Research Article Open Access
Ecological Health Status of the Fosu Lagoon, Southern Ghana II: Environmental and Human Health Risk Assessment
Frederick A. Armah1,2*, Isaac Luginaah1, Markku Kuitunen3 and Paul Mkandawire1
1Department of Geography, University of Western Ontario, Canada
2Department of Environmental Science, School of Biological Sciences, University of Cape Coast, Ghana
3Department of Biological and Environmental Science, University of Jyväskylä, Ambiotica, Finland
- *Corresponding Author:
- Frederick A. Armah
Department of Geography
University of Western Ontario, Canada
E-mail: farmah@ucc.edu.gh
Received Date: January 23, 2012; Accepted Date: January 26, 2012; Published Date: January 29, 2012
Citation: Armah FA, Luginaah I, Kuitunen M, Mkandawire P (2012) Ecological Health Status of the Fosu Lagoon, Southern Ghana II: Environmental and Human Health Risk Assessment. J Ecosys Ecograph 2:107. doi:10.4172/2157-7625.1000107
Copyright: © 2012 Armah FA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
This study set out to assess the levels, distribution and human health risk of Pb, As, Cd, Mn and polycyclic aromatic hydrocarbons (PAHs) in sediments from the Fosu Lagoon in Ghana. This was coupled with environmental fate modeling to investigate biodegradation behaviour of PAHs in sediment. Generally, the order of heavy metal enrichment for the sampling locations was Mn>Cd>Pb>As respectively. Only Mn showed significant enrichment at all sites. The observed PAH contamination demonstrated potential anthropogenic impact. The phenanthrene-anthracene ratio was less than 10 indicating that the PAHs originate from combustion. Benzo[b]fluoranthrene is the only carcinogenic PAH that was detected in the study. Regarding heavy metals in children, the total Hazard Index (HI) for ingestion and dermal route was 3.4×10-2 and 8.2×10-3 respectively for Central Tendency Exposure (CTE) whereas it was 2.1×10-1 and 1.0×10-1 for the Reasonable Maximum Exposure (RME) scenario. In adults, the total HI was 1.0×10-3 and 1.2×10-3, for ingestion and dermal routes respectively under the CTE scenario. For RME scenario, ingestion and dermal exposure had risk values of 2.2×10-2 and 3.1×10-2, respectively. Regarding PAHs, the total carcinogenic risk (RME) for children and adults were estimated as 1.02×10-5 and 8.38×10-6 respectively. The carcinogenic risk derived from the ingestion and dermal absorption of metals exceeded the generally acceptable risk level of 10-6 for individual chemicals, being slightly above 10-4. That risk level, basically due to the ingestion of As and Mn through sediments suggests potential harm to human health. The concentrations of PAHs in sediments did not present signiï¬Âcant non-carcinogenic and carcinogenic risk for the local population. The fate modelling however, suggests that naphthalene, anthracene, benzo[b]fluoranthene, pyrene, fluorene and phenanthrene tend to sorb onto soil or sediment in the environment and biologically ‘‘do not degrade fast’’. This implies the need to implement long term monitoring of these compounds in order to sustain this fragile ecosystem.
Keywords
Sediments; Heavy metals; PAHs; Fosu lagoon; Estimation Programs Interface (EPI suite); Health risks; Fate and transport modelling
Introduction
In aquatic ecosystems, heavy metals and polycyclic aromatic hydrocarbons (PAHs) are in dynamic equilibrium with pore water, and the underlying water columns have pathways that are primarily associated with sediment substrate [1]. Consequently, heavy metals are among the pollutants within sediment substrate that should be monitored in order to obtain a logical and wide-ranging overview of the inhabitable condition for certain aquatic systems, particularly lagoons [2]. Knowledge of the distribution and concentrations of heavy metals and PAHs in sediments gives information on the nature and kind of source inputs in aquatic systems [3]. Sediment is recognized as an important sink for heavy metals and PAHs emitted from anthropogenic sources, as well as a potential non-point pollution source which may directly affect overlying waters and aquatic organisms [4]. Due to the close connection between the sediments and water quality, contaminated sediments can be a long-lasting source of diffusion of contaminants, even if the pollution of this water is considerably reduced [2]. Sediments exhibit variations in grain size, mineral, organic matter content, and so on, which can also create anomalously high heavy metals concentration as well as anthropogenic contamination [5]. PAHs are integrated into bottom sediments, for the most part by removal from the water column through their association with particulate matter. The partitioning behavior of PAHs is such that they tend to accumulate in sediments to levels that are many times higher than in the adjoining water and their deposition rates are generally connected to their rates of input into the immediate area [6]. Many of the polycyclic aromatic hydrocarbons are considered genotoxic carcinogens, depending on their molecular structure. PAHs are usually dissimilar in their molecular weights [7,8]. Whereas the 2 or 3 aromatic hydrocarbon rings PAHs are regarded as low molecular weight, their 4 aromatic hydrocarbon rings and more counterparts are regarded as high molecular weight PAHs. The latter group is considered to be less acutely toxic but more carcinogenic and teratogenic [9]. The US Environmental Protection Agency (USEPA) has classified seven PAH compounds as probable human carcinogens; benzo [a] anthracene, benzo [a] pyrene, benzo [b] fluoranthene, benzo [k] fluoranthene, chrysene, dibenzo [a,h] anthracene, and indeno [1,2,3-cd] pyrene [10]. Previous research has shown that the total concentrations of heavy metals and PAHs in sediments alone cannot provide sufficient information about their impact on the ecosystem and humans since the mobility, bioavailability and toxicity of metals is partially dependent on their total concentrations and partly dependent on the geochemical fractions in which they occur [11]. Consequently, there is the need to assess the risk heavy metals and PAHs pose to both ecological and human receptors.
About 90 lagoons exist along the coastline of Ghana and some of them particularly the Korle and Fosu lagoons are regarded as polluted [4,12] because they have become receptacles of heavy metals and PAHs. Traditionally, Lagoons in Ghana have important cultural and environmental significance (mangroves associated with these lagoons provide indirect protective effects) and also are frequently local fishing grounds for surrounding communities. The Fosu lagoon has been added to the list of water bodies with ‘dead zones’ and this is a source of concern to individuals who depend on it for their livelihood and regulatory agencies as well [12]. ‘Dead zones’ in this context are areas where the bottom water (the water at the lagoon floor) is anoxic with very low concentrations of dissolved oxygen [12]. Previous studies have indicated the Fosu lagoon has high level of suspended solids and dissolved metals. For example, Gilbert et al. [1], Dodoo and Adjei [13], reported significantly high concentrations of copper and zinc in the Fosu lagoon especially at points adjacent to human settlements. However, the distribution and risk assessment of PAHs and heavy metals (cadmium, manganese, arsenic) in sediments from the lagoon with potential health impacts on the surrounding populations have not been extensively studied. This research addresses this gap by: determining the levels and distribution of Lead, Cadmium, Arsenic and Manganese and PAHs in sediments from the Fosu lagoon; conducting source apportionment for these heavy metals and PAHs; determining the enrichment factor, geo-accumulation index and metal pollution index for these heavy metals; and assessing the health risk to resident adults and children on exposure to the heavy metals and PAHs in sediments using the Risk-Integrated Software for Clean-Ups (RISC 4.02).
This study will extend our understanding of the distribution of these metals and PAHs, and the risk they pose to adult and children within surrounding populations and provide policy options for a sustainable management of the Fosu Lagoon.
Materials and Methods
Study area
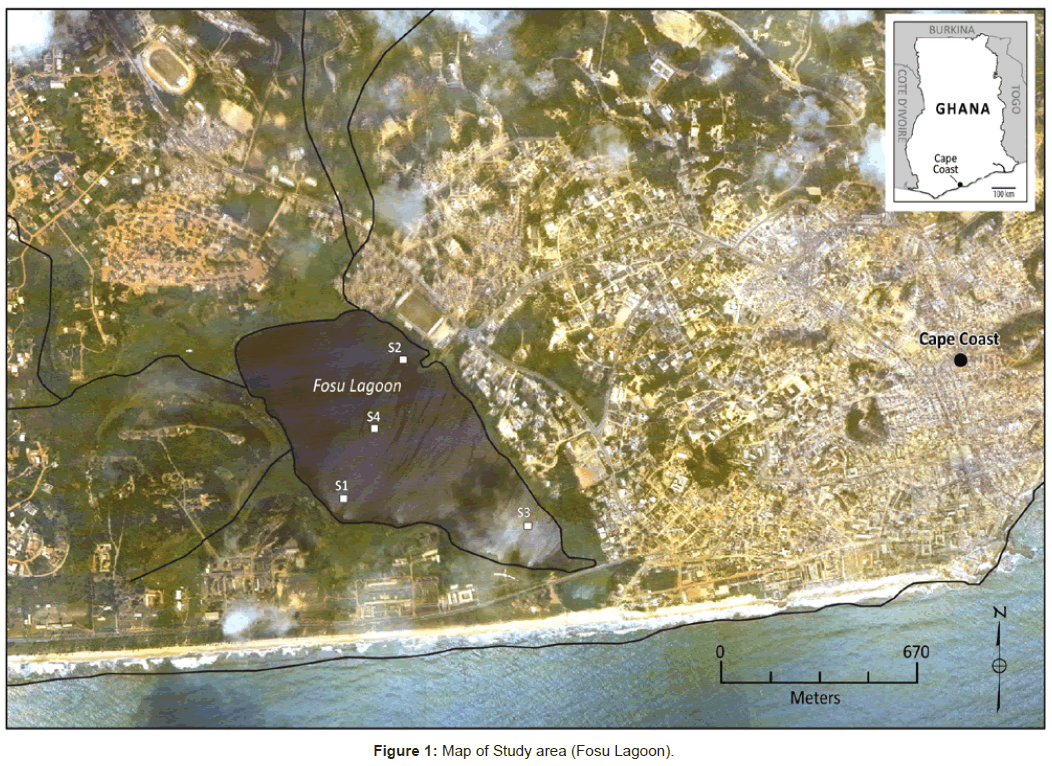
Fosu lagoon (5°6’17.87” N, 1°15’ 18.68” W) is in the metropolis of Cape Coast, Ghana in West Africa. The region receives two wet seasons in a year, the major one from April to July and a minor season from September to November [1]. The lagoon is surrounded by many sites that act as point sources for discharge of pollutants. This includes domestic waste discharges from a highly polluted area, a metropolis transport garage on the northern side of the lagoon, and an industrial waste discharge from mechanical workshops on the north eastern side. Drains from an educational institution and a nearby hospital, household dumping and sewages characterize the environs of the lagoon. The human activities in the study area is immense and eutrophication has caused massive sedimentation and especially in the more populated northern sector, one can walk many meters on waterweeds as the lagoon has been transformed into waste and marsh land (Figure 1).
Data collection
Twenty-four sediment samples were collected from January to March 2011, at four demarcated sites with six samples from each demarcated site. Twelve samples each were collected for heavy metals and PAHs analyses. Sampling was done at various sites of the lagoon where refuse and other effluents were discharged. Typically, the sampling sites were less than forty meters from the respective refuse dumping sites or effluent entry points. The sample sites were thus presumed as the discharge point with the corresponding areas as follows;
S1: close to direct discharge of untreated laboratory and hospital effluents respectively from the college and the hospital located on the western and south western side of the lagoon.
S2: close to industrial waste discharge from a cluster of mechanical and spraying workshops on the north eastern side.
S3: close to household dumping and sewages from the southern end.
S4: middle of the Fosu lagoon.
An Eckman grab (15 cm×15 cm) was used to collect the sediment samples at an average depth of 10 cm into a clean polythene bag and tied. The samples were then kept at 4°C prior to being analysed.
Sample preparation
The sediment samples collected from each sampling site were free from pieces of roots, pebbles, and other foreign objects. The samples were air dried for 72 hours and then oven dried for 24 hours to a constant weight at 50°C. The dried samples were ground and homogenized in a porcelain mortar and sieved with a 0.5 mm mesh as composite sample for the site. The final composite samples weighed between 5 to 10 g. The homogenized samples were digested for the determination of the various heavy metals. The sediment was categorized as being gravel, sand, silt or clay depending on the particle size diameters: ≥2.00 mm, 1.00-0.125, 0.05, <0.05 respectively. The particle size composition plays an important role in the absorption and accumulation of heavy metals and PAHs [14]. The nature of the Fosu lagoon sediments was coarse sand to very coarse gravel.
Heavy metal analysis
Five grams of the prepared smooth soil sample was weighed into a 250 ml beaker. 10 ml of concentrated nitric acid and 3 ml of concentrated hydrogen peroxide were added to the sample, stirred and covered with glassware. The beaker and its contents were heated on a hot plate until the fumes of the 1:1 ratio of nitric acid and hydrogen peroxide were given off. The sample was digested three times. The digest was allowed to cool in a fume chamber and filtered using the Whatman filter paper into the 50 ml volumetric flask. The beaker was thoroughly washed with 10 % v/v nitric acid into the flask to the 50 ml mark. The solution was stored in a plastic container for subsequent analysis with the Flame Atomic Absorption Spectrophotometer Shimadzu model 6401F. The same procedure was repeated for all the 12 sediment samples. For quality control and assurance, reproducibility and recovery studies were conducted. The spiking method was used for quality assurance tests because of the absence of reference materials. About 1 mg each of Pb as Pb(NO3)2, of Cd as cadmium metal, Mn as MnSO4.H2O were added to the weighed sample. The mixture in each case was subjected to the same analysis protocol as that adopted for sediments and the recovery of the added elements was determined. Five replicate addition experiments were performed for each of the sediments. Percentage recovery from spiked sediment samples for Pb, Cd and Mn were 97.1 ± 0.3, 94.5 ± 0.4, 98.2 ± 0.2 respectively. A blank solution was prepared using the same procedure without the sample. The blank was also stored in a plastic container for AAS analysis. In all cases the standard error was less than 1, indicating that the analytical method used was reproducible.
PAHs analysis
Ten grams of sodium sulfate and 3 g of baby powder (talcum powder) were put in a glass bottle with PTFE foil and screw cap. The glass bottle was shaken to ensure uniform mixture of the sodium sulfate and the baby powder and was cooled to about 4°C. 10 g of the sediment sample was added to the contents of the glass bottle and shaken. This step was repeated with the other 11 sediment samples. The bottles were then shaken for 4 hours and placed in the refrigerator at 4°C. The bottles were allowed to stand undisturbed for about 16 hours. The content of the bottles was then transferred into polyethylene bottles and cooled in the refrigerator for another 12 hours at a temperature of -20°C. The same procedure was followed for the blank, where shore sand was used instead of the sediment sample. About 10 g of sediment sample was weighed into a 10 ml Erlenmeyer flask with glass stopper. 50 ml of petroleum ether was added and shaken for 10 minutes. After shaking, the Erlenmeyer flask was allowed to stand undisturbed ensuring separation of the layers. The separated petroleum fraction was then transferred to the Kuderna Danish equipment, using a funnel with glass wool. The extraction was repeated with another 50 ml of petroleum ether and the extract was also added to the Kuderna Danish equipment. Again the Erlenmeyer was rinsed twice with 10 ml of petroleum ether and added to the extract in the Kuderna Danish apparatus. The extract was evaporated to a volume of about 10 ml, using the Kuderna Danish concentrator and a water bath of 70-75°C. The extract was further evaporated to a volume smaller than 1 ml, using the cool air from the air conditioner at room temperature. Some amount of petroleum ether was added to the evaporated extract till the volume was 1 ml. An absorption column was prepared by putting some glass wool in a chromatography tube where 2 g of deactivated aluminum oxide was also added. Some amount of sodium sulfate was added to the content of the mounted column. The extract was then transferred to the absorption column by means of a pipette. The Kuderna Danish apparatus was again rinsed twice with 1 ml of petroleum ether and transferred to the column at the moment that the liquid had penetrated the column. About 12 ml of petroleum ether was used to elude the content of the column. The eluate was collected in a calibrated tube of 20 ml. The collected eluate was evaporated with cool air from the air conditioner at room temperature to an end volume of 1 ml. Finally, the extract was transferred into auto sampler bottles with screw cap. This was stored in the refrigerator before gas chromatography analysis.
Calculations
Metal Pollution Index (MPI) of heavy metals: The MPI was calculated using the formula [15] (Table 1)
MPI = (Cf1 X Cf2 ………. X Cfn)1/n
Where Cfn=concentration of metal and n = number of metals
| MPI Values | MPI Class | Intensity of Pollution |
| MPI<1 | 1 | Low pollution |
| 1<MPI<2 | 2 | Medium pollution |
| MPI>2 | 3 | High pollution |
*Based on Chen et al. (2004)
Table 1: Classification of MPI values*.
Enrichment factor (EF) of heavy metals:
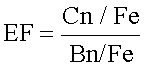
Where Cn=concentration of metal and Bn=background value
The enrichment factor (EF) (Table 2), which is the index of contamination for the sediment samples, was calculated using the heavy metal average concentrations at S1 to S4 and with USEPA (1989) guidelines values as baseline levels. According to Tam and Wong [15], metal loads from natural and anthropogenic sources accumulate together; therefore, normalizing tools are needed in order to differentiate the fraction of the element concentration originating from natural sedimentary sources from the anthropogenic fraction. Fe as a conservative element was used to evaluate anthropogenic sources of the metals.
| Enrichment values | Level of enrichment |
| EF≤2 | Depletion to minimal enrichment |
| EF=2-5 | Moderate enrichment |
| EF=5-20 | Significant enrichment |
| EF=20-40 | Very high enrichment |
| EF>40 | Extremely High Enrichment |
*Based on Sutherland (2000)
Table 2: Enrichment factor categories*.
Geo-accumulation index of heavy metals (Igeo):
Igeo = logCn / 1.5Bn
Where Cn is concentration of the metal and Bn is the background value of the metal [15] (Table 3).
| Igeo value | Igeo class | Intensity of pollution |
| <0 | 1 | Practically unpolluted |
| 0≤Igeo≤1 | 2 | Unpolluted to moderately polluted |
| 1≤Igeo≤ 2 | 3 | Moderately polluted |
| 2≤Igeo≤ 3 | 4 | Moderately to strong polluted |
| 3≤Igeo≤4 | 5 | Strongly polluted |
| 4≤Igeo≤5 | 6 | Strong to very strongly polluted |
| > 5 | 7 | Very strongly polluted |
Tam and Wong (1995)*
Table 3: Classification of Index of Geo-accumulation*.
Human health risk assessment methodology
The exposure of humans to metals through sediment was assessed under a residential scenario. The metal intake through sediment was calculated by applying an existing methodology USEPA [16]. The exposure of the local population to metals through sediment was estimated by considering two main routes: ingestion and dermal absorption. Non-carcinogenic and carcinogenic risks were assessed separately. Adults and children were evaluated. Children were chosen as special target populations taking into account that they are among the most sensitive groups. Deterministic simulations were applied to calculate carcinogenic and non-carcinogenic risks. Deterministic analysis is usually performed to take into account the variability and certainty associated with each parameter [4,17,18]. The deterministic parameter distribution for metal exposure and risk input data is shown in Table 4.
| Receptor type | Child RME | Adult RME | Child Typical | Adult Typical |
| Life Time (years) | 70 | 70 | 70 | 70 |
| Body weight | 15 | 70 | 15 | 70 |
| Exp. Frequency for soil (events / yrs) | 350 | 350 | 130 | 40 |
| Exp. Duration for Soil(yr) | 6 | 9 | 6 | 9 |
| Ingestion rate for soil (mg/Kg) | 200 | 100 | 90 | 40 |
| Total Skin Surface area (cm2) | 7280 | 23000 | 6800 | 18400 |
| Fraction skin exposed to soil | 0.55 | 0.25 | 0.13 | 0.11 |
| Skin adherence factor(mg/cm2) | 0.2 | 0.2 | 0.2 | 0.2 |
*Based on default values in RISC 4.02 software
(RME=Reasonable Maximum Exposure; Typical=CTE=Central Tendency
Exposure)
Table 4: Receptor specific exposure data*.
Non-cancer risks: The Hazard Quotient (HQ) - a numeric estimate of the systemic toxicity potential posed by a single chemical within a single route of exposure was calculated by comparing the environmental predicted exposure with the reference dose (RfD) for each element. The RfDs for Pb, Cd, Mn and As respectively are as follows: 6.0×10-2, 5.0×10-3 1.4×10-1, 3.0×10-4. The Hazard Index (HI) was calculated by summing HQs for each exposure pathway. The HI is a numeric estimate of the systemic toxicity potential posed by all chemicals reaching a receptor through a single exposure route. HI(ing), and HI(derm) are numeric estimates of the systemic toxicity potential posed to a receptor by exposure to all chemicals through ingestion and dermal absorption respectively. The HI (total) was calculated by summing HIs for each route of exposure [19]. The HI (total) is often called an estimate of “total non carcinogenic risk.” When HQ or HI exceeds unity, there may be concern for potential human health effects caused by exposure to non-carcinogenic substances [19].
Cancer risks: The chemical-specific excess cancer incidence (ELCRi) is an estimate of the increased cancer incidence (probability) to a receptor resulting from an exposure to a single chemical within a single exposure route. For the ingestion and dermal absorption routes of exposure, the ELCR was calculated by multiplying the daily exposure and the ingestion or dermal slope factor, respectively [20]. The total pathway-specific excess cancer incidence for all chemicals within a single exposure route was calculated as the sum of ELCRs to all chemicals for a single route. Finally, the total excess cancer incidence posed by all chemicals over all routes, which is an estimate of the increased cancer incidence resulting from the exposure to all chemicals reaching a receptor over all routes, was calculated as a sum of all ELCRs [19].
Environmental fate ad transport modeling: Quantitative Structure Activity Relationship (QSAR) estimates were complemented with experimental data to investigate biodegradation behaviour of PAHs in sediment. Quantitative structure-activity relationship (QSAR) analyses were performed to estimate the physical-chemical properties and fate data of PAHs using the software EPI Suite version 4.10 and Persistent Bio-accumulative and Toxicity (PBT) Profiler from the United States Environmental Protection Agency (US EPA). The EPI (Estimation Programs Interface) Suite is a Windows based suite of physical/chemical property and environmental fate estimation models developed by the US EPA’s Office of Pollution Prevention Toxics and Syracuse Research Corporation. EPI Suite uses a single input to run the following estimation models: KOWWINTM, AOPWINTM, HENRYWINTM, MPBPWINTM, BIOWINTM, PCKOCWINTM, WSKOWWINTM, BCFWINTM, HYDROWINTM, and STPWINTM, WVOLWINTM, and LEV3EPITM. The PBT Profiler was developed by the US EPA and it can be used to predict environmental persistence (half-life), bio-concentration factor (BCF), and fish chronic toxicity (ChV) characteristics from chemical structure [21,22]. In the present study, biodegradation, bioaccumulation and aquatic toxicity estimates were obtained using BioWin, BCFWin and ECOSAR of the EPI Suite.
Results
Descriptive statistics of heavy metal and PAHs concentrations
The mean concentrations and standard deviation of the four heavy metals in sediment samples collected in the area under the investigation are summarized in Table 5. The mean concentrations and standard deviation of the PAHs in sediment samples collected in the area under the investigation are summarized in Table 6.
| Site Number | Pb (mg/kg) | Cd (mg/kg) | As (mg/kg) | Mn (mg/kg) | MPI |
|---|---|---|---|---|---|
| 1 | 21.24+9.37 | 1.22+0.14 | 0.52+0.44 | 298.34+42.95 | 7.90 |
| 2 | 51.08+26.38 | 0.93+0.32 | 1.22+0.06 | 377+8.24 | 12.16 |
| 3 | 33.80+16.45 | 1.20+0.24 | 0.84+0.10 | 128.25+60.35 | 8.13 |
| 4 | 23.04+9.46 | 1.09+0.17 | 1.01+0.06 | 253.02+69.22 | 8.95 |
Table 5: Mean concentration and standard deviation of heavy metals in each site.
| PAH Compounds |
S1 | S2 | S3 | S4 |
| Naphthalene | 5.71±3.07 | 10.03±0.58 | 10.76±0.17 | 1.58±1.71 |
| Anthracene | 0.03±0.01 | 0.06±0.02 | 0.02±0.00 | ND |
| Benzo[b]fluoranthene | 3.35±1.63 | ND | 3.94±0.56 | 1.80±0.33 |
| Pyrene | ND | 0.02±0.00 | ND | ND |
| Fluorene | ND | ND | 0.01±0.00 | ND |
| Phenanthene | 0.03±0.01 | 0.08±0.03 | 0.03±0.00 | 0.25±0.48 |
Acenaphthalene, Chrysene, Benzo[a]pyrene, Dibenzo[a,h]anthracene, Benzo[ghi]perylene and Indeno[123-cd]pyrene were not detected in any of the sites and so are not shown in Table 6.
Table 6: Mean concentration and standard deviation of PAHs in each site (mg/kg).
All sites in the present study recorded low degree of contamination for Pb, As and Cd with intensity of unpolluted to moderately polluted but Mn recorded high degree of contamination which appears that sediments in the lagoon are somewhat impacted by human activities. Almost all the sites showed MPI values > 2 confirming there were considerable heavy metal contamination. The proximity of the road to the lagoon perhaps contributes to the enrichment factors for Pb, Cd and As. The high enrichment of sediments by Mn could be attributed to anthropogenic processes. The order of magnitude in terms of enrichment factor for the various sampling locations was as follows; S1: Mn >Cd>Pb>As; S2: Mn >Pb>Cd>As; S3: Mn >Cd>Pb>As; S4: Mn >Cd>Pb>As. Except Mn that showed significant enrichment at all sites, all the other metals showed minimal enrichment at all locations (Table 2). Results of geo-accumulation index indicate that all the sites are unpolluted to moderately-polluted by Pb, Cd and As. S3 is moderately to strongly-polluted by Mn whereas S1, S2 and S4 are very strongly polluted by Mn (Table 3). MPI calculated for the heavy metals indicate that all sites exhibit high levels of pollution i.e. MPI>2 (Table 1).
Acenaphthalene, Chrysene, Benzo [a] pyrene, Dibenzo [a,h] anthracene, Benzo [ghi] perylene and Indeno[123-cd]pyrene were not detected in any of the sites whereas pyrene and fluorene were detected in only 1 site. Anthracene and Benzo [b] fluoranthene were detected in three locations. Naphthalene had the highest concentration within all the PAHs detected.
Risk Assessment results for heavy metals and PAHs in sediments
From Table 7, none of the metals had HQ greater than 1. This suggests that non-carcinogenic risk posed by As, Pb, Cd, Mn via dermal and ingestion modes under both central tendency and reasonable maximum exposure scenarios, is low. The USEPA considers carcinogenic risk of between 1 person out of 10,000 and 1,000000 (10-4 and 10-6) to be acceptable. In Table 7, none of the metals recorded a carcinogenic risk value of more than 1 in 10,000 (10-4). Carcinogenic and non-carcinogenic risks for both adults and children were generally higher for ingestion than dermal contact.
| Hazard quotients for receptor, route and chemical (non-carcinogenic risk) | Carcinogenic risk for receptor, route and chemical | |||||||||||
| Resident CTE | ||||||||||||
| Child | Adult | Child | Adult | |||||||||
| Metals | Ingestion | Dermal | Total | Ingestion | Dermal | Total | Ingestion | Dermal | Total | Ingestion | Dermal | Total |
| As | 6.4 ҳ 10-3 | 3.8 ҳ 10-4 | 6.8 ҳ 10-3 | 1.9 ҳ 10-4 | 5.7 ҳ 10-5 | 2.4 ҳ 10-4 | 2.5 ҳ 10-7 | 1.5 ҳ 10-8 | 2.6 ҳ 10-7 | 1.1 ҳ 10-8 | 3.3 ҳ 10-9 | 1.4 ҳ 10-8 |
| Cd | 4.7 ҳ 10-3 | 9.3 ҳ 10-6 | 4.8 ҳ 10-3 | 1.4 ҳ 10-4 | 1.4 ҳ 10-6 | 1.4 ҳ 10-4 | - | - | - | - | - | - |
| Pb | 2.0 ҳ 10-2 | 3.8 ҳ 10-4 | 2.0 ҳ 10-2 | 5.7 ҳ 10-4 | 5.8 ҳ 10-5 | 6.3 ҳ 10-4 | - | - | - | - | - | - |
| Mn | 3.8 ҳ 10-3 | 7.4 ҳ 10-3 | 1.1 ҳ 10-2 | 1.1 ҳ 10-4 | 1.1 ҳ 10-3 | 1.2 ҳ 10-3 | 2.4 ҳ 10-9 | 4.8 ҳ 10-9 | 7.2 ҳ 10-9 | 1.1 ҳ 10-1 | 1.1 ҳ 10-9 | 1.2 ҳ 10-9 |
| Total | 3.4 ҳ 10-2 | 8.2 ҳ 10-3 | 4.3 ҳ 10-2 | 1.0 ҳ 10-3 | 1.2 ҳ 10-3 | 2.2 ҳ 10-3 | 2.5 ҳ 10-7 | 1.9 ҳ 10-8 | 2.7 ҳ 10-7 | 1.1 ҳ 10-8 | 4.4 ҳ 10-9 | 1.5 ҳ 10-8 |
| Resident RME | ||||||||||||
| As | 3.8 ҳ 10-2 | 4.6 ҳ 10-3 | 4.3 ҳ 10-2 | 4.1 ҳ 10-3 | 1.4 ҳ 10-3 | 5.5 ҳ 10-3 | 1.5 ҳ 10-6 | 1.8 ҳ 10-7 | 1.8 ҳ 10-6 | 7.9 ҳ 10-7 | 2.7 ҳ 10-7 | 1.1 ҳ 10-6 |
| Cd | 2.8 ҳ 10-2 | 1.1 ҳ 10-4 | 2.8 ҳ 10-2 | 3.0 ҳ 10-3 | 3.5 ҳ 10-5 | 3.1 ҳ 10-3 | - | - | - | - | - | - |
| Pb | 1.2 ҳ 10-1 | 4.7 ҳ 10-3 | 1.2 ҳ 10-1 | 1.3 ҳ 10-2 | 1.4 ҳ 10-3 | 1.4 ҳ 10-2 | - | - | - | - | - | - |
| Mn | 2.3 ҳ 10-2 | 9.0 ҳ 10-2 | 1.1 ҳ 10-1 | 2.4 ҳ 10-3 | 2.8 ҳ 10-2 | 3.0 ҳ 10-2 | 1.4 ҳ 10-6 | 5.8 ҳ 10-8 | 7.2 ҳ 10-10 | 7.8 ҳ 10-9 | 8.9 ҳ 10-8 | 9.8 ҳ 10-9 |
| Total | 2.1 ҳ 10-1 | 1.0 ҳ 10-1 | 3.1 ҳ 10-1 | 2.2 ҳ 10-2 | 3.1 ҳ 10-2 | 5.3 ҳ 10-2 | 2.9 ҳ 10-6 | 2.4 ҳ 10-7 | 1.7 ҳ 10-6 | 8.0 ҳ 10-7 | 3.6 ҳ 10-7 | 1.2 ҳ 10-6 |
Table 7: Carcinogenic and non-carcinogenic risk values for heavy metals via CTE and RME Scenarios.
Discussion
Distribution of sediment-based heavy metals in the lagoon
The highest concentrations for Pb (77.46 mg/kg), Mn (385.24 mg/kg) and as (1.28 mg/kg) were obtained at S2 that is around the mechanical and spraying workshop. The concentrations were greater than the USEPA baseline levels except for As. The highest concentration of Cd was found at S1, which is around the hospital and the educational institution although the levels are comparable to background values reported in the literature. The quotient of enrichment factor of different metals and background values at S1 followed the order: Mn>Cd>Pb>As, whereas at S2, the order changed into Mn>Pb>Cd>As. It is evident that the contaminants were unevenly distributed and the concentrations of Mn, Pb, Cd, and As in sediments from S2 were observed to be more enriched than any other sample site.
Metal load assessment based on enrichment factor
All the heavy metals in all sites had EF values ≤2 which is depletion to minimal enrichment except for Mn which had EF values of 5-20 (significant enrichment). Even though the calculated EF (except for Mn) could be underestimated values given that sediments from which background values were derived probably contained metals from anthropogenic sources, it also allowed for the identification of places where anthropogenic inputs of particular metals were introduced. The industrial activities at the north eastern sector of the lagoon, especially the cluster of mechanical workshops at Siwdu is likely the main source of Mn, Pb, As and Cd discharge to the lagoon; although effluents from the hospital, garbage dumps and domestic effluents from Adisadel Housing Estates and a vague source from the southern segment all contribute to anthropogenic loads of Mn into the lagoon. This finding is consistent with the conclusion of Gilbert et al. [1].
Metal load assessment based on geoaccumulation index
Geoaccumulation index is used to assess whether or not sediments have been contaminated by heavy metals or otherwise. All sites were unpolluted to moderately polluted by Pb, Cd and As (i.e. 0 ≤ Igeo ≤ 1) even as geoaccumulation index for Mn in all sites except S3 showed very strong pollution loads with values greater than 5, but S2 (cluster of mechanical workshops at Siwdu) had the highest value of 8.34.
Metal load assessment based on metal pollution index
Metal Pollution Index (MPI) for the investigated sites is illustrated in Table 1. All the sites can be classified as high contamination or pollution areas i.e. MPI > 2 according to the classification of Chen et al. [5], with the highest pollution area at S2 (MPI=12.16) which may due to the industrial wastes discharged from a cluster of mechanical and spraying workshop on the north eastern side. The lowest value was recorded at S3 i.e. around household discharge points for effluents and sewage from the southern end.
Non-cancer risks from exposure to heavy metals
The calculated risk for adults and children from exposure to heavy metals in sediments via ingestion and dermal route are shown in Table 7. Generally, the estimated risk for metals through sediment ingestion was greater than that for dermal exposure. Important differences in metal ingestion and dermal exposure between adults and children were noted. In turn, because of their greater susceptibility, children (Table 7) showed a greater absorption of metals than adults. In children, the total HI for ingestion and dermal exposure were 3.4×10-2 and 8.2×10- 3 respectively for CTE while the RME scenario had 2.1×10-1 and 1.0×10-1 for ingestion and dermal exposure respectively. In adults the total HI was 1.0×10-3 and 1.2×10-3 for ingestion and dermal exposure respectively for the CTE scenario. For RME scenario, ingestion and dermal exposure had 2.2×10-2 and 3.1×10-2 respectively. Both HQ and HI values were below the safety level of 1.0 (HQ or HI<1). Consequently, sediments were not found to be a medium of concern for human exposure to metals. Regarding heavy metals, children are more at risk than adults. The maximum quotient (total for ingestion and dermal) was recorded for Pb (2.0×10-2 and 1.2×10-1) under CTE and RME scenarios respectively. Contribution to risk in children is in the order Pb>Mn>As>Cd. In adults the maximum quotient (total for ingestion and dermal) was recorded for Mn (1.2×10-3 and 3.0×10-3) under CTE and RME scenarios respectively. Contribution to risk in children is in the order Mn>Pb>As>Cd. Pb is known to bioaccumulate in the body which culminates in lead poisoning in children.
According to the Agency for Toxic Substances and Disease Registry (ATSDR 2000) [23] gastrointestinal effects are seen primarily after As ingestion, and less often after dermal absorption. Pigment changes and hyperkeratosis are characteristic of chronic As exposure. These changes have been observed in populations chronically consuming fishes infested with As in lagoons [18].
Cancer risk from exposure to heavy metals
Of the four target chemicals, carcinogenicity values have only been reported for As and Mn (Table 7). The total cancer risk of As and Mn exposure in the Fosu lagoon was estimated to be 2.5×10-7 and 1.9×10-8 through ingestion and dermal absorption of sediments, respectively for children in the CTE scenario. For RME total cancer risk was 2.9×10-6 and 2.4×10-7 respectively for ingestion and dermal routes. In adults, the total cancer risk through ingestion and dermal route was1.1×10-8 and 4.4×10-9 respectively for CTE while the RME scenario had risk of 8.0×10-7and 3.6 ×10-7. Although the concentration of these elements should obviously be zero to get the maximum protection for human health, lifetime risks of 10-6to 10-4 are considered acceptable for carcinogens in water and sediments [16]. The As-related cancer risk was 2.6×10-7 and 1.7×10-6 for children under CTE and RME scenarios respectively whereas for adults, it was 1.4×10-8 and 1.1×10-6 for CTE and RME. The carcinogenic risk derived from the ingestion and dermal absorption of metals greatly exceeded the generally acceptable risk level of 10-6 for individual chemicals, being slightly above 10-4. That risk level, basically due to the ingestion of As and Mn through sediments would mean a concern for human health. Consequently, it would be advisable to perform a continuous research of these elements, as well as to control exhaustively the degree of exposure of the population to As and Mn. In the present study no differentiation between organic and inorganic As in water and sediment samples was performed. Taking into account that only inorganic As is carcinogenic, a clear overestimation of the risk might have been done in this work. Therefore, an As speciation study would be of great importance in order to establish much more reliable values of cancer risk.
Nature and kind of source inputs and distribution of PAHs in sediments
The PAH compounds with molecular weights between 166 and 202 (that is fluorene, phenanthrene, anthracene and pyrene) had low concentrations in the lagoon sediment. The highest PAH concentration was recorded for naphthalene (molecular weight=128). Samples collected at S2 and S3 exhibited relatively high levels of PAH contamination clearly demonstrating anthropogenic impact. The magnitude of PAH from S2 reflects the heavy load from the automobile repair and spraying activities in the northern part of the lagoon. Incinerated household garbage, effluents and sewage discharge, unburnt petroleum products from two filling stations and vehicular emission from the heavily trafficked bridge with the sediments as an ultimate sink appear to be main sources of the PAHs. This finding confirms the work of Gilbert et al. [1]. Generally, the sources of PAHs may be categorized into two: combustion/pyrolysis and petrogenesis. By and large, when phenanthrene-anthracene ratio is more than 10, the PAH source is considered to be petrogenesis whereas the source is considered to be combustion or pyrolysis when the ratio is less than 10. Again, a fluoranthrene-pyrene ratio greater than 1 suggests a pyrolytic derivation. The phenathrene-anthracene ratios for S1, S2 and S3, were less than 10 indicating that the PAHs obtained in this study originate from combustion. As indicated above, the combustion could be related to vehicular emissions and tyre burning which is widespread at the Siwdu mechanical workshops.
Non-cancer and cancer risks from exposure to PAHs
Benzo[b]fluoranthrene is the only carcinogenic PAH that was detected in the study. The total non-cancer risk for children and adults under the RME scenario were estimated as 2.54×10-2 and 4.16×10-3 respectively. On the other hand, the total carcinogenic risk for children and adult resident-RME were estimated as 1.02×10-5 and 8.38×10-6 respectively (Table 8). From the results obtained in the PAH-based risk calculations, it appears that the non-cancer and cancer risk to children were greater than that of the adult residents. This is expected as children are considered to be more vulnerable to chemical-induced risk than their adult counterparts [18]. The risk values for anthracene and fluorene were very small as compared to the threshold (HQ < 1) hence posed insignificant health risk to both adults and children under the CTE scenario. Pyrene also constituted little non-cancer health risk to children.
| PAH Compounds | Hazard index for receptor, route and chemical | Carcinogenic risk for receptor, route and chemical | |||||||||||
| Resident CTE | |||||||||||||
| Child | Adult | Child | Adult | ||||||||||
| Ingestion | Dermal | Total | Ingestion | Dermal | Total | Ingestion | Dermal | Total | Ingestion | Dermal | Total | ||
| Anthracene | 2.58×10-6 | 5.08×10-7 | 3.09×10-6 | 7.58×10-8 | 7.67×10-8 | 1.53×10-7 | - | - | - | - | - | - | |
| Naphthalene | 3.00×10-3 | 5.90×10-4 | 3.59×10-3 | 8.80×10-5 | 8.90×10-5 | 1.77×10-4 | - | - | - | - | - | - | |
| Pyrene | 1.24×10-5 | 2.43×10-6 | 1.48×10-5 | 3.63×10-7 | 3.68×10-7 | 7.31×10-7 | - | - | - | - | - | - | |
| Fluorene | 5.29×10-7 | 1.04×10-7 | 6.33×10-7 | 1.55×10-8 | 1.57×10-8 | 3.12×10-8 | - | - | - | - | - | - | |
| Benzo(b)fluoranthene | - | - | - | - | - | - | 1.22×10-6 | 2.39×10-7 | 1.46×10-6 | 5.34×10-8 | 5.41×10-8 | 1.08×10-7 | |
| Total | 3.02×10-3 | 5.93×10-4 | 3.61×10-3 | 3.00×10-3 | 8.95×10-5 | 3.09×10-3 | 1.22×10-6 | 2.39×10-7 | 1.46×10-6 | 5.34×10-8 | 5.41×10-8 | 1.08×10-7 | |
| Resident RME | |||||||||||||
| Anthracene | 1.55×10-5 | 6.19×10-6 | 2.17×10-5 | 1.66×10-6 | 1.91×10-6 | 3.57×10-6 | - | - | - | - | - | - | |
| Naphthalene | 1.80×10-2 | 7.19×10-3 | 2.52×10-2 | 1.92×10-3 | 2.21×10-3 | 4.13×10-3 | - | - | - | - | - | - | |
| Pyrene | 7.42×10-5 | 2.97×10-5 | 1.04×10-4 | 7.95×10-6 | 9.14×10-6 | 1.71×10-5 | - | - | - | - | - | - | |
| Fluorene | 3.16×10-5 | 1.27×10-5 | 4.43×10-5 | 3.39×10-6 | 3.90×10-6 | 7.29×10-6 | - | - | - | - | - | - | |
| Benzo(b)fluoranthene | - | - | - | - | - | - | 7.27×10-6 | 2.91×10-6 | 1.02×10-5 | 3.90×10-6 | 4.48×10-6 | 8.38×10-6 | |
| Total | 1.81×10-2 | 7.24×10-3 | 2.54×10-2 | 1.93×10-3 | 2.22×10-3 | 4.16×10-3 | 7.27×10-6 | 2.91×10-6 | 1.02×10-5 | 3.90×10-6 | 4.48×10-6 | 8.38×10-6 | |
Table 8: Carcinogenic and non-carcinogenic risk values for PAHs via CTE and RME Scenarios.
The quantitative risk estimates are based on a considerable number of assumptions, extrapolations and uncertainties. Areas of uncertainty are associated with most aspects of the project including sampling and analysis, data evaluation, estimating exposure point concentrations, quantifying exposure parameters and quantifying toxicity dose-response evaluations. Each of these areas may result in an under- or overestimate of risk as described below. The data used to estimate exposure point concentrations were from sampling rather than population data. Because PAHs tend to accumulate in sediments, benthic organisms may be continuously exposed to the contaminants. However, sediment-sorbed PAHs have only limited bioavailability to marine organisms, which greatly reduces their potential toxicity [24]. Sediment samples were collected from known or suspected areas of contamination and may not accurately reflect actual exposure to various receptors. In addition, long term exposure was evaluated based on current conditions with no correction for chemical dilution, dispersion or degradation. It is extremely unlikely that site conditions will remain unchanged for the next 25 to 30 years. Notwithstanding these limitations the study is very relevant and holds implications for policy and decision-making regarding the use and management of the lagoon.
Environmental fate and transport modelling
Table 9 shows physical-chemical properties of naphthalene, anthracene, benzo [b] fluoranthene, pyrene, fluorene and phenanthrene estimated using the US EPA’s QSAR Software (EPI Suite). Except naphthalene, all the PAHs have low vapour pressures and low water solubilities, suggesting that they are not volatile and not very water soluble compounds. High Kow (octanol-water partition coefficient) and Koc (soil adsorption coefficient) values also suggest that they have a tendency to partition onto soil or sediment in the environment. Computer modelling using the PBT Profiler estimated fish bioconcentration factor values (log BCF 3.481 for benzo [b] fluoranthene and 3.271 for phenanthrene), suggesting that both compounds have the potential to bioaccumulate while naphthalene (log BCF 1.844) is the least bioaccumulative. Generally, ChV (mg/L) of less than 0.1 indicates high concern level whereas ChV (mg/L) greater than 10 indicates low concern. ChV (mg/L) ranging from 0.1 to 10 reflects moderate concern. The concern levels for benzo[b]fluoranthene and pyrene are high (Table 9). The concern levels for the other PAHs are moderate.
| Property | Naphthalene | Anthracene | Benzo[b]fluoranthene | Pyrene | Fluorene | Phenanthrene |
| CAS number | 91-20-3 | 120-12-7 | 205-99-2 | 129-00-0 | 86-73-7 | 85-01-8 |
| Molecular formula | C10H8 | C14H10 | C20H12 | C16H10 | C13H10 | C14H10 |
| Boiling point (°C) | 231.64 | 327.31 | 442.75 | 371.85 | 292.57 | 327.31 |
| Melting point (°C) | 5.01 | 78.09 | 169.41 | 119.90 | 63.69 | 78.09 |
| Vapour pressure mmHg at 25°C | 0.0404 | 2.17E-06 | 2.49E-08 | 3.44E-07 | 3.3 E-04 | 4.32E-05 |
| Water solubility mg/L at 25°C | 142.1 | 0.6905 | 0.02065 | 0.2249 | 1.339 | 0.677 |
| LogKow | 3.17 | 4.35 | 6.11 | 4.93 | 4.02 | 4.35 |
| LogKoc | 2.864 | 3.862 | 5.016 | 4.235 | 3.627 | 3.870 |
| LogBCFa | 1.844 | 2.603 | 3.481 | 2.887 | 2.425 | 3.271 |
| ChV (mg/L)b | 1.042 | 0.145 | 0.006 | 0.052 | 0.257 | 0.145 |
aFish bioconcentration factor estimated by the PBT Profiler
bFish chronic toxicity value estimated by the PBT Profiler
Table 9: General properties of the PAHs calculated using the US EPA Estimation Programs Interface Suite (EPI Suite v4.10) and PBT Profiler.
Aerobic biodegradability of the PAHs was predicted using six different BIOWIN models. All models gave ‘‘does not biodegrade fast’’ estimation for the PAH compounds with primary biodegradation half lives of “days-weeks’’ and ‘‘weeks’’ and ultimate biodegradation halflives of “weeks-months” and ‘‘months’’ (Table 10).
| Compound | BIOWIN models | |||
| Linear | Non-linear | Survey model | ||
| Primary | Ultimate | |||
| Naphthalene | NBFa | NBF | Days-weeks | Weeks-months |
| Anthracene | NBF | NBF | Weeks | Months |
| Benzo[b]fluoranthene | NBF | NBF | Weeks | Months |
| Pyrene | NBF | NBF | Weeks | Months |
| Fluorene | NBF | NBF | Days-weeks | Weeks |
| Phenanthrene | NBF | NBF | Weeks | Months |
a Does not biodegrade fast
Table 10: Estimated reactivity data (EPI Suite v4.10).
According to Carlsen and Walker [22], BIOWIN estimates biodegradation probabilities and probabilities lower than 0.5 signify that the compound does not biodegrade fast. This implies that estimated probabilities for all the PAHs were below 0.5. In the cases of primary biodegradation and ultimate biodegradation, predicted values in the ranges of 5.0-4.0, 4.0-3.0, 3.0-2.0, 2.0-1.0, and <1.0 indicate that biodegradation will occur within hours, days, weeks, months, longer than months, respectively [22].
STPWIN32 (modeling software for removal in wastewater treatment) predicted that approximately 55% of the phenanthrene mass partitioned into sludge, only about 0.5% of the mass was biodegraded in wastewater treatment. No more than 9% of the naphthalene mass partitioned into sludge, and only about 0.13% of the mass was biodegraded in wastewater treatment whereas 15% was lost to air. About 52% of the anthracene mass partitioned into sludge, only about 0.5% of the mass was biodegraded in wastewater treatment. A very high fraction (90%) of benzo [b] fluoranthene mass was partitioned into sludge whereas only 0.8% of the mass was biodegraded in wastewater. Nearly 74% of the pyrene mass partitioned into sludge whereas only about 0.7% of the mass was biodegraded in wastewater treatment. Regarding fluorene, mass partitioned into sludge and mass biodegraded are 38% and 0.4% respectively.
Level III fugacity model and the PBT Profiler generated similar results on partitioning of the PAH mass in air, water, soil and sediment. More than 80% of the mass of all the PAHs was found in the soil and sediment compartments, which will have a significant influence on the overall persistence of these compounds [21,22]. The mass of each of the following PAHs: pyrene, anthracene and benzo [b] fluoranthene found in the soil and sediment was higher than 90%. Consequently, information on the soil and sediment half-lives is important to understand the fate of these two compounds in the environment. The half-lives for the PAH compounds were estimated to be between 120 and 541 days in the sediment compartment.
Policy implications and recommendations
In the face of increasing pollution of the Fosu Lagoon, local community initiatives have over the years, been restricted to episodic clearing of aquatic weeds (invasive species) from the lagoon. For fishermen, who depend on the lagoon for livelihood, they recognized the fact that the catch per unit effort and fish abundance have both reduced over the years. Yet, they hastened to add that comparatively, the fingerlings (mpatoa) from the Fosu Lagoon were still the tastiest. The Water Resources Commission, the statutory government regulatory body, has done little regarding remediation besides cautioning the public not to eat fish from the lagoon [12]. Fishermen continue to fish and sell the ‘mpatoa’ from the lagoon defying the Ghana EPA caution. Opinion leaders among the fisher folk perceive there was no way the lagoon was polluted considering the fact that they had worked in the lagoon for the past 35 years and were eating the fish and were very healthy. The fishermen expressed their displeasure over the GEPA caution of the public, adding that there were no prior consultations before the warning was sounded. They indicated the GEPA public statement to the effect that the lagoon was one of the most polluted in the country and eating fish from it could cause cancers and nervous system breakdowns, had greatly affected sale of the fingerlings on the market, and that many of the over 200 fishermen who plied their trade in the lagoon had been encountering very difficult financial problems since the caution was issued.
It would seem that one way to inform public decision-making on the matter would be to characterize the type and quantify the amount of biological and chemical contaminants in fish samples from the lagoon. To consolidate the findings of the proposed study, there may be also the need to assess the fish consumption related risk posed to different stakeholder groups in the surrounding population. Armah et al. [12], Darkwa and Smardon [25], have severally highlighted salient knowledge systems, management and policy implications regarding the context of the Fosu Lagoon. It is obvious that the Ghana Government needs to resource the WRC and the metropolitan assembly financially and logistically in order to accomplish its pollution monitoring and remediation, and legislation enforcement mandate. In many ways, the interactive effects of the number and diversity of stakeholders in the Fosu Lagoon introduces complexity into the management decision making process [12]. The aforementioned issues hold implications for policy outcomes. Several management practices particularly those that are attuned to the cultural dispositions of the indigenous population are embedded in the knowledge systems of these communities [12,25]. Apart from the enactment of by-laws on the utilization of the Fosu Lagoon resources by the local government, we recommend the reinforcement of these practices to ensure that the Fosu Lagoon is managed sustainably.
Conclusion
Geo-accumulation index, Metal pollution index, and Enrichment factor were successfully applied for the assessment of heavy metal and PAH contamination of sediments from Fosu lagoon. Also, human health risk assessment from exposure to these contaminants was carried out. All sites in the present study recorded low degree of contamination for Pb, As and Cd with intensity of unpolluted to moderately polluted but Mn recorded high degree of contamination indicating that sediments in the lagoon are somewhat impacted by human activities. Almost all the sites showed MPI values > 2 confirming there were considerable heavy metal contamination. The proximity of the road to the lagoon perhaps contributes to the enrichment factors for Pb, Cd and As. The high enrichment of sediments by Mn could be attributed to anthropogenic processes. Benzo[b]fluoranthrene is the only carcinogenic PAH that was detected in the study. The phenanthrene-anthracene ratio was less than 10 indicating that the PAHs originate from combustion. The carcinogenic risk derived from the ingestion and dermal absorption of metals exceeded the generally acceptable risk level for individual chemicals. That risk level, basically due to the ingestion of As and Mn through sediments would mean a concern for human health. Although the concentrations of PAHs in sediments were quite high, the risk assessment indicates they do not present significant non-carcinogenic and carcinogenic risk for the local population. The fate modelling however, suggests that naphthalene, anthracene, benzo [b] fluoranthene, pyrene, fluorene and phenanthrene tend to sorb onto soil or sediment in the environment and biologically ‘‘do not degrade fast’’. This suggests the need to implement long term monitoring of these compounds in order to sustain this fragile ecosystem.
Acknowledgements
The authors thank Charity Amegah and Philip Baidoo for assisting us in data collection and processing. We also acknowledge Karen Van Kerkoerle of the Cartography Unit, UWO; Canada for drawing the map of the study area.
References
- Gilbert E, Dodoo DK, Okai-Sam F, Essuman K, Quagraine EK (2006) Characterization and Source Assessment of Heavy Metals and Polycyclic Aromatic Hydrocarbons (PAHs) in Sediments of the Fosu Lagoon ,Ghana. J Environ Sci Health A Tox Hazard Subst Environ Eng 41: 2747-2775.
- Simpson SL, Batley GE, Chariton AA, Stauber JL, King CK, et al. (2005) Handbook for Sediment Quality Assessment CSIRO Bangor NSW.
- Silva N, Haro J, Prego R (2009) Metals background and enrichment in the Chiloe. Interior Sea sediments (Chile). Is there any segregation between fjords, channels and sounds? Estuar Coast Shelf Sci 82: 469-476.
- Armah FA, Obiri S, Yawson DO, Onumah EE, Yengoh GT, et al. (2010) Anthropogenic sources and environmentally relevant concentrations of heavy metals in surface water of a mining district in Ghana: a multivariate statistical approach. J Environ Sci Health A Tox Hazard Subst Environ Eng 45:1804-1813.
- Chen ZY, Saito Y, Kanai Y, Wei TY, Li LQ, et al. (2004) Low concentration of heavy metals in the Yangtze estuarine sediments, China: A diluting setting. Estuar Coast Shelf Sci 60: 91-100.
- Forstner U (1990) Inorganic Sediments Chemistry and Elemental Speciation. In: Buado R, Giesy J, Muntau H (Eds) Sediments: Chemistry and Toxicity of in-place pollutants. Lewis Publishers Inc, Boca Racon, pp: 61-105.
- Ingersoll CG (1995) Sediment tests in: Rand GM (Ed) Fundamentals of Aquatic Toxicology (2nd Ed) Effects Environmental Fate, and Risk assessment Taylor and Francis, New York, pp: 231-255.
- Obiri S, Cobbina SJ, Armah FA, Naangmenyele Z (2011) Quantification and characterisation of vehicle-based polycyclic aromatic hydrocarbons (PAHs) in street dust from the Tamale metropolis, Ghana. Environ Sci Pollut Res Int 18: 1166-1173.
- Baars AJ, Theelen RMC, Janssen PJCM, Hesse JM, Van Apeldoon ME et al. (2001) Re-evaluation of Human Toxicological maximum Permissable risk levels.
- Fetzer JC (2000) The Chemistry and Analysis of the Large Polycyclic Aromatic Hydrocarbons. Polycyclic Aromatic Compounds (New York: Wiley) 27:143
- Ure AM, Davidson CM (2002) Chemical speciation in soils and related materials by selective chemical extraction. In: AM Ure CM, Davidson (Eds.) Chemical Speciation in the Environment (2nd Ed.) Blackwell Scientific Publications Oxford.
- Armah FA, Yawson DO, Pappoe ANM, Afrifa EKA (2010) Participation and Sustainable Management of Coastal Lagoon Ecosystems: The Case of the Fosu Lagoon in Ghana. J Sustainability 2: 383-399.
- Dodoo DK, Adjei GA (1995) Copper and zinc in the sediments of the Fosu Lagoon. Ghana Journal of Chemistry 1: 507-514.
- Ruellet T, Dauvin JC (2007) Benthic indicators: analysis of the threshold values of ecological quality classifications for transitional waters. Mar Pollut Bull 54: 1707-1714.
- Tam NFY, Wong YS (1995) Spatial and temporal variations of heavy metals contamination in sediments of a mangrove swamp in Hong Kong. Mar Pollut Bull 31: 254-261.
- USEPA (2005b) The Risk Assessment Information System. RAIS.
- USEPA (1997) Guiding Principles for Monte Carlo Analysis. EPA/630/R-97/001. Risk assessment Forum Washington DC, USA.
- Obiri S, Dodoo DK, Essumang DK, Armah FA (2010) Cancer and Non-Cancer Risk Assessment from Exposure to Arsenic, Copper, and Cadmium in Borehole, Tap, and Surface Water in the Obuasi Municipality, Ghana. International J Hum Ecol Risk Assess 16: 651-665.
- USEPA (1989) Risk Assessment Guidance for Superfund: Volume 1-Human Health Evaluation Manual. Interim Final EPA/540/1-89/002. Office of Emergency and Remedial Response Washington DC, USA.
- USEPA (2002) Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites. Appendix D-Dispersion Factors Calculations. OSWER 9355: 4-24 Washington DC USA.
- Ying GG, Yu XY, Kookana RS (2007) Biological degradation of triclocarban and triclosan in a soil under aerobic and anaerobic conditions and comparison with environmental fate modelling. Environ Pollut 150: 300-305.
- Carlsen L, Walker JD (2003) QSARs for prioritizing PBT substances to promote pollution prevention. QSAR Comb Sci 22: 49-57.
- Agency for Toxic substances and Disease Registry (ATSDR) 2000. "Toxicology profile for Polycyclic Aromatic Hydrocarbons (PAHs)" Atlanta, GA: US Department for health and public health Service.
- Salazar-Coria L, Amezcua-Allieri MA, Tenorio-Torres M, González-Macías C (2007) Polyaromatic hydrocarbons (PAHs) and metal evaluation after a diesel spill in Oaxaca, Mexico. Bull Environ Contam Toxicol 79: 462-467.
- Darkwa S, Smardon RC (2010) Ecosystem restoration: Evaluating local knowledge and management systems in Fosu Lagoon, Ghana. Environ Pract 12: 202-213.
- Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environmental Geology 39: 611-627.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 16046
- [From(publication date):
March-2012 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 11340
- PDF downloads : 4706