adherent smoking alcoholics would be necessary to achieve 80% power with an effect size fixed at 25%, [Pillai’s V = 0.06, Wilks’ λ = 14.06, F (6, 218) = 2.14].
Discussion
Similar to other studies that have investigated the role of ondansetron in smoking cessation [13,14], our study showed that ondansetron at dosage of 16 mg/day was no more effective than placebo in decreasing the mean number of cigarettes smoked and increasing the abstinence proportion among adherent alcoholic smokers.
Ondansetron had a favorable side-effect profile. In this study, although the most common adverse effects of ondansetron were headache and constipation, there were no differences between both medication groups. One could presume that ondansetron at a full-dose can have contributed to the high dropout rates in this study. Despite this, the reported side-effects between the placebo and ondansetron groups were not different. In addition, other studies on ondansetron efficacy in treating depressive and anxiety symptoms (concomitant to or even derived from other illnesses) have successfully tested doses between 8 and 24 mg daily, reporting a reduction in depressive symptoms [21,34], decrease of anxiety in subjects with Obsessive Compulsive Disorder [35], and even improvement on cognitive impairments among schizophrenics [36]. Also, 8 mg ondansetron (but not 4 mg) was shown to be useful for decreasing opiate withdrawal signs [37].
Nevertheless, the optimal dose of ondansetron to treat alcoholism has yet to be determined, mainly because this drug may be useful for different types of alcoholics on a dose-dependent basis. For early onset alcoholics a U-shaped non-monotonic dose-response has been observed, with 4 mcg/Kg b.i.d. dose working better than a higher (16 mcg/Kg b.i.d) or a lower dose (1 mcg/Kg b.i.d) [12,38]. As to the treatment for smoking cessation, further investigations are required, involving larger samples and different oral dosages of ondansetron.
| Characteristic | Completers (n = 38) | Non-completers (n = 27) | p |
|---|---|---|---|
| Age, mean (SD) | 44.66 (8.20) | 40.78 (9.82) | t = 1.73, 63 df, p = 0.09 |
| Marital status, n (%)
Married Single Separated / Widowed |
23 (60.53) 3 (7.89) 12 (31.58) |
18 (66.67) 3 (11.11) 6 (22.22) |
χ2 = 0.85, 2 df, p = 0.69 |
| Education, n (%)
12th grade or less High school or more |
20 (52.63) 18 (47.37) |
14 (51.85) 13 (48.15) | χ2 < 0.01, 1 df, p > 0.99 |
| Quantity of ethanol per day (in grams), mean (SD)a |
288.42 (155.10) |
343.93 (236.83) |
t = -1.14, 63 df, p = 0.26 |
| Years since alcohol-related problems occurred, mean (SD) | 13.37 (8.79) | 12.33 (11.01) | t = 0.42, 63 df, p = 0.68 |
| Early Onset Alcoholics, n (%) | 15 (39.47) | 13 (48.15) | χ2 = 0.48, 1 df, p = 0.49 |
| Family history of alcoholism, n (%) | 31 (81.58) | 21 (77.78) | χ2 = 0.14, 1 df, p = 0.71 |
| Previous treatments for alcoholism, n (%) | 28 (73.68) | 15 (55.56) | χ2 = 2.32, 1 df, p = 0.13 |
| Monthly income (in R$, the Brazilian currency) mean (SD) |
1476.32 (1006.40) |
1577.22 (1444.29) |
t = - 0.33, 63 df, p = 0.74 |
| Cigarettes smoked/day mean (SD) |
15.79 (11.22) |
16.67 (10.41) |
t = - 0.32, 63 df, p = 0.75 |
aIndicates alcohol usage during the last three months preceding the study.
Table 3: Baseline psychosocial characteristics and alcohol use-related aspects between completers and non-completers of treatment.
Some authors report a mixed role of smoking in alcoholism treatment outcomes [39]. Despite the devastating effects of tobacco on health in general, smoking can help alcoholics to cope with alcohol withdrawal symptoms and consequently show better outcomes. This can be particularly true when a certain type of treatment is not effective to decrease alcohol craving and anxiety. Thus, those alcoholics who smoke could remain longer in an ineffective treatment for alcoholism than those who do not smoke.
There are some explanations for the inefficacy of ondansetron at a dosage of 16 mg/day in this study. First, this study was under-powered and of insufficient sample size. Second, previous studies have suggested that ondansetron does not work very well when used to treat smokers. Third, our sample mostly comprised severely alcohol-dependent individuals. According to Sellers et al. [40], ondansetron appears to be less effective for treating heavy drinkers than light drinkers.
Caution is warranted when interpreting the results of this study. Our sample size is not large enough to affirm that ondansetron at dosage of 16 mg/day is ineffective to treat alcoholic smokers. Theoretically, a sample size six times larger would show superiority of ondansetron over placebo in terms of smoking reduction.
This study has several weaknesses that need to be further considered:
1. There were no other psychotherapeutic procedures associated with the pharmacological treatment and behavioral management which could increase the compliance of the patients and the efficacy of the treatment;
2. The number of dropouts was high in this study, probably as a result of its design, which allowed patients to follow the standard community-based programs of treatment, without norms to increase patient retention. Although this approach to trial design, which allows normal life events to influence trial outcome, probably enhances external validity, it can lead to considerable difficulties in interpreting data, such as motives for relapse and premature discontinuation of follow-up;
| Characteristic | Completers (n = 38) | Non-completers (n = 27) | p |
|---|---|---|---|
| Plasma GGT, U/L; mean (SD) (reference range 8-61) | 212.95 (452.14) | 114.30 (137.74) | t = 1.10, 63 df, p = 0.28 |
| Plasma ALT, U/L; mean (SD) (reference range < 41) | 34.76 (26.66) | 37.07 (30.21) | t = - 0.33, 63 df, p = 0.75 |
| Plasma AST, U/L; mean (SD) (reference range < 37) | 41.74 (40.54) | 39.67 (31.99) | t = 0.22, 63 df, p = 0.83 |
| Plasma MCV, f/L; mean (SD) (reference range 80-100) | 95.94 (5.20) | 94.30 (9.83) | t = 0.85, 63 df, p = 0.40 |
| CIWA-AR, mean (SD) | 13.58 (9.70) | 13.81 (8.54) | t = -0.10, 63 df, p = 0.92 |
| SADD, mean (SD) | 25.97 (7.15) | 27.33 (9.07) | t = - 0.67, 63 df, p = 0.50 |
| OCDS, mean (SD) | 46.39 (8.67) | 46.41 (11.09) | t = < -0.01, 63 df, p > 0.99 |
| HDRS, mean (SD) | 9.50 (5.79) | 9.70 (4.70) | t = - 0.15, 63 df, p = 0.88 |
Biological markers were measured and instruments were applied over two weeks before the beginning of double-blind study. GGT, Gamma-Glutamyl Transferase; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; MCV: Mean Cellular Volume; CIWA-AR: Clinical Institute Withdrawal Assessment; SADD, Short Alcohol Dependence Data; OCDS: Obsessive-Compulsive Drinking Scale; HDRS: Hamilton Depression Rating Scale.
Table 4: Baseline hepatic indices and psychometric measures between completers and non-completers of treatment.
| Clinical event | Ondansetron (n = 32) n (%) | Placebo (n = 33) n (%) | p (1df) |
|---|---|---|---|
| Nothing reported | 13 (40.62) | 17 (51.51) | χ2 = 0.77, p = 0.38 |
| Somnolence | 1 (3.12) | 0 | χ2 = 1.05, p = 0.49 |
| Headache | 5 (15.62) | 5 (15.15) | χ2 < 0.01, p > 0.99 |
| Dyspepsia | 2 (6.25) | 3 (9.09) | χ2 = 0.18, p > 0.99 |
| Diarrhea | 1 (3.12) | 2 (6.06) | χ2 = 0.32, p > 0.99 |
| Constipation | 5 (15.62) | 1 (3.03) | χ2 = 3.08, p = 0.10 |
| Genitourinary symptoms | 1 (3.12) | 2 (6.06) | χ2 = 0.32, p > 0.99 |
| Pruritus | 2 (6.25) | 2 (6.06) | χ2 < 0.01, p > 0.09 |
Table 5: Side-effect profile of subjects receiving ondansetron or placebo.
3. Our service is exclusively dedicated to the treatment of males with alcohol and/or other drug problems. Therefore, our findings cannot be extended to women;
4. A larger sample size would have been required to provide higher power for a comparison between both medication groups;
5. Although we used ECG and clinical evaluation of our patients through anamnesis and cardiac auscultation at screening, only clinical evaluation, but not ECG was conducted at each appointment during the study;
6. Breath carbon monoxide level and serum cotinine level were not included in this research plan. Although this can be considered a serious methodological flaw, we have obtained information related to alcohol and tobacco consumption through standardized interviews with these patients and their family members at each appointment.
References
- Monteiro CA, Cavalcante TM, Moura EC, Claro RM, Szwarcwald CL (2007) Population-based evidence of a strong decline in the prevalence of smokers in Brazil (1989-2003). Bull World Health Organ 85: 527-534.
- Meyerhoff DJ, Tizabi Y, Staley JK, Durazzo TC, Glass JM, et al. (2006) Smoking comorbidity in alcoholism: neurobiological and neurocognitive consequences. Alcohol Clin Exp Res 30: 253-264.
- Hendrickson LM, Guildford MJ, Tapper AR (2013) Neuronal nicotinic acetylcholine receptors: common molecular substrates of nicotine and alcohol dependence. Front Psychiatry 4: 29.
- Larsson A, Edström L, Svensson L, Söderpalm B, Engel JA (2005) Voluntary ethanol intake increases extracellular acetylcholine levels in the ventral tegmental area in the rat. Alcohol Alcohol 40: 349-358.
- Johnson BA (2004) Topiramate-induced neuromodulation of cortico-mesolimbic dopamine function: a new vista for the treatment of comorbid alcohol and nicotine dependence? Addict Behav 29: 1465-1479.
- Lê AD, Li Z, Funk D, Shram M, Li TK, et al. (2006) Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci 26: 1872-1879.
- Funk D, Marinelli PW, Lê AD (2006) Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res Health 29: 186-192.
- Garbutt JC, Kampov-Polevoy AB, Gallop R, Kalka-Juhl L, Flannery BA (2010) Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind, placebo-controlled trial. Alcohol ClinExp Res 34: 1849-1857.
- Rosner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, et al. (2010) Acamprosate for alcohol dependence. Cochrane Database Syst Rev CD004332.
- Johnson BA, Ait-Daoud N, Akhtar FZ, Javors MA (2005) Use of oral topiramate to promote smoking abstinence among alcohol-dependent smokers: a randomized controlled trial. Arch Intern Med 165: 1600-1605.
- Baltieri DA, Daró FR, Ribeiro PL, Andrade AG (2009) Effects of topiramate or naltrexone on tobacco use among male alcohol-dependent outpatients. Drug Alcohol Depend 105: 33-41.
- Kranzler HR, Pierucci-Lagha A, Feinn R, Hernandez-Avila C (2003) Effects of ondansetron in early- versus late-onset alcoholics: a prospective, open-label study. Alcohol ClinExp Res 27: 1150-1155.
- West R, Hajek P (1996) Randomised controlled trial of ondansetron in smoking cessation. Psychopharmacology (Berl) 126: 95-96.
- Zacny JP, Apfelbaum JL, Lichtor JL, Zaragoza JG (1993) Effects of 5-hydroxytryptamine3 antagonist, ondansetron, on cigarette smoking, smoke exposure, and mood in humans. PharmacolBiochemBehav 44: 387-391.
- Ye JH, Ponnudurai R, Schaefer R (2001) Ondansetron: a selective 5-HT(3) receptor antagonist and its applications in CNS-related disorders. CNS Drug Rev 7: 199-213.
- McNulty R (2007) Are all 5-HT3 receptor antagonists the same? J NatlComprCancNetw 5: 35-43.
- Mahesh R, Viyogi S, Pandey DK, Yadav S (2010) Evaluation of anti-depressant and analgesic-like activity of ondansetron in rodents model of co-morbid pain and depression. Indian J Pharm Educ Res 44: 160-170.
- Gil-Bea FJ, Domínguez J, García-Alloza M, Marcos B, Lasheras B, et al. (2004) Facilitation of cholinergic transmission by combined treatment of ondansetron with flumazenil after cortical cholinergic deafferentation. Neuropharmacology 47: 225-232.
- Rajkumar R, Mahesh R (2010) The auspicious role of the 5-HT3 receptor in depression: a probable neuronal target? J Psychopharmacol 24: 455-469.
- Ramamoorthy R, Radhakrishnan M, Borah M (2008) Antidepressant-like effects of serotonin type-3 antagonist, ondansetron: an investigation in behaviour-based rodent models. BehavPharmacol 19: 29-40.
- Faris PL, Eckert ED, Kim SW, Meller WH, Pardo JV, et al. (2006) Evidence for a vagal pathophysiology for bulimia nervosa and the accompanying depressive symptoms. J Affect Disord 92: 79-90.
- Friedberg G, Zoldan J, Weizman A, Melamed E (1998) Parkinson Psychosis Rating Scale: a practical instrument for grading psychosis in Parkinson's disease. ClinNeuropharmacol 21: 280-284.
- Sirota P, Mosheva T, Shabtay H, Giladi N, Korczyn AD (2000) Use of the selective serotonin 3 receptor antagonist ondansetron in the treatment of neuroleptic-induced tardive dyskinesia. Am J Psychiatry 157: 287-289.
- Lacerda JF, Martins C, Carmo JA, Lourenço MF, Araújo Pereira ME, et al. (2000) Randomized trial of ondansetron, granisetron, and tropisetron in the prevention of acute nausea and vomiting. Transplant Proc 32: 2680-2681.
- McFee RB, McGuigan MA (2004) Antiemetic drugs. In Dart RC, Caravati EM, McGuigan MA, Whyte IM, Dawson AH, et al. (edr.), Medical Toxicology. Lippincott Williams & Wilkins, Philadelphia.
- Skinner HA (1982) The drug abuse screening test. Addict Behav 7: 363-371.
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 84: 1353-1357.
- Raistrick D, Dunbar G, Davidson R (1983) Development of a questionnaire to measure alcohol dependence. Br J Addict 78: 89-95.
- Annis HM, Sobell LC, Ayala-Velazquez H, Rybakowski JK, Sandahl C, et al. (1996) Drinking-related assessment instruments: cross-cultural studies. Subst Use Misuse 31: 1525-1546.
- HAMILTON M (1960) A rating scale for depression. J NeurolNeurosurg Psychiatry 23: 56-62.
- Anton RF, Moak DH, Latham P (1995) The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol ClinExp Res 19: 92-99.
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K (1987) The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. ActaPsychiatrScandSuppl 334: 1-100.
- Hedeker D, Gibbons RD (1997) Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Meth 2: 64-78.
- Piche T, Vanbiervliet G, Cherikh F, Antoun Z, Huet PM, et al. (2005) Effect of ondansetron, a 5-HT3 receptor antagonist, on fatigue in chronic hepatitis C: a randomised, double blind, placebo controlled study. Gut 54: 1169-1173.
- Soltani F, Sayyah M, Feizy F, Malayeri A, Siahpoosh A, et al. (2010) A double-blind, placebo-controlled pilot study of ondansetron for patients with obsessive-compulsive disorder. Hum Psychopharmacol 25: 509-513.
- Bennett AC, Vila TM (2010) The role of ondansetron in the treatment of schizophrenia. Ann Pharmacother 44: 1301-1306.
- Chu LF, Liang DY, Li X, Sahbaie P, D'arcy N, et al. (2009) From mouse to man: the 5-HT3 receptor modulates physical dependence on opioid narcotics. Pharmacogenet Genomics 19: 193-205.
- Johnson BA (2000) Serotonergic agents and alcoholism treatment: rebirth of the subtype concept--an hypothesis. Alcohol ClinExp Res 24: 1597-1601.
- Schmidt LG, Smolka MN (2007) Results from two pharmacotherapy trials show alcoholic smokers were more severely alcohol dependent but less prone to relapse than alcoholic non-smokers. Alcohol Alcohol 42: 241-246.
- Sellers EM, Toneatto T, Romach MK, Somer GR, Sobell LC, et al. (1994) Clinical efficacy of the 5-HT3 antagonist ondansetron in alcohol abuse and dependence. Alcohol ClinExp Res 18: 879-885.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 14768
- [From(publication date):
August-2013 - Jul 17, 2024] - Breakdown by view type
- HTML page views : 10369
- PDF downloads : 4399