Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
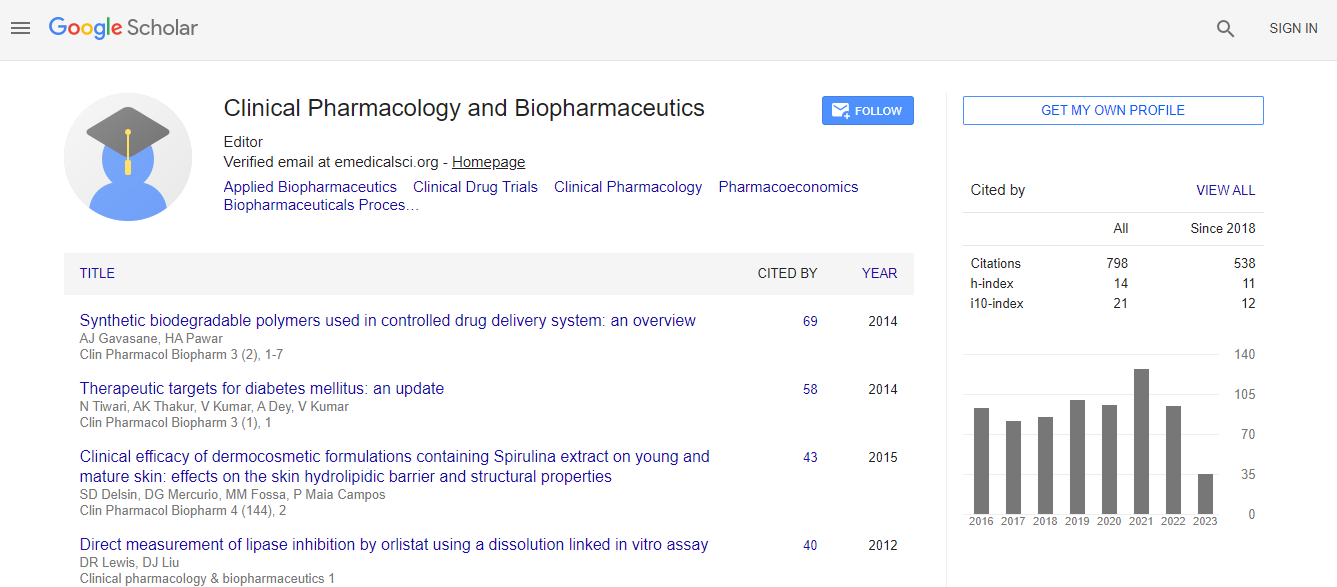
Google Scholar citation report
Citations : 1089
Clinical Pharmacology & Biopharmaceutics received 1089 citations as per Google Scholar report
Clinical Pharmacology & Biopharmaceutics peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
SNEHAPRAVA MAHARANA

Biography
I am Qualified Pharmaceutical professional with 9 years of experience in Quality Assurance, Pharmacovigilance processes and Regulatory compliance in the Pharmacological sector and Medical device sector. Proficient in initiating, investigating, root cause analysis and closure of market complaints within stipulated timelines. Skilled in documentation, verification and approval of Standard Operating Procedure (SOP) or creating new procedures. Sound exposure in process and system inspection/audit to identify non-compliance and improve efficiency. Demonstrated ability to face regulatory audits including USFDA,PIC/s, WHO Geneva, NABL India, ISO 9001:2015,ISO22715, ISO 13485: 2016 (Medical Device) and Health Canada as well as various Customer audits. Team player with ability to lead a team by example and motivate them to achieve desired objectives.
Research Interest
Pharmacovigilance, Pharmaceutics, Medical device

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi