Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
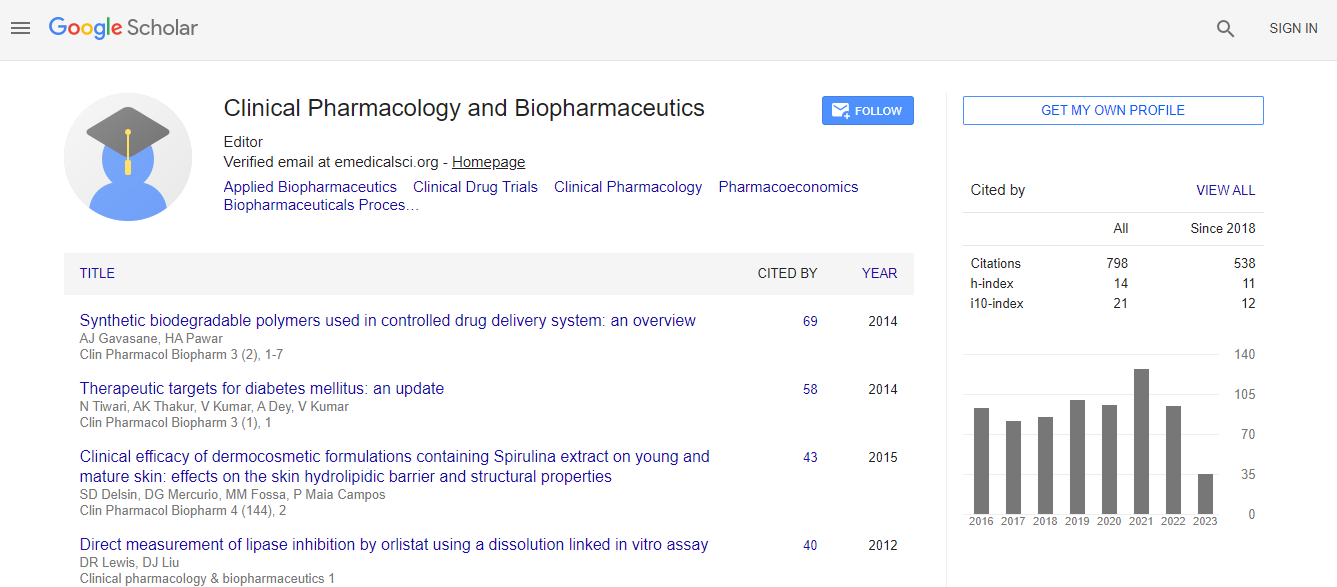
Google Scholar citation report
Citations : 1089
Clinical Pharmacology & Biopharmaceutics received 1089 citations as per Google Scholar report
Clinical Pharmacology & Biopharmaceutics peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Irfan A Mohammed

Associate Director, Operations Asset Leader,
New Haven,
United States
Biography
Irfan A. Mohammed is a CMC Development Scientist/ Leader with comprehensive experience in ideating, designing, developing, and scaling innovative and complex drug development programs ranging from oral solids to sterile dosage forms (Injectables and Ophthalmics) from pre-clinical stage to commercialization. Irfan A. Mohammed has led the development of drug products in various disease spaces, such as, oncology, hematology, neurology, ophthalmology, hospital, and critical care medicine, including rare diseases. He is an inventor/ co-inventor on 30+ patents awarded/ pending in the US and rest of the world (ROW).
Currently serving as an Associate Director, CMC Development Leader at Alexion Pharmaceuticals, his work focuses on leading global Chemistry, Manufacturing and Controls (CMC) development teams, such as, drug substance, drug product , analytical sciences, device, clinical manufacturing, clinical supply chain, regulatory CMC and quality management teams to advance innovative therapies for rare diseases from R&D to clinics. His experience includes leading drug product design and development (pre-formulation and formulation), manufacturing science and technology (MS&T), such as, manufacturing process development, characterization, verification, validation, and formulation/fill/finish operations), regulatory CMC and compliance. His research experience encompasses both academia and industry focused on developing robust drug product formulation and process strategies for new molecular entities and already approved drug substances, primarily within sterile dosage forms, such as, injectables and ophthalmic drug products, including aqueous, non-aqueous, lyophilized, suspensions, emulsions formulations, pre-filled syringes/ combination products, blow fill seal (BFS) and infusion bags. His work aims to develop drug products to improve the overall quality of life in patients, adding value to the patients, healthcare providers and the healthcare system.
Irfan is passionate to contribute and add value to the overall healthcare ecosystem, beginning from drug development to healthcare delivery and health outcomes leveraging new and innovative approaches, such as, digital applications in drug development and healthcare.
Irfan A. Mohammed is also currently pursuing his Doctorate Degree in Pharmaceutics from Philadelphia College of Pharmacy, University of Sciences, where his research work focuses on developing long acting injectables drug delivery systems. He obtained his Master’s degree in Pharmaceutics and Industrial Pharmacy from Long Island University, Brooklyn, NY, where his research work focused on development, characterization and understanding the process variables in the preparation of stable polymeric nanoparticles using wet media milling to improve the drug delivery of an antifungal agent through topical/transdermal and oral solid dosage forms utilizing and comparing regular form of drug, nanoparticles and solid dispersion technologies. He graduated with a Bachelor’s degree in Pharmacy from Jawaharlal Nehru Technological University, Hyderabad, India
Research Interest
Pharmaceutics, Chemistry, Manufacturing and Controls, Advance Medicine, Analytical Sciences

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi