Research Article Open Access
Effect of Compression Pressure on Dissolution and Solid State Characterization of Cefuroxime Axetil
Basavaraj K Nanjwade*, Shamrez Ali M, Veerendra K Nanjwade and FV ManviDepartment of Pharmaceutics, KLE University’s College of Pharmacy, Belgaum, Karnataka, India
- *Corresponding Author:
- Prof. Dr. Basavaraj K Nanjwade
Department of Pharmaceutics
KLE University’s College of Pharmacy
Belgaum, Karnataka, India
Tel: +919742431000
Fax: +91-8312472387
E-mail: bknanjwade@yahoo.co.in
Received date: October 12, 2010; Accepted date: November 30, 2010; Published date: December 02, 2010
Citation: Nanjwade BK, Ali MS, Nanjwade VK, Manvi FV (2010) Effect of Compression Pressure on Dissolution and Solid State Characterization of Cefuroxime Axetil. J Anal Bioanal Tech 1:112. doi: 10.4172/2155-9872.1000112
Copyright: © 2010 Nanjwade BK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The current work aims to investigate the sudden drop of dissolution with respect to higher compression pressure and hardness of tablet with a good and comparative less significant disintegration time and to understand an affect of pressure on drug with respect to compression behavior on dissolution and other properties. Where Cefuroxime Axetil was compressed at different pressure in form of pellets and tablets using hydraulic press and compression machine respectively, and characterized to understand any physical and chemical change using X-ray powder diffraction, differential scanning calorimetry, and Dissolution and Scanning Electron microscopy. Scanning electron microscopy studies of pellets revealed that the change in surface morphology from opaque to translucent clear with increase in breaking force, these different pressure compressed pellets were subjected to uniform reduction of particle size. The similarity factor f2 values of drug release profile were comparably same. The breaking force and pellet hardness had indicated a very firm intrinsic binding force exist with Cefuroxime Axetil, study had investigated the decrease in-vitro dissolution is marked by the compact binding property of individual particles of Cefuroxime Axetil between the excipient due to the increase in compression force.
Keywords
Pressure effect; Compression; Dissolution; Cefuroxime Axetil; Thermal analysis
Introduction
The preferred physical form of a pharmaceutical product is often the solid form, e.g. granules, capsules, tablets and powders for reconstitution into different dosage forms. During the manufacturing of a solid product, drugs and excipients are subjected to mechanical stresses, e.g. during charging and discharging, grinding, mixing, extrusion, fluidization, dispensing, compression and coating. It is thus the concern of the formulation and manufacturing scientists to understand the response of solids to mechanical stresses during the development of a product and in the production line. Two aspects of such a response have been subjects of considerable interest in powder and pharmaceutical science for some time: the mechanics [1] and the mechanical activation [2] of particles during mechanical straining. Understanding, characterizing and predicting material properties are important aspects in the current evolution of the conceptual view of the pharmaceutical development and manufacturing, reflected in several initiatives such as formulation by Quality by Design (QbD) [3].
A variety of methods and procedures are used to assess the mechanical properties of drugs and excipients. Mechanical testing of small particles is not trivial, and the use of compression parameters derived from powder compression curves, i.e. relationships between the volume of the powder bed and the applied pressure, is an attractive approach of both experimental and statistical reasons. The problem with this approach is the question whether the derived compression parameters indicate a physically defined mechanical property, i.e. whether the parameters can represent indications of the plasticity and brittleness of the particles. In order to enable the use of compression parameters as descriptors of mechanical properties of powders, our understanding of their physical interpretation needs to be improved.
For the preparation of pharmaceutical forms only the amorphous form is used. In comparison with the crystalline form that one has better physicochemical and biological properties, e.g. significantly higher solubility and bulk density as well as a higher absorption degree after oral administration [4]. A 10 to 1600 -fold increase in the predicted solubility can be obtained from the amorphous state, whereas only one to four fold increase from metastable polymorphs is obtained compared to the stable crystalline counterpart [5]. On the other hand, due to the higher potential energy the amorphous samples are more physically unstable than the crystalline forms and they tend to crystallize to a more stable crystalline form [6- 8]. It has also been shown that the increase in molecular mobility decreases chemical stability [9]. This creates a problem during manufacturing and storage. Therefore, the storage and processing conditions have to be controlled carefully [10]. An increase in either temperature or humidity increases the molecular mobility of the sample. Amorphous samples are hygroscopic and, therefore, the absorbed moisture acts as a plasticizer. An increase in molecular mobility can lead to crystallization of the sample. A well known example of the stability problems related to amorphous formulations is the case of the cholesterol-lowering drug product Lipitor® [11]. During the development phase the API (atorvastatin) was formulated as an amorphous salt. However, during Phase III studies the API crystallized. This increased the costs of product development, since new studies had to be conducted before the drug could be formulated in a crystalline form. Even though the drug remains amorphous during storage and processing, there is still the possibility that the amorphous drug crystallizes in the gastrointestinal tract when in contact with the fluids [5,12]. If the amorphous drug crystallizes during the dissolution, the dissolution rate will gradually change. The aim of the study was to evaluate the stability of CFA in solid state and to estimate the effect of pressure.
Materials and Methods
Materials
Cefuroxime Axetil (Amorphous) was kindly donated by (Aurobindo Pharma, India), Microcrystalline cellulose were provided by (FMC India), Croscarmellose sodium, Type A (JRS PHARMA GmbH & Co. KG), Sodium lauryl sulphate (Cognis, Turkey) , Silica Colloidal Anhydrous (Evonik, India), Opaspray white (Coloron , India), Aluminum foil strips (Associated capsules group , India) and all other chemicals used were of Analytical grade and procured from commercial sources, Compression machine with Sense Force HMI instrumentation package (Remik-II, Karnavati Eng, India), Hydraulic press Perkin Elmer, Multi mill (Bectochem India).
Formulation of tablets: The batches of Cefuroxime Axetil tablet were manufactured by direct compression method the required quantities of the ingredients were weighed as mentioned in Table 1 and blended to form a homogenous powder mix. The blends were then compressed on 19.2 x 5.9-mm oval, caplet shaped punch set on a Rotary Compression machine (Remik-II, Karnavati Engineering, India) Approximately 500 tablet in a batch with 3 reproducible batches of single hardness were compressed , each tablet was made to contain 500mg of cefuroxime theoretical weight and at approximately equal hardness. Sense force-II (Karnaavati Engineering), was used to determine the compression force required to produce tablets of approximately equal hardness, and mentioned in Table 2.
| Ingredient | Batch code | |||||
|---|---|---|---|---|---|---|
| ( mg/tab) | F1 | F2 | F3 | F4 | F5 | F6 |
| Cefuroxime Axetil | 544 | 544 | 544 | 544 | 544 | 544 |
| MCC (102) | 400 | 400 | 400 | 400 | 400 | 400 |
| Sodium lauryl sulphate | 20 | 20 | 20 | 20 | 20 | 20 |
| Collidol silicon dioxide | 5 | 5 | 5 | 5 | 5 | 5 |
| Croscarmellose sodium, Type A | 30 | 30 | 30 | 30 | 30 | 30 |
| Magnesium Stearate | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Opaspray white | 45 | 45 | 45 | 45 | 45 | 45 |
Table 1: Composition of CFA tablets compressed.
| Formulation | Weight(mg) Mean ± S.D |
Thickness (mm) Mean ± S.D |
Length(mm) Mean ± S.D |
Hardness (Kp) Mean ± S.D |
Disintegration time (min) Mean ± S.D |
|---|---|---|---|---|---|
| F1 | 1053.615 ± 1.894 | 6.522 ± 0.020 | 19.221 ± 0.010 | 12.03 ± 0.019 | 1.8 ± 0.029 |
| F2 | 1051.769 ±1.964 | 6.519 ± 0.021 | 19.223 ± 0.009 | 12.10 ± 0.026 | 1.9 ± 0.012 |
| F3 | 1053.538 ± 2.183 | 6.522 ± 0.019 | 19.225 ± 0.011 | 12.20 ± 0.018 | 2.0 ± 0.03 |
| F4 | 1053.231 ± 2.314 | 5.041 ± 0.131 | 19.207 ± 0.049 | 18.23 ± 0.022 | 3.1 ± 0.018 |
| F5 | 1052.769 ± 2.047 | 5.062 ± 0.131 | 19.225 ± 0.011 | 19.22 ± 0.013 | 3.2 ± 0.019 |
| F6 | 1052.769 ± 1.832 | 5.060 ±0.154 | 19.224 ± 0.011 | 20.02 ± 0.012 | 3.5 ± 0.030 |
Table 2: CFA Tablet Physical characteristic of Batch (F1-F6).
Compression of pellets: Accurately weighed quantity of theCefuroxime Axetil 614 mg was filled in the die cavity of the hydraulic press (Perkin Elmer), which was compressed at various pre-determined pressures, 200, 400 , 600 , 800 and 1200 Psi. With following parameters mentioned in Table 3 and these pellets were further crushed in multi mill with knife forward action and the resultant crushed particle were resized by passing it through No 100 Mesh and the powder was packed in amber colored glass vial with stopped were stored in silica gel desiccators prior to use.
| Pellets | Weight(mg) Mean ± S.D |
Thickness (mm) Mean ± S.D |
Diameter (mm) Mean ± S.D |
Hardness (Kp) |
|---|---|---|---|---|
| 200 PSI | 614.00 ± 0.002 | 4.30 ± 0.021 | 13.07 ± 0.001 | 12.03 ± 0.019 |
| 400 PSI | 614.00 ± 0.013 | 3.86 ± 0.020 | 13.07 ± 0.001 | 12.10 ± 0.026 |
| 600 PSI | 614.00 ± 0.011 | 3.56 ± 0.022 | 13.07 ± 0.001 | 12.20 ± 0.018 |
| 800 PSI | 614.00 ± 0.010 | 3.42 ± 0.019 | 13.07 ± 0.001 | 18.23 ± 0.022 |
| 1200 PSI | 614.00 ± 0.012 | 3.40 ± 0.019 | 13.07 ± 0.001 | 19.22 ± 0.013 |
Table 3: CFA pellets compressed at different pressure.
Breaking force determination: The breaking force of the prepared tablets was determined after compression using Erweka hardness tester (Erweka TBH 310 MD), which also measures the diameter of the tablets. Ten tablets from each batch were tested for tablet strength, and the mean and standard deviation were calculated.
Disintegration time: Disintegration times of the prepared tablets were measured in 900 mL of purified water with disc at 37°C using Erweka TAR series tester. Disintegration times of six individual tablets were recorded.
In Vitro Dissolution Studies
Dissolution of CFA tablets
The dissolution studies of the prepared tablets were carried out using USP Apparatus 2 (Pharma test PTWS 310, Germany), Dissolution was performed in 0.07 N HCl (900 mL each) at 37±0.5°C at the paddle speed 55 rpm, determination of amount of Cefuroxime (C15H16N4O6S) dissolved by employing UV absorption at a wavelength of maximum absorbance at about 278 nm on the filtered portion of solution under test, suitably diluted with dissolution medium , in comparison with standard solution having a known concentration of Cefuroxime Axetil RS, equivalent to 0.01 to 0.02 mg of Cefuroxime (C15H16N4O8S), samples were analyzed at 15 min and 45 min, using (Jasco V-350, Japan) spectrophotometer, dissolution test were carried out in triplicate and showed a high repeatability, the mean values of the triplicate were used for plotting the data.
Dissolution of compressed compact pellets
The dissolution studies of the compact pellets were not possible, because of the integrity of the pellet and lack of self disintegration, hence the pellets were milled and pass through Sieve No 100, to get the uniformity, and weight quantity of 545 mg of powder was brought in contact with dissolution medium and dispersed in a suspension before transferring it into the dissolution medium 0.07 N HCl (900 mL each) at 37±0.5°C at the paddle speed 55 rpm, and the suspension were made simultaneously. Fixed volume of 5ml medium was removed and 5ml of dissolution medium was replaced to the dissolution apparatus to maintain constant sink volume, determination of amount of Cefuroxime (C15H16N4O6S) dissolved by employing UV absorption at a wavelength of maximum absorbance at about 278 nm on the filtered portion of solution under test, suitably diluted with dissolution medium, in comparison with standard solution having a known concentration of Cefuroxime Axetil RS, equivalent to 0.01 to 0.02 mg of Cefuroxime (C15H16N4O8S), samples were analyzed at 15, 30 and 45 min, using (Jasco V-350, Japan) spectrophotometer, dissolution test were carried out in triplicate and showed a high repeatability, the mean values of the triplicate were used for plotting the data.
Structural analysis
X-ray powder diffraction: X-ray powder patterns were obtained using Philips X-ray diffractometer (Model: PW 1710) with a copper target. The conditions were: voltage - 15 kV; scanning speed 1º per minute; temperature of acquisition - room temperature; detector: scintillation counter detector; sample holder non-rotating holder.
Thermal analysis
Differential scanning calorimetry: Differential scanning calorimetry (DSC) measurements were carried out in triplicate using a Perkin Elmer Series 7 DSC thermal analysis system (Perkin Elmer Ltd., Beaconsfield, UK). Samples between 2 and 10 mg were crimped and sealed in aluminium DSC pans with vented lids that were placed in sample cells under nitrogen. Samples were scanned from 25°C to 400°C at 5°C/min.
Infrared (IR) spectroscopy
IR spectroscopy was conducted using a Shimadzu FTIR 8300 Spectrophotometer (Shimadzu, Tokyo, Japan) and the spectrum was recorded in the wavelength region of 4000-400 cm-1. The procedure consisted of dispersing a sample in KBr and compressing into discs by applying a pressure of 5 ton for 5 min in a hydraulic press. The pellet was placed in the light path and the spectrum was recorded. All spectra were collected as an average of three scans.
Scanning electron microscopy
Scanning electron microscopy (SEM) was used to visualize particle surface characteristics and obtain particle size comparisons. Samples were photographed using Hitachi S520 SEM (Hitachi, Tokyo, Japan). Samples were mounted onto a graphite layer on an aluminium stub and gold coated under vacuum using a Polaron SEM cool sputter coater (Polaron, Hailsham, UK), prior to analysis.
Result and Discussions
In vitro dissolution studies
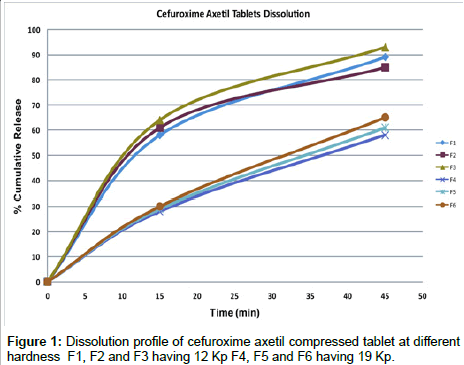
Effect of hardness on dissolution: All the formulation were compressed individually, to get predetermined hardness, as investigated the effect of hardness on the Cefuroxime Axetil release, it is clear from the dissolution profile (Figure 1) that the tablet F1, F2 and F3 , which were compressed at lower hardness has shown desired release within 15 minutes which was a average of 61% of the labeled amount of C16 H10N4O8S and further it was an average of 89% after 45 minutes, which passes the requirement of Cefuroxime Axetil tablet monograph in USP 28, by reducing the thickness and increasing the tonnage on the compression machine cams higher hardness was achieved, for the other three formulations F4, F5 an F6 were subjected to in-vitro release, the behavior was completely different irrespective of the disintegration time, the release after 15 minutes an average was 29% and 61.3% after 45 minutes. This clearly indicates that there is sharp decline in the release of the drug when the tablet of similar size and shape but with different hardness as this phenomenon is normal in all other tablet dosage form, here the disintegration time difference is less than 1 minute, which indicates the disintegrating is assisted by super disintegrant, hence there is certain effect on Cefuroxime Axetil leading to sharp decline of dissolution. Further similarity factor (f2) and dissimilarity factor (f1) was calculated [13,14] for the average of lower hardness tablets Vs the higher hardness tablets, similarity factor (f2) was 29.05, which should ideally between 50-100 as shown in Table 4.
| Time | Reference (Average % Release of F1, F2 and F3) | Test (Average % Release of F3, F4 and F5) |
|---|---|---|
| 15 minutes | 65.00 | 29.00 |
| 45 minutes | 89.00 | 61.333 |
| Similarity factor (f2) (50-100) |
29.05 | |
| Dissimilarity factor (f1) (0-15) | 41.34 |
Table 4: Similarity and dissimilarity factor f1 and f2 for Cefuroxime Axetil tablets compressed at various hardness.
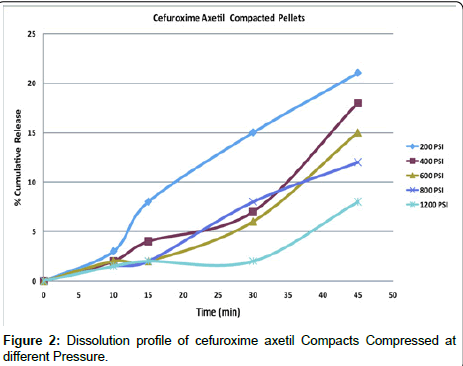
Effect of pressure on dissolution of compacted pellets: Dissolution of the compacts was difficult, because of its brittle nature and lack of self disintegration ability , still one such attempt was made to check the release behavior, the erosion of the Cefuroxime Axetil was directly proportional to the compressed pressure, hence the disintegration ability was poor, the dissolution profile of various compacts have shown in Figure 2, the concentration of the dissolved Cefuroxime in 0.07 N Hcl at 10 minutes where highest release was 3% and consecutively for 15, 30 and 45 minutes was 8, 15 and 21% respectively which is more half less than the initial 15 minutes of compressed tablets, sink condition was maintained for dissolution according to USP method [15] the release was unlikely to influence to such an extent, which indicates the Cefuroxime Axetil is having strong intrinsic binding properties, it was observed that Cefuroxime Axetil is having a strong binding and gelling property, which is responsible for the good compaction, brittleness and non- disintegrating behavior of compact. Increment of release of drug from higher hardness to lower hardness signifies surface erosion is responsible for the release, as there is an overall significant drop of the release which clearly indicates that there is effect of pressure on release behavior.
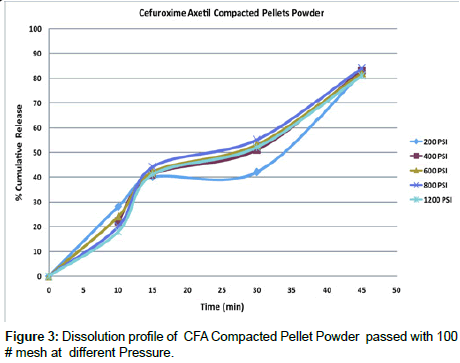
Dissolution studies of compacted pellet powder: All individual set of the pellets were crushed using multi mill and passed through No 100 Mesh to get the particle size uniformity, this method was employed to see the effect of dissolution of particles after compaction, at various pressure 200, 400, 600, 600, 800 and 1200 PSI, and the results very satisfactory and the difference between the lowest pressure 200 PSI is comparatively similar to the highest pressure 1200 PSI which was 57.20 and the dissimilarity factor (f1) between two profile was also less significant Table 5 and Figure 3.
| Time | Reference (Average % Release of 200 PSI) | Test (Average % Release of 1200 PSI) |
|---|---|---|
| 10 minutes | 28.00 | 18.00 |
| 15 minutes | 40.00 | 41.00 |
| 30 minutes | 42.00 | 52.00 |
| 45 minutes | 82.00 | 81.00 |
| Similarity factor (f2) (50-100) |
57.20 | |
| Dissimilarity factor (f1) (0-15) | 11.45 |
Table 5: Similarity and dissimilarity factor f1 and f2 for Cefuroxime Axetil tablets powder of compacted pellets.
Following the above result there were challenges ahead for to understand the mechanism of decrease in dissolution and this process enabled to simulates the conditions and helped to understand, can reprocessing Recoverable residue which are generated in routine industrial production of tablet will give us the desired dissolution.
From present study it can be postulated that there should be three main factors responsible for a decrease in dissolution rate are (a) Good compression property of the Cefuroxime Axetil itself; (b) an increase in the binding property due to increase of pressure (c) Pressure sensitive compaction lead binding even in presence of excipients.
Structural analysis
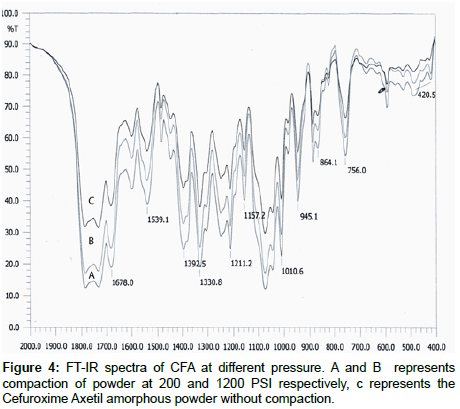
IR studies: Comparative IR spectra were taken for any possible change with the drug, showed major peaks at 1678.9, 1539.1, 1392.5, 1330.8, 1211.2, 1157.2, 1010.6, 945.1, 864.1, 756.0, 420.5 cm-1. The results revealed no considerable changes in the IR peaks of Cefuroxime Axetil when compared between different pressures. Shown in Figure 4.
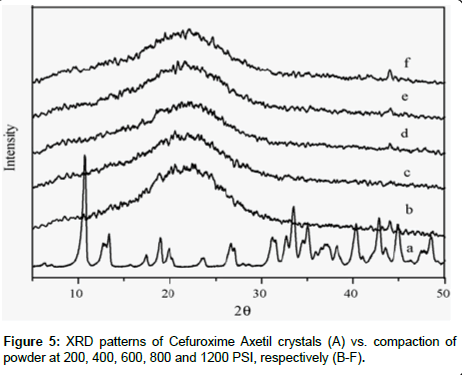
XRD Analysis: XRD was performed to investigate the effect of the pressure on the crystallinity of Cefuroxime Axetil with increase of pressure, in order to simulates and understand decrease in the dissolution with increase in hardness of tablets. XRD patterns are shown in the Figure 5 of the compressed pellets of amorphous Cefuroxime Axetil was compares with Cefuroxime Axetil crystalized, showed entirely different pattern as well as line intensities, the crystalline CFA showed intense peaks at between 10 and 30O theta , where as there were no significant pattern as that of the crystalline. This signifies that there is no substantial conversion to crystallinity, after the compression pressure on amorphous Cefuroxime Axetil.
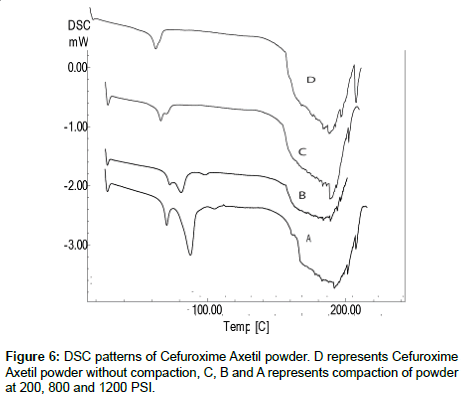
Differential scanning calorimetry: DSC was performed to investigate the effect of the pressure on the crystallinity of Cefuroxime Axetil with increase of pressure, in order to simulates and understands decrease in the dissolution with increase in hardness of tablets. DSC patterns are shown in the Figure 6 a characteristic glass transitions for an amorphous solid were observed as the temperature increased appears as step along with base line.
As the temperature increases, an amorphous solids will become less viscous, which will trigger the molecules to obtain enough freedom of motion to spontaneously arrange themselves into a crystalline form, as due to the increased binding property of A, B and C when compared to D there might be no enough freedom of motion, which might be showing the slight pseudo-crystallization effect.
Scanning Electron Microscopy: SEM was performed to investigate the effect of the pressure on the surface of Cefuroxime Axetil with increase of pressure, SEM pictures are shown in the Figure 7, demonstrates the clear effect of pressure on the cefuroxime Axetil and due to the pressure, there is a visible change in texture and integrity of shape, hence increase in binding property when A, B, and C is compared with D which is cefuroxime axetil amorphous uncompressed powder.
Conclusion
Amorphous Cefuroxime Axetil when subjected to a strong compression force revels that there is significant decrease in dissolution of Cefuroxime Axetil in tablet form, which was reinvestigated using compressed pellets the dissolution data offered an interesting results. All the pellets which were subjected to different pressure and uniform particle size later has shown similar results which was mathematically compared using similarity and dissimilarity factor (f1 & f2), this has indicated uniform the particle size similar the drug release irrespective of the compression pressure applied. Further breaking force and pellet hardness had indicated a very firm intrinsic binding force exist with Cefuroxime Axetil, study had investigated the decrease in-vitro dissolution is marked by the compact binding property of individual particles of Cefuroxime Axetil between the excipient due to the increase in compression force.
Hence a compression pressure lead compaction of Cefuroxime Axetil is a major reason for a drastic drop of dissolution, by the above fact we can conclude any recoverable residue generated during the production process of tablets can be crushed to a validated can be reutilized.
References
- Roberts RJ, Rowe RC (1987) Brittle/ductile behaviour in pharmaceutical materials used in tabletting. Int J Pharm 36: 205-209.
- Hüttenrauch R, Fricke S, Zielke P (1985) Mechanical activation of pharmaceutical systems. Pharm Res 2: 302-306.
- Huang J, Kaul G, Cai C, Chatlapalli R, Hernandez-Abad P, et al. (2009) Quality by design case study: an integrated multivariate approach to drug product and process development. Int J Pharm 382: 23-32.
- Tejchman B, Jaromin´ ska M, Hordecka M, Oszczapowicz I (1995) Evaluation of stability of cefuroxime axetil in solid state. Acta Polon Pharm 52: 477-/482.
- Hancock BC, Parks M (2000) What is the true solubility advantage for amorphous pharmaceuticals? Pharm Res 17: 397-404.
- Yu L (2000) Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev 48: 27-42.
- Cui Y (2007) A material science perspective of pharmaceutical solids. Int J Pharm 339: 3-18.
- Craig DQ, Royall PG, Kett VL, Hopton ML (1999) The relevance of the amorphous state to pharmaceutical dosage forms: glassy drugs and freeze dried systems. Int J Pharm 179: 179-207.
- Guo Y, Byrn SR, Zografi G (2000) Physical characteristics and chemical degradation of amorphous quinapril hydrochloride. J Pharm Sci 89: 128-143.
- Zhang GG, Law D, Schmitt EA, Qiu Y (2004) Phase transformation considerations during process development and manufacture of solid oral dosage forms. Adv Drug Deliv Rev 56: 371-390.
- Gardner CR, Walsh CT, Almarsson O (2004) Drugs as materials: valuing physical form in drug discovery. Nat Rev Drug Discov 3: 926-934.
- Debnath S, Predecki P, Suryanarayanan R (2004) Use of glancing angle X-ray powder diffractometry to depth-profile phase transformations during dissolution of indomethacin and theophylline tablets. Pharm Res 21: 149-159.
- Moore JW, Flanner HH (1996) Mathematical comparison of curves with an emphasis on in-vitro dissolution profiles. Pharm Tech 20: 64-74.
- Staff (1997) Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms.US Food and Drug Administration, Rockville, MD, USA.
- Staff (2007) United States Pharmacopeia USP-30 NF25 <1088>.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 20275
- [From(publication date):
December-2010 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 15194
- PDF downloads : 5081