Rapid Communication Open Access
Effect of Anticoagulants on Amyloid®-Protein Precursor and Amyloid Beta Levels in Plasma
Cara J. Westmark1*, Crystal M. Hervey3, Elizabeth M. Berry-Kravi3,4,5 and James S. Maltera2
1Waisman Center for Developmental Disabilities
2Department of Pathology & Laboratory Medicine, University of Wisconsin, Madison, WI 53705
3Department of Pediatrics, Rush University Medical Center, Chicago, IL 60612 Running Title: Blood Anticoagulants and AD Biomarkers
4Biochemistry, Rush University Medical Center, Chicago, IL 60612 Running Title: Blood Anticoagulants and AD Biomarkers
5Neurological Sciences, Rush University Medical Center, Chicago, IL 60612 Running Title: Blood Anticoagulants and AD Biomarkers
- Corresponding Author:
- Dr. Cara J. Westmark
Waisman Center for Developmental Disabilities
Room T507, 1500 Highland Avenue
Madison, WI 53705
Tel: (608) 262-9730
Fax: (608) 263-0529
E-mail: westmark@wisc.edu
Received date: July 15, 2011;Accepted date: July 22, 2011; Published date: July 24, 2011
Citation: West mark CJ, Hervey CM, Berry-Kravisc EM, Maltera JS (2011) Effect of Anticoagulants on Amyloid®-Protein Precursor and Amyloid Beta Levels in Plasma. J Alzheimers Dis 1:101. doi: 10.4172/2161-0460.1000101
Copyright: © 2011 Westmark CJ. This is an open-access article distributed un der the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Altered levels of amyloid β-protein precursor (AβPP) and/or amyloid beta (Aβ) are characteristic of several neurological disorders including Alzheimer's disease (AD), Down syndrome (DS), Fragile X syndrome (FXS), Parkinson's disease (PD), autism and epilepsy. Thus, these proteins could serve as valuable blood-based biomarkers for assessing disease severity and pharmacological efficacy. We have observed significant differences in Aβ1-42 levels in human plasma dependent on the anticoagulant utilized during blood collection. Our data suggests that anticoagulants alter AβPP processing and that care needs to be used in comparing published studies that have not utilized the same blood collection methodology.
Keywords
Alzheimer's disease; Amyloid β-Protein Precursor; Amyloid beta; Biomarker; ELISA
Abbreviations
AβPP: Amyloid β -protein precursor; Aβ: Amyloid beta; AD: Alzheimer's disease; CTF: C-Terminal Fragmentsp; DS: Down syndrome; FXS: Fragile X Syndrome; PBMC: Peripheral Blood Mononuclear Cells; PD: Parkinson's disease; RUMC: Rush University Medical Center
Introduction
The dysregulated expression of AβPP and its proteolytic products has been implicated in the pathology of several neurological disorders. Aβ senile plaques are found in brain autopsy tissue from individuals with AD as well as in a significant percentage of patients with DS [1], PD [2] and temporal lobe epilepsy [3]. AβPP and Aβ are elevated in the brain of a mouse model of FXS [4], and sAβPPα is elevated in blood plasma from autistic children [5,6]. Thus, AβPP and its proteolytic derivatives could serve as valuable blood-based biomarkers for disease progression and therapeutic efficacy in some or all of these disorders.
During AD progression, there is a shift in the Aβ1-42/Aβ1-40 ratio. Plasma Aβ1-42 levels are increased in patients with mild cognitive impairment, but drop to control levels by the time of AD diagnosis [7]. Similarly, in DS, elevated plasma Aβ1-42 is associated with earlier onset of AD [8] and the Aβ1-42/Aβ1-40 blood plasma ratio is lower than in controls [9]. We wanted to determine if plasma levels of sAβPPα and Aβ could be used as biomarkers for FXS. We found a reduced Aβ1-42/Aβ1- 40 ratio in FXS patients compared to control plasma (Westmark et al, manuscript submitted), which was consistent with other amyloidogenic diseases. During the course of this work, we collected control blood samples from two clinical sites and discovered a large variation in Aβ levels dependent on where the blood was collected. The two sites utilized different anticoagulants during blood collection, and herein, we demonstrate that blood plasma levels of sAβPPα and Aβ levels are significantly altered dependent on the type of anticoagulant used during blood collection.
Materials and Methods
Donors were recruited from the FXS Clinic at Rush University Medical Center (RUMC) in Chicago, IL. The study was approved by the RUMC Institutional Review Board and all donors signed the appropriate consent forms for study participation. Donors (age 23-33) were normal volunteers working at RUMC and had no history of cognitive or mental health disorders. To avoid differences in blood collection technique or methodology, all blood samples for the experiments shown herein were collected at the same site on the same day by a single phlebotomist. Blood from each of 5 donors was drawn into blood collection vacutainers containing various anticoagulants [144 USP units lithium heparin (Becton Dickinson, Franklin Lakes, NJ, USA, product #367880), 10.8 mg K2EDTA (Becton Dickinson, Franklin Lakes, NJ, USA, product #367863), 68 SP units sodium heparin (Becton Dickinson, Franklin Lakes, NJ, USA product #367871) or 0.105 M sodium citrate (Becton Dickinson, Franklin Lakes, NJ, USA, product #369714)]. The blood was spun at 1,500 rpm and the plasma supernatant removed and frozen at –80°C. The remaining anticoagulated blood was shipped by overnight delivery from RUMC to the University of Wisconsin- Madison where peripheral blood mononuclear cells (PBMC) were isolated within 24 hr as previously described [10] and cultured for 24 hr prior to harvesting the culture media for ELISA analyses. For the assessment of sAβPPα, Aβ1-40 and Aβ1-42 by ELISA, plasma was thawed and clarified at 12,000 rpm for 10 min at 4°C. ELISAs were performed per the manufacturer’s instructions (BioSource, Int., Camarillo, CA, USA, catalog #KHB0051, KHB3482, KHB3442) with the following modifications for the Aβ assays: (1) the sample volume was doubled from 50 µL to 100 µL, (2) the incubation time was extended from 3 hr to overnight at 4°C, and (3) after the overnight incubation, the samples were removed from the antibody-coated wells prior to addition of the detection antibody.
Results and Discussion
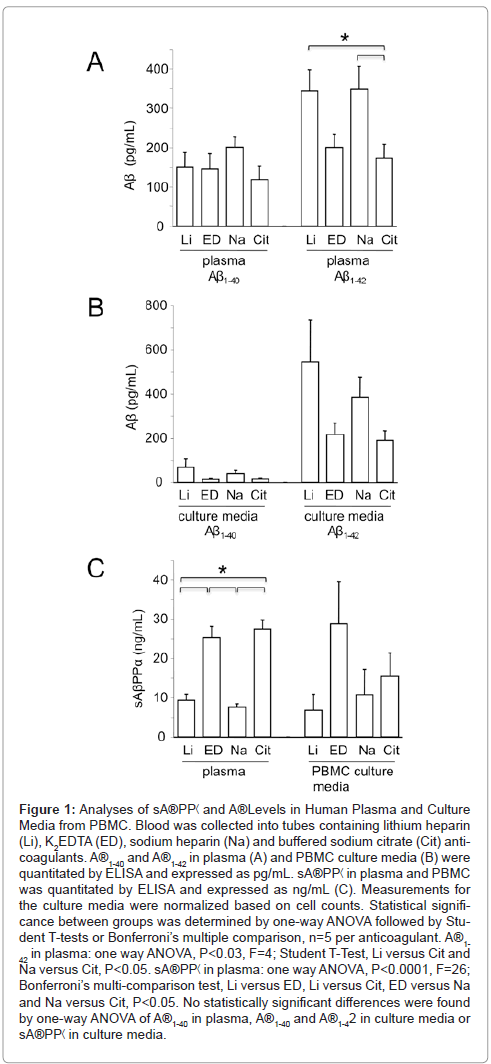
We compared the effect of anticoagulant on Aβ levels in both plasma and PBMC culture media. Aβ1-42 levels were significantly lower in plasma derived from whole blood exposed to K2EDTA or buffered sodium citrate compared with heparin (Figure 1A). Aβ1-40 levels in plasma were equivalent with all anticoagulants tested (Figure 1A). In culture media from PBMC, there was a trend for increased levels of both Aβ1-40 and Aβ1-42 with the lithium and sodium heparin anticoagulants; however, the differences were not statistically significant (Figure 1B). AβPP is processed by β- and γ-secretases to generate multiple species of Aβ as well sAβPPα and C-terminal fragments (CTF) or by α-secretase, which cleaves within the Aβ region and generates sAβPPα and CTF. To confirm that AβPP processing is altered in response to blood anticoagulants, we assessed sAβPPα levels in plasma, which were significantly elevated in samples derived from blood exposed to K2EDTA or buffered sodium citrate in comparison to the heparin samples (Figure 1C). Differences in sAβPP secreted into the media from cultured PBMC were not statistically significant. These data strongly suggest that post-blood collection processing of AβPP occurs with heparin anticoagulants favoring β-secretase (amyloidogenic) processing and K2EDTA and buffered sodium citrate favoring γ-secretase (nonamyloidogenic) processing.
Figure 1: Analyses of sA®PP< and A®Levels in Human Plasma and Culture Media from PBMC. Blood was collected into tubes containing lithium heparin (Li), K2EDTA (ED), sodium heparin (Na) and buffered sodium citrate (Cit) anticoagulants. A®1-40 and A®1-42 in plasma (A) and PBMC culture media (B) were quantitated by ELISA and expressed as pg/mL. sA®PP< in plasma and PBMC was quantitated by ELISA and expressed as ng/mL (C). Measurements for the culture media were normalized based on cell counts. Statistical significance between groups was determined by one-way ANOVA followed by Student T-tests or Bonferroni’s multiple comparison, n=5 per anticoagulant. A®1- 42 in plasma: one way ANOVA, P<0.03, F=4; Student T-Test, Li versus Cit and Na versus Cit, P<0.05. sA®PP< in plasma: one way ANOVA, P<0.0001, F=26; Bonferroni’s multi-comparison test, Li versus ED, Li versus Cit, ED versus Na and Na versus Cit, P<0.05. No statistically significant differences were found by one-way ANOVA of A®1-40 in plasma, A®1-40 and A®1-42 in culture media or sA®PP< in culture media.
These results have important implications regarding the use of sAβPPα and Aβ as blood-based biomarkers. There have been several studies documenting plasma levels of Aβ during AD progression with Aβ isoforms elevated or equivalent between controls and patients dependent on the study [11-14]. It is generally accepted in the AD field that protocols and platforms for Aβ measurements need to be standardized to allow for multi-site comparisons of data; however, anticoagulants are not considered a variable. At first our results appear inconsistent with a rigorous study by Okereke and colleagues in which multiple AD laboratories across the U.S. tested aliquots of the same plasma samples in five different ELISA protocols to assess intra-assay reproducibility, the impact of K2EDTA versus heparin anticoagulant tubes and the effect of processing time on Aβ determinations [15]. Similar to our protocol, blood was collected from 5 control individuals on the same occasion into different types of blood collection tubes and plasma was separated from whole blood within a few hours of collection and aliquoted and frozen. They reported that Aβ1-40 and Aβ1-42 values were generally similar for EDTA and heparin samples within ELISA protocols [15]; however, we observed a significant elevation in Aβ1-42 when heparin was used as the anticoagulant. We used a commercially available ELISA kit from BioSource that utilizes a monoclonal antibody specific for the NH2-terminus of human Aβ and a rabbit detection antibody specific for the COOH-terminus of Aβ1-42. This ELISA protocol most closely resembled their Protocol B, which gave a 32% decrease in Aβ1-42 in EDTA compared to heparin samples as well as the highest percent recovery of spiked Aβ1-42. Thus, we both observed decreased Aβ1-42levels when EDTA was used as the anticoagulant.
In conclusion, a robust and reliable blood-based biomarker is needed to assess the progression of amyloidogenic diseases and therapeutic efficacy. Our results demonstrate that blood collection methodology affects downstream ELISA measurements of Aβ1-42 and sAβPPα and that the choice of anticoagulant should be added to the list of variables that needs to be standardized in developing a blood-based Aβ biomarker assay.
Acknowledgements
We thank the donors who participated in this study. The work was supported by FRAXA Research Foundation (http://www.FRAXA.org) (C.J.W.), the Illinois- Eastern Iowa District Kiwanis International Spastic Paralysis and Allied Diseases of the Central Nervous System Foundation (E.B.K.) and National Institutes of Health Grant P30 HD03352 (Waisman Center).
References
- Wisniewski KE, Dalton AJ, McLachlan C, Wen GY, Wisniewski HM (1985) Alzheimer's disease in Down's syndrome: clinicopathologic studies. Neurology 35: 957-961.
- Alves G, Bronnick K, Aarsland D, Blennow K, Zetterberg H, et al. (2010) CSF amyloid-beta and tau proteins, and cognitive performance, in earlyand untreated Parkinson's disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry 81: 1080-1086.
- Mackenzie IR, Miller LA (1994) Senile plaques in temporal lobe epilepsy. Acta Neuropathol 87: 504-510.
- Westmark CJ, Malter JS (2007) FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol 5: e52.
- Sokol DK, Chen D, Farlow MR, Dunn DW, Maloney B, et al. (2006) Highlevels of Alzheimer beta-amyloid precursor protein (APP) in children with severely autistic behavior and aggression. J Child Neurol 21: 444-449.
- Bailey AR, Giunta BN, Obregon D, Nikolic WV, Tian J, et al. (2008) Peripheral biomarkers in Autism: secreted amyloid precursor protein-alpha as a probable key player in early diagnosis. Int J Clin Exp Med 1: 338-344.
- Assini A, Cammarata S, Vitali A, Colucci M, Giliberto L, et al. (2004) Plasma levels of amyloid beta-protein 42 are increased in women with mild cognitive impairment. Neurology 63: 828-831.
- Schupf N, Patel B, Pang D, Zigman WB, Silverman W, et al. (2007) Elevated plasma beta-amyloid peptide Abeta(42) levels, incident dementia, and mortality in Down syndrome. Arch Neurol 64: 1007-1013.
- Mehta PD, Capone G, Jewell A, Freedland RL (2007) Increased amyloid beta protein levels in children and adolescents with Down syndrome. J Neurol Sci 254: 22-27.
- Westmark CJ, Malter JS (2001) Extracellular-regulated kinase controls betaamyloid precursor protein mRNA decay. Brain Res Mol Brain Res 90: 193-201.
- Ringman JM, Younkin SG, Pratico D, Seltzer W, Cole GM, et al. (2008) Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology 71: 85-92.
- van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM (2006) Plasma Abeta(1-40) and Abeta(1-42) and the risk of dementia: a prospective casecohort study. Lancet Neurol 5: 655-660.
- Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, et al. (2000) Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol 57: 100-105.
- Hansson O, Zetterberg H, Blennow K (2008) Evaluation of plasma Abeta40 and Abeta42 as predictors of conversion to Alzheimer's disease in patients with mild cognitive impairment. Neurobiol Aging 31: 357-367
- Okereke OI, Xia W, Irizarry MC, Sun X, Qiu WQ, et al. (2009) Performance characteristics of plasma amyloid-beta 40 and 42 assays. J Alzheimer's Dis 16: 277-285.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 14826
- [From(publication date):
August-2011 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10096
- PDF downloads : 4730