Research Article Open Access
Early Phase Clinical Trials: Referral Barriers and Promoters among Physicians
Amelie G. Ramirez1*, Patricia Chalela1, Lucina Suarez2, Edgar Muñoz1, Brad H. Pollock3, Steven D. Weitman4 and Kipling J. Gallion1
1Institute for Health Promotion Research, The University of Texas Health Science Center at San Antonio,7411 John Smith Dr. Suite 1000, San Antonio, Texas 78229, USA
2Texas Department of State Health Services, Environmental Epidemiology & Disease Registries Section T-711, MC 1964,1100 West 49th Street, Austin, Texas 78756, USA
3Department of Epidemiology and Biostatistics, The University of Texas Health Science Center at San Antonio,7703 Floyd Curl Ave., Mail Code 7933, San Antonio, Texas 78229-3901, USA
4The University of Texas Health Science Center at San Antonio, Cancer Therapy & Research Center, Institute for Drug Development, 7979 Wurzbach Road, Room Z459, San Antonio, Texas 78229, USA
- *Corresponding Author:
- Amelie G. Ramirez, DrPH
University of Texas Health Science Center at San Antonio
Institute for Health Promotion Research
7411 John Smith Dr., San Antonio
Texas 78229, USA
Tel: 210-562-6500
Fax: 210-562-6545
E-mail: ramirezag@uthscsa.edu
Received date: August 30, 2012; Accepted date: September 22, 2012; Published date: September 24, 2012
Citation: Ramirez AG, Chalela P, Suarez L, Muñoz E, Pollock BH, et al. (2012) Early Phase Clinical Trials: Referral Barriers and Promoters among Physicians. J Community Med Health Educ 2:173. doi: 10.4172/2161-0711.1000173
Copyright: © 2012 Ramirez AG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
Background: Physician referral is among the most effective means of recruiting patients into cancer clinical trials. Therefore, to increase minority representation in early-phase clinical trials (EPCTs), specifically accrual of Latinos, it is first necessary to examine physicians’ attitudes and practices regarding these studies and factors that influence physicians’ referral decisions.
Methods: This study surveyed oncologists (N=111) from a Texas Medical Association mailing list to examine barriers and promoting factors associated with physician referral of patients to EPCTs and identify areas for intervention to increase accrual of Latinos and other minorities into clinical research. Exploratory factor analysis was conducted to identify underlying dimensions, and significant factors that promote or deter physicians from referring patients to EPCTs were assessed through multiple logistic regression.
Results: Burden of the clinical trial process was the only significant dimension associated with referring patients to EPCTs. Physicians who agreed with this set of logistical barriers—such as diverting time and resources away from their practice—were less likely to refer patients than physicians with opposing opinions (OR= 0.28, 95% CI= 0.08-0.94).
Conclusion: This study, one of the first to identify physician barriers for referring patients to EPCTs in Texas, highlights potential focal areas for physician and community-based education and communication to promote clinical trial opportunities among both minority and non-minority patients. Given that Texas physicians deal with a large proportion of Latino patients, such efforts could also address ethnic disparities in clinical trial participation, which will become increasingly important as the Latino population continues to grow.
Keywords
Early phase; Clinical trials; Physicians; Barriers; Promoters; Referral
Introduction
Almost two decades after the National Institutes of Health (NIH) mandate to ensure inclusion of women and minorities in clinical research, Latinos and other minority groups continue to be critically underrepresented in the research arena [1,2]. Of all patients enrolled in publicly funded National Cancer Institute (NCI) clinical trials, only 8% were African American and 5% were Latinos [1,3]. These populations experience disparate cancer incidence, mortality, survival, and other cancer care [4-7].
Adequate inclusion of minorities in clinical research is an essential step to developing novel cancer treatments, improving health care overall and addressing minorities’ disproportionate cancer burden [1,2,4]. Without adequate minority representation in early-phase clinical trials (EPCTs), researchers cannot assess differential effects among groups or ensure the generalizability of trial results [1,2,8].
Provider perceptions and attitudes play a significant role in trial enrollment for underrepresented populations [1,2]. Physicians act as gatekeepers and provide a majority of patient education regarding clinical trials; therefore, they influence recruitment and patient decision-making. Research has shown that participation and recruitment barriers include physicians’ lack of clinical trial awareness, attitudes, level of comfort explaining the trial, fear of losing patients, mistrust of medical institutions and researchers, lack of time and structural support, limited resources for data management, complexity of the study protocol and level of burden, feelings about the patient’s age and comorbid conditions, and lack of adequate compensation for involvement [1,2,8-20]. Physicians may also feel that referring patients to trials might negatively affect and threaten their relationship with patients, manifesting in patients either blaming the physician for any negative consequences of study participation or threatening the physician’s income as patients seek increasing care via trial physicians [17,21-23].
Physicians may not offer or even discuss clinical trials as a treatment option with minority patients specifically, because they may perceive that these patients face unique barriers: mistrust of researchers and the medical system; lack of interest, awareness/information and resources; insufficient health literacy to understand the trial; and limited protocol compliance [2,8,9,11,12,14,17,20,24]. Other known barriers to the referral of minorities include providers’ inadequate language proficiency [20,25,26] and inadequate means of presenting information to patients [2,9,20,24,27].
Physician referrals are among the most effective means of recruiting patients into cancer clinical trials [1,2,8,12]. Therefore, to increase minority representation in EPCTs, specifically Latinos, it is first necessary to examine physicians’ attitudes and practices regarding EPCTs. The study aimed to examine physicians’ knowledge, attitudes and practices toward EPCTs. Assessing barriers and promoting factors associated in physicians’ referrals to EPCTs is crucial for identifying appropriate areas for intervention to increase accrual of Latinos and other minorities in clinical research, promote equity in clinical trial participation, and reduce disparities in cancer outcomes and survivorship.
Materials and Methods
Participants and data collection
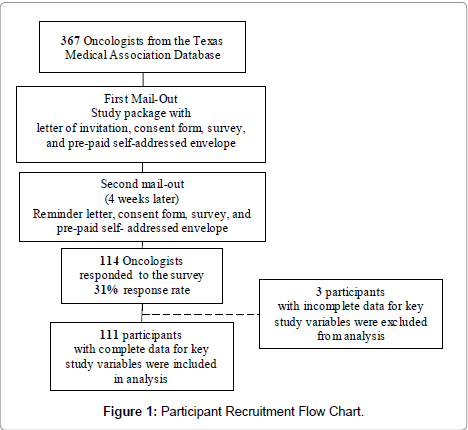
A list of 367 eligible physicians—those categorized as medical oncologists, hematologist-oncologists or radiation oncologists—was obtained from the Texas Medical Association. Physicians were mailed survey packets in December 2004 and again in January 2005 (for initial non-respondents). Survey packets included a letter of invitation, consent form, survey, and return postage-paid self-addressed envelope. Physicians were informed of the survey’s anonymity, purpose (identify promoters/barriers oncologists face in referring patients to EPCTs) and use of study results (guiding an intervention to reduce barriers and increase EPCT accrual). After the two mail-outs, 114 physicians returned surveys (31% response rate). The study was approved by the corresponding Institutional Review Board. Respondents participated in three raffles of $500 each as an incentive for their time (Figure 1).
Survey instrument
Based on a literature review, questionnaires from other investigators [28-30], the research team’s own experience and discussion with oncologists at the Cancer Therapy & Research Center in San Antonio, Texas, a survey questionnaire was created, reviewed and approved. The 10-minute survey covered participants’ background and characteristics, EPCT experience, information about clinical trials, doctor/patient communication, and promoters and barriers that influence oncologists’ decisions to refer patients to EPCTs.
Measures
The present study’s outcome variable of interest was assessed by one item: “Last year have you referred patients to clinical trials (CTs)?”
Relevant demographic and background variables included information about the participant’s age, gender, ethnic identification, specialty, years practicing oncology, practice setting, ethnicity of patients served, and Spanish language proficiency.
Information about clinical trials included six items related to sources of information about EPCTs, frequency and amount of information received, most effective ways of receiving information, and familiarity with local EPCTs.
Doctor/patient relationship included one item: “In your opinion, how easy is it to communicate information about clinical trials to your patients?”
Promoters that encourage physicians to refer patients to EPCTs included five items about direct therapeutic or psychosocial benefit to patients, one item on patient/family desire to try something new, one item related to close follow-up of patients by medical team, and six items related to physician’s professional benefit.
Barriers included nine items about logistical factors (e.g., “No staff support available”), 11 items on personal factors (e.g., “Lack of awareness about the trial”), and eight items on trial-related factors (e.g., “Eligibility criteria too strict/stringent”).
Finally, two questions measured most difficult ethnic and age groups to refer to EPCTs.
Data analysis
The analysis included 111 physicians with complete data for key study variables. Descriptive statistics were used to summarize sociodemographic items, practice characteristics, and familiarity with local clinical trials. Univariate analysis was used to assess differences between referring and non-referring physicians regarding specific EPCT referral promoters and barriers, including t-tests for continuous variables, and Chi-square statistics for categorical variables (exact Chi-square test for nominal variables and Chi-square test for trend for ordinal variables). Given the small sample size and the number of promoter and barrier items, an exploratory factor analysis with alpha factoring extraction method, based on maximizing the reliability of factors, was conducted to identify correlated items that could represent one or more underlying dimensions of promoters and barriers [31]. Anderson-Rubin and simple sum methods were used to obtain factor scores for each dimension, and were evaluated using means testing [32]. In addition, a multiple logistic regression analysis was conducted to assess significant factors that promote or deter physicians from referring patients to EPCTs. Selected physician sociodemographic characteristics known as potential confounders, promoters and three barrier dimensions were included as covariates in the multiple logistic regression model.
Standardized scores for predictor variables were used for multiple logistic regression analysis to place the predictors on a common scale so that each has the same mean and standard deviation [33,34].
All analyses were conducted using PASW version 19 [35]. All statistical tests were two-sided and a p value of 0.05 was considered statistically significant.
Results
Table 1 shows the demographic characteristics of participants. Most were males (76.6%) with more than five years of practice (82.8%). Two-thirds (66.7%) were non-Hispanic Whites, 16.2 percent Asian, and 12.6 percent Hispanic. Most were medical oncologists (80%) or hematologists-oncologists (15.3%), and most were in private (57.7%) or academic (34.2%) practice. Only 17 percent spoke Spanish fluently or well enough to interview patients, and half reported they were very familiar with clinical trials in their area.
| Characteristics | Referral Status | Total (N=111) |
p | ||||
| YES (N = 96) |
NO (N=15) |
||||||
| N | % | N | % | N | % | ||
| Age: | |||||||
| 40-49 | 31 | 32.3 | 5 | 33.3 | 36 | 32.4 | |
| 50-59 | 33 | 34.4 | 5 | 33.3 | 38 | 34.2 | |
| 60+ | 13 | 13.5 | 2 | 13.3 | 15 | 13.5 | |
| Gender: | |||||||
| Male | 74 | 77.1 | 11 | 73.3 | 85 | 76.6 | 0.750 |
| Female | 22 | 22.9 | 4 | 26.7 | 26 | 23.4 | |
| Ethnicity: | |||||||
| Asian/Pacific Islander | 15 | 15.6 | 3 | 20.0 | 18 | 16.2 | 0.615 |
| Hispanic/Latino | 11 | 11.5 | 3 | 20.0 | 14 | 12.6 | |
| White non-Hispanic | 65 | 67.7 | 9 | 60.0 | 74 | 66.7 | |
| Other | 5 | 5.2 | 0 | 0.0 | 5 | 4.5 | |
| Speak Spanish: | |||||||
| Fluently | 10 | 10.4 | 2 | 13.3 | 12 | 10.8 | 0.858 |
| Well enough to interview patients | 6 | 6.2 | 1 | 6.7 | 7 | 6.3 | |
| Limited to basic conversation | 18 | 18.8 | 2 | 13.3 | 20 | 18.0 | |
| Not very well, just a few words | 41 | 42.7 | 5 | 33.3 | 46 | 41.4 | |
| Not at all | 21 | 21.9 | 5 | 33.3 | 26 | 23.4 | |
| Specialty: | |||||||
| Medical oncologist | 78 | 81.2 | 11 | 73.3 | 89 | 80.2 | 0.767 |
| Radiation oncologist | 4 | 4.2 | 1 | 6.7 | 5 | 4.5 | |
| Hematologist/oncologist | 14 | 14.6 | 3 | 20.0 | 17 | 15.3 | |
| Practice Setting: | |||||||
| Academic/Teaching hospital | 36 | 37.5 | 2 | 13.3 | 38 | 34.2 | 0.258 |
| Community-based hospital | 6 | 6.3 | 1 | 6.7 | 7 | 6.3 | |
| Private practice | 52 | 54.2 | 12 | 80.0 | 64 | 57.7 | |
| Other | 2 | 2.0 | 0 | 0.0 | 2 | 1.8 | |
| <5 | 15 | 15.6 | 4 | 26.7 | 19 | 17.1 | 0.31 |
| 5-10 | 17 | 17.7 | 3 | 20.0 | 20 | 18.0 | |
| 11-15 | 10 | 10.4 | 2 | 13.3 | 12 | 10.8 | |
| 16-20 | 19 | 19.8 | 1 | 6.7 | 20 | 18.0 | |
| 21-25 | 17 | 17.7 | 3 | 20.0 | 20 | 18.0 | |
| >25 | 18 | 18.8 | 2 | 13.3 | 20 | 18.0 | |
| Familiarity with Local CTs: | |||||||
| Very familiar/Familiar | 54 | 56.8 | 1 | 6.7 | 55 | 50.0 | <0.001 |
| Somewhat familiar | 27 | 28.4 | 3 | 20.0 | 30 | 27.3 | |
| Not very familiar/Totally unfamiliar | 14 | 14.8 | 11 | 73.3 | 25 | 22.7 | |
| Frequency of CT Information: | |||||||
| Very often/Often | 39 | 40.6 | 3 | 20.0 | 42 | 37.8 | 0.126 |
| Not very often/Never | 57 | 59.4 | 12 | 80.0 | 69 | 62.2 | |
Table 1: Characteristics of Physicians by Referral Status.
Nearly 87 percent have referred patients to clinical trials during the past year. In general, no significant differences existed in demographic and background characteristics between physicians who referred patients and those who did not, except for familiarity with local clinical trials. As expected, those who referred patients were more familiar with clinical trials in their area than those who did not refer patients (88.2% vs. 77.3%, p<0.001).
Table 2 shows the dimensions identified through exploratory factor analysis for promoter and barrier items included in the original survey as well as the stand-alone items that were not associated with any of the dimensions but were included in subsequent analysis given that literature suggests their association with referring patients to clinical trials [9,12-14,17,23,36].
| Dimension | Original Survey Items |
|---|---|
| Promoters: | |
| Physician personal benefits | •Local trials keep patients in the community •Frequent contact with PI/researchers •Trial participation gives prestige •Frequent exposure to EPCT information |
| Trial treatment benefits | •Possibility of therapeutic benefit to patient •Possibility that experimental treatment is better than standard treatment •Trial provides the best and most current treatment |
| Patient psychosocial benefits | •Possibility of psychological benefit to patient •Patient/family wants to try something new |
| •Comfortable explaining EPCTsa •Patients are followed very closelya |
|
| Logistical barriers: | |
| Clinical trial process burden | •Paperwork of referral process is too time-consuming •Too much time required to explain an EPCTs •Excessive details of protocols •No staff support available •Referral procedures are too complex •Referring patients to EPCTs is extra work |
| Coordination/Involvement with clinical trial site | •Contacting the PI is too difficult •Contacting the referral office at CT site is too difficult •The institution does not keep me in the loop regarding my patients |
| Personal Barriers: | |
| Personal/Attitudes | •Don’t want to increase patient’s anxiety •Language barriers with minority patients •Patients will blame the referral oncologist for any adverse effects •Hassle of convincing patients to participate •Difficulty explaining medical uncertainty •Referring patients to trials might negatively affect relationship with patient •Distrust of researchers conducting the trial |
| Lack of personal benefits | •Lack of rewards and recognition •Loss of personal income as a result of patients seeking treatment from trial physicians •Fear loss of control over patient care |
| •Lack of awareness about EPCTsa | |
| Protocol-Related Barriers: | |
| Protocol complexity | •Misinterpretation of study protocol •Consent procedure too difficult or complex •Trial protocol too complex •Misinterpretation of eligibility of patients •Eligibility criteria too strict/stringent |
| Trial treatment | •Toxicity/side effects of experimental TT outweighs possible benefits •Trial therapy not as good as standard therapy •Low probability of therapeutic benefit to the patient |
Table 2: Dimensions Identified through Exploratory Factor Analysis and Original Survey Items.
Differences between physicians who referred patients to clinical trials and those who did not were assessed via means testing (Table 3). A summary score was created for items measuring the same underlying dimension and stand-alone items identified under major categories but not related to any of the specific dimensions also included in the analysis.
| Factors | Referral Status | t- test | p-value | 95% CI | Min. | Max. | |||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n= 96) |
No (n= 15) |
||||||||
| Mean | SD | Mean | SD | ||||||
| Promotersa | |||||||||
| Physician benefits summary score | 10.74 | 3.07 | 11.33 | 2.87 | 0.697 | 0.487 | -1.08 – 2.24 | 4.00 | 20.00 |
| Trial benefits summary score | 6.72 | 2.35 | 7.20 | 2.43 | 0.741 | 0.460 | -0.81 – 1.78 | 3.00 | 15.00 |
| Patient psychosocial benefits summary score | 4.27 | 1.16 | 4.87 | 0.92 | 1.903 | 0.060 | -0.03 – 1.22 | 2.00 | 7.00 |
| Patients are followed closely | 2.42 | 0.87 | 2.47 | 0.64 | 0.214 | 0.831 | -0.41 – 0.51 | 1.00 | 5.00 |
| Comfortable explaining trial to patients | 2.35 | 0.67 | 2.33 | 0.82 | -0.109 | 0.913 | -0.40 – 0.36 | 1.00 | 4.00 |
| Logistical Barriersa | |||||||||
| Clinical trial process burden summary score | 16.14 | 4.73 | 12.00 | 3.34 | -3.26 | 0.001 | -6.65 – -1.62 | 6.00 | 30.00 |
| Coordination/Involvement with clinical trial site summary score | 8.15 | 2.87 | 7.07 | 1.75 | -2.00 | 0.055 | -2.18 – 0.26 | 3.00 | 15.00 |
| Personal Barriersa | |||||||||
| Physician attitudes items summary score | 24.58 | 5.02 | 22.27 | 4.27 | -1.693 | 0.093 | -5.03 – 0.40 | 7.00 | 35.00 |
| Lack of personal benefits items summary score | 11.40 | 2.54 | 9.20 | 2.60 | -3.105 | 0.002 | -3.60 – -0.79 | 3.00 | 15.00 |
| Lack of awareness | 2.45 | 0.99 | 2.33 | 0.62 | -0.61 | 0.549 | -0.50 – 0.27 | 1.00 | 5.00 |
| Trial Protocol-Related Barriersa | |||||||||
| Protocol complexity items summary score | 14.60 | 3.52 | 12.27 | 3.04 | -1.434 | 0.017 | -4.24 – -0.43 | 5.00 | 25.00 |
| Trial treatment items summary score | 9.09 | 2.48 | 9.60 | 2.56 | 0.732 | 0.466 | -0.86 – 1.88 | 3.00 | 15.00 |
Table 3: Means Comparison of Summary Scores of Promoters and Logistical, Personal and Trial-Related Barriers by Referral Status.
No significant mean differences existed between the two groups for promoting factors, and in general most physicians tend to agree with all promoters.
Mean differences between oncologists who referred patients to trials and those who did not were significant for at least one of the dimensions of logistical, personal, and trial-related barriers, specifically those related to clinical trial process burden, lack of personal benefits, and protocol complexity. Physicians who referred patients tended to disagree more with these factors than those who did not.
Individual items comprising the summary score of those significant barriers-related factors were examined to identify where the largest differences occurred (data not shown). Significant mean differences were found for four specific items within the clinical trial process burden factor.
Oncologists who did not refer patients to trials tended to strongly agree or agree more with barriers of excessive paperwork associated with the referral process (t=-5.376; p=<0.001), excessive details of protocols (t=-3.261; p=0.001), no staff support (t=-2.829; p=<0.006), and extra work referring patients (t=-2.365; p=<0.026). On the other hand, those who did refer patients tended to have mean scores neither agreeing nor disagreeing with these logistical barriers.
Significant mean differences were found for all three items on the lack of personal benefits factor. Oncologists who did not refer patients to trials tended to have more neutral opinions about personal barriers, while oncologists who did refer patients tended to have mean scores disagreeing with these barriers. Significant barriers included lack of rewards and recognition (t=-2.469; p=<0.015), loss of personal income (t=-3.483; p=<0.001), and fear of loss of control over patient care (t=- 2.197; p=<0.030).
Finally, significant mean differences were found for three of the five items within the trial protocol complexity factor. Physicians who did not refer patients to trials tended to agree more with barriers of misinterpretation of study protocol (t=-2.157; p=<0.033), complexity of consent procedures (t=-2.886; p=<0.005), and complexity of trial protocol ( t =-3.011; p=<0.003), while physicians who did refer tended to have more neutral opinions.
Bivariate and multiple logistical regression analysis (Table 4) included selected demographic characteristics, factors identified by exploratory factor analysis, and the three stand-alone items.
| Factors | Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |||
| Demographic Characteristics | ||||||||
| Age | 1.000 | 0.838 | ||||||
| 40-49 vs. 30-39 | 0.98 | 0.21 | 4.57 | 0.978 | 1.80 | 0.20 | 16.42 | 0.604 |
| 50-59 vs. 30-39 | 1.04 | 0.22 | 4.85 | 0.958 | 3.17 | 0.26 | 38.81 | 0.367 |
| 60+ vs. 30-39 | 1.03 | 0.15 | 7.02 | 0.979 | 2.96 | 0.13 | 66.31 | 0.493 |
| Female vs. Male | 0.82 | 0.24 | 2.82 | 0.750 | 0.72 | 0.12 | 4.42 | 0.727 |
| Ethnicity | 0.655 | 0.916 | ||||||
| API/other vs. White | 0.92 | 0.23 | 3.74 | 0.911 | 1.31 | 0.14 | 12.58 | 0.816 |
| Hispanic/Latino vs. White | 0.51 | 0.12 | 2.17 | 0.361 | 0.78 | 0.08 | 7.77 | 0.829 |
| Hematologist-Oncologist/Radiation Oncologist vs. Medical Oncologists | 0.63 | 0.18 | 2.22 | 0.477 | 0.83 | 0.11 | 6.48 | 0.862 |
| Academic/Teaching Hospital/Other vs. Private Practice | 3.38 | 0.90 | 12.76 | 0.072 | 3.66 | 0.58 | 23.13 | 0.168 |
| Promoters | ||||||||
| Personal benefits | 1.15 | 0.67 | 1.97 | 0.604 | 1.29 | 0.55 | 3.02 | 0.553 |
| Trial treatment benefits | 1.23 | 0.74 | 2.05 | 0.414 | 1.46 | 0.64 | 3.34 | 0.373 |
| Comfortable explaining CT to patients | 1.14 | 0.65 | 1.97 | 0.651 | 0.71 | 0.28 | 1.80 | 0.472 |
| Patients are followed closely | 0.97 | 0.56 | 1.68 | 0.919 | 0.89 | 0.36 | 2.19 | 0.800 |
| Psychosocial benefit | 1.86 | 1.06 | 3.27 | 0.029 | 2.09 | 0.80 | 5.47 | 0.135 |
| Logistical barriers | ||||||||
| CT process burden | 0.35 | 0.17 | 0.70 | 0.003 | 0.28 | 0.08 | 0.94 | 0.040 |
| Coordination with CT site/involvement | 0.84 | 0.48 | 1.44 | 0.523 | 1.53 | 0.57 | 4.09 | 0.394 |
| Personal barriers | ||||||||
| Personal/attitudes | 0.87 | 0.51 | 1.47 | 0.597 | 2.04 | 0.72 | 5.77 | 0.180 |
| Lack of personal benefits | 0.41 | 0.23 | 0.74 | 0.003 | 0.49 | 0.20 | 1.19 | 0.116 |
| Lack of awareness about EPCT | 1.11 | 0.64 | 1.94 | 0.714 | 1.31 | 0.53 | 3.22 | 0.554 |
| Trial-related barriers | ||||||||
| Protocol complexity | 0.40 | 0.20 | 0.79 | 0.008 | 0.53 | 0.13 | 2.15 | 0.376 |
| Trial treatment | 1.43 | 0.79 | 2.59 | 0.239 | 0.79 | 0.31 | 1.99 | 0.615 |
Table 4: Unadjusted and Adjusted Odds Ratios for Promoting Factors and Logistical, Personal/Attitudes and Trial-Related Barriers Associated with Physician Referral Status.
Bivariate analysis shows that there was no significant association between physicians’ demographic characteristics and referral of patients to EPCTs. Within promoters, agreement with the possibility of psychosocial benefit to patients was positively related to referral. In contrast, agreement with the barriers of clinical trial process burden, lack of personal benefits, and protocol complexity were inversely associated with referring patients to EPCTs.
As seen in Table 4, one standard deviation increase in agreeing that the possibility of psychosocial benefits to patients encourages physician referrals to clinical trials was associated with a 1.86 increase (OR=1.86, 95% CI=1.06-3.27) in the odds of referring patients.
Regarding barriers, among physicians who considered the clinical trial process a burden, one standard deviation increase in their agreement was associated with a 65 percent decrease (OR=0.35, 95% CI=0.17-0.70) in the odds of referring patients to trials. For physicians who agreed with lack of personal benefits as a deterrent to referral, one standard deviation increase in their agreement produced a 59 percent decrease (OR=0.41, 95% CI=0.23-0.74) in their odds of referring patients. In addition, one standard deviation increase in physicians’ agreement with the complexity of clinical trial protocols as a barrier was associated with a 60 percent decrease (OR=0.40, 95% CI=0.20- 0.79) in their odds of referring patients.
Multiple logistic regression analysis, when adjusting for all predictors, identified “burden of the clinical trial process” as the only significant dimension associated with referral to EPCTs. Physicians who agreed with this set of logistical barriers were less likely to refer patients than physicians with opposing opinions. For one standard deviation increase in their agreement, a 72 percent decrease occurred (OR= 0.28, 95% CI=0.08-0.94) in their odds of referring patients.
Discussion
Cancer clinical trials are essential to develop new effective treatments and improve cancer patient outcomes and survival. However, the rates of minority patient enrollment in trials, specifically into early-phase clinical trials (EPCTs), are unacceptably low.
While researchers have focused on later-phase clinical trials, very little is known about the factors that impact accrual of Latinos and other minorities into EPCTs. Although later-phase trial research may increase knowledge about EPCT accrual programs possibly due to similar factors, differences in EPCTs—eligibility criteria, expected clinical outcomes, and geographic availability—may limit the utility of inferences. In contrast to later phase trials, Phase I and II trial cancer patients usually have no known effective treatment options, have had multiple-relapses or were refractory, and have exhausted nearly all conventional options without success, and therefore face distinctive concerns, including end-of-life issues [37].
Physicians play a vital role in successfully recruiting underrepresented patients to cancer clinical trials, given that they can introduce the option of clinical trials. Patients might be more open to participating if physicians were willing to talk with them about clinical research and provide educational materials [38].
In this study, 83 percent either did not speak Spanish well enough to interview patients or did not speak the language at all, even though 33 percent served Hispanic patients. Language complicates patientprovider communications, trial recruitment, and patient eligibility [36,39]. Specifically, language factors may make it difficult for providers to communicate with patients about consent forms and clinical trial documents (i.e., translation and back-translation of materials), while many U.S. trials require English proficiency of participants. Training and recruitment bilingual teams and oncologists would remove the language barrier, as would developing multilingual services and alerting community providers of their availability for non-Englishspeaking patients.
Barriers related to lack of awareness are among the easiest to overcome. This study found that familiarity with clinical trials and their local availability was significantly related to referring patients. To increase awareness, physicians suggested utilizing the Internet (personalized e-mails, websites, E-newsletters), hand- or mail-delivered brochures, colleagues, and cancer organizations. Efforts should not only employ these multiple channels to raise awareness of trial availability among physicians, but also should involve physicians as part of the clinical trial team and maintain two-way communications about the patients they refer. This might include helping physicians stay up-todate with scientific advances, inviting them to scientific forums, and keeping them informed of the progress of their referrals. In addition, developing a user-friendly, up-to-date, and easily accessible centralized registry could improve both physician and patient awareness of available trials [8].
A significant proportion (almost half) of physicians still found it difficult to talk to their patients about CTs. Their methods of presentation or communication skills may prevent or promote patient enrollment. Physicians should be able to describe trial facts, risks, and benefits in a culturally sensitive, accessible way to patients of different educational and health literacy levels [11]. Interventions should empower physicians to discuss trial issues and share potential therapeutic benefits with patients. Having multilingual staff available may also help ease physicians’ communication abilities or concerns. In addition, having multilingual, easy-to-follow educational materials to give Latino patients may facilitate the communication process and encourage patients to learn more about clinical trials. Other studies have shown that communication skills training can also alter physicians’ attitudes and beliefs [40], positively influence the number of patients offered a trial by physicians, and improve the quality of communication about trials [8]. Additional strategies that may help physicians tell Latino and non-Latino patients about clinical trials include educating physicians about: the benefits, limitations, and overall scientific value of clinical trials; informed consent procedures; the role of Institutional Review Boards; and the experimental subjects’ bill of rights.
Many physicians believed that trial referrals are complicated, and motivating and referring a patient consume time and resources from their practice. In fact, the burden of the clinical trial process (comprised of six logistical barriers) was the only statistically significant dimension associated with referral of patients to clinical trials. These issues demonstrate the remove the physician’s processing burden and expedite the research team’s contact with patients so as to directly explain the details and risks/benefits of participating in EPCTs to patients. However, a good rapport between treating physician and researcher is necessary for the former to quickly entrust patients to the latter. The long-term relationship with patients, ethical responsibility for what happens to patients as a consequence of a referral, and assurance that the patients will be treated as well are top concerns for a physician when making any referral.
Although none of the promoting factors was significantly associated with referral status, practically all physicians endorsed all items to a large extent. The factors most highly endorsed were the patient/family wanting to try something new and the possibility of therapeutic benefits for the patient. This suggests that perceptions of their patients’ desires and trial benefits are leading factors that may prevent or promote physician referral of patients to trials.
In addition, to increase physicians’ referral of patients to EPCTs, it is important to foster a culture of research and encourage professional responsibility to support clinical research within the medical and professional schools that train physicians [38], and to emphasize the development of bilingual professionals. From their academic formation, physicians should be introduced to the basic scientific and ethical principles of clinical and translational research, including how it is conducted, evaluated, explained to patients, and applied to patient care [41].
There are several limitations to consider when interpreting study results. First, participating physicians did not comprise a nationally representative sample, and their referral status may differ from those who did not respond to the survey. The small sample size may have limited the power of the study to detect statistically significant differences and associations between the selected predictors and the outcome variable. In addition, study findings cannot be generalized to the broader community of non-oncology physicians and responses of participants may not completely reflect those of physicians in Texas or beyond. The data were cross-sectional; thus causal inferences cannot be made. Because this study was based on self-report data, there is potential for respondent bias. The physicians who responded to the survey may in fact participate in research to a greater degree, which would result in a sample biased toward reporting participation in clinical trials, thus possibly overestimating the extent to which physicians participate in general. The possibility also exists that physicians responded in a socially desirable manner.
If community oncologists know about CTs available in the area, feel confident and have multilingual staff and/or material resources to discuss CT issues with their patients, trust the research team or center, see the personal and patient benefits of referrals, and have a quick, easy way to refer patients without losing them or increasing their workload, they may be much more likely to consider referring their minority patients to trials.
Conclusion
More research is needed to better understand physicians’ attitudes and beliefs regarding clinical research, and to examine specific promoting factors and barriers that facilitate or hinder physicians’ participation in clinical research and referral of patients to clinical trials. Such research could lead to culturally tailored interventions and education that may simultaneously increase opportunities to involve patients and physicians in clinical trials, while ensuring that the benefits of participation are equitably distributed to patients. In addition to outreach and education for physicians, creating bilingual study teams could solve language barriers and enhance the ability of Latinos to participate in clinical trials.
This study, one of the first to identify physician barriers for referring patients to EPCTs in Texas, highlights potential focus areas on physician and community-based education to promote communication and clinical trial opportunities among both their minority and non-minority patients. Given that Texas physicians deal with a large proportion of Latino patients, such efforts could also serve to address ethnic disparities in clinical trial participation. These disparities will become increasingly important as the Latino population continues to grow.
Acknowledgements
The study was funded by the National Cancer Institute (Grant No. R21CA101717), the Cancer Therapy & Research Center (Grant No. 2 P30 CA054174-17) and Redes En Acción: National Latino Cancer Research Network (Grant No. U54CA153511). The authors wish to thank all physicians who participated in the survey for sharing their thoughts and experiences with the research team. Special thanks to Drs. Meropol, Daugherty, and Tomamichel for kindly sharing their study questionnaires with us, and to Cliff Despres for his assistance editing the manuscript.
References
- Instituteof Medicine (2010) A national cancerclinical trials system for the 21st Century: Reinvigorating the NCI CooperativeGroup Program.The National Academies Press, Washington, DC.
- Ford JG,Howerton MW, Bolen S, Gary TL, Lai GY, et al. (2005) Knowledge and access toinformation on recruitment of underrepresented populations to cancer clinicaltrials. Evid Rep Technol Assess 122:1-11.
- Coalitionof Cancer Cooperative Groups (2006) Baseline study of patient accrual ontopublicly sponsored trials: An analysis conducted for the Global Access Projectof the National Patient Advocate Foundation. Coalition of Cancer CooperativeGroups, Philadelphia, MA.
- Institute of Medicine(1999) The unequal burden of cancer. An assessment of NIH research and programsfor ethnic minorities and medically underserved. National Academy Press,Washington, DC.
- Howe HL, Wu X, Ries LA, Cokkinides V, Ahmed F, et al. (2006) Annualreport to the nation on the status of cancer, 1975-2003, featuring cancer amongU.S. Hispanic/Latino populations Cancer 107:1711-1742.
- Vélez LF, Chalela P, Ramirez AG(2008) Hispanic/Latino health and disease: an overview. In: Kline MV, Huff RM,editors. Health Promotion in Multicultural Populations: A Handbook forPractitioners and Students. (2nd edn ),SAGE Publications, ThousandOaks, CA.
- Ward E, Jemal A, CokkinidesV, Singh GK, Cardinez C, et al. (2004)Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin54:78-93.
- Ford E, Jenkins V,Fallowfield L, Stuart N., Farewell D, et al. (2011) Clinicians’ attitudestowards clinical trials of cancer therapy. Br J Cancer104:1535-1543.
- Ford JG, Howerton MW, LaiGY, Gary TL, Bolen S, et al. (2008) Barriers to recruiting underrepresentedpopulations to cancer clinical trials: a systematic review. Cancer112:228-242.
- Lara PN Jr, Higdon R, Lim N,Kwan K, Tanaka M, et al. (2001) Prospective evaluation of cancer clinical trialaccrual patterns: Identifying potential barriers to enrollment. J Clin Oncol19:1728-1733
- Howerton MW, Gibbons MC,Baffi CR, Gary TL, Lai GY, et al. (2007) Provider roles in the recruitment ofunderrepresented populations to cancer clinical trials.Cancer109:465-476.
- Hudson SV,Momperousse D, Leventhal H (2005) Physician perspectives on cancer clinicaltrials and barriers to minority recruitment. Cancer Control12:93-96.
- Tournoux C,Katsahian S, Chevret S, Levy V (2006) Factors influencing inclusion of patientswith malignancies in clinical trials. Cancer106:258-270.
- Fayter D, McDaidC, Eastwood A (2007) A systematic review highlights threats to validity instudies of barriers to cancer trial participation. J Clin Epidemiol60:990-1001.
- Klabunde CN, Keating NL,Potosky AL, Ambs A, He Y, et al. (2011) A population-based assessment of specialty physician involvementin cancer clinical trials. J Natl Cancer Inst103:384-397.
- Kaanoi M, BraunKL, Gotay CC, Abrigo L (2002) Oncologists’ knowledge, attitudes and practicesrelated to cancer treatment trials. Hawaii Med J61:91-95.
- McCaskill-Stevens W, PintoH, Marcus AC, Comis R, Morgan R, et al. (1999) Recruiting minority cancerpatients into cancer clinical trials: a pilot project involving the EasternCooperative Oncology Group and the National Medical Association. J Clin Oncol17:1029-1039.
- Somkin CP,Altschuler A, Ackerson L, Geiger AM, Greene SM, et al. (2005) Organizationalbarriers to physician participation in cancer clinical trials. Am J Manag Care11:413-421.
- EDICT (2008) The EDICTProject: Policy Recommendations to Eliminate Disparities in Clinical Trials.EDICT Project, Houston, TX.
- Ramirez AG, Wildes K,Talavera G (2008) Clinical Trials attitudes and practices of Latino physicians.Contemp Clin Trials 29:482-492.
- Ellis PM, Butow PN,Tattersall MH, Dunn SM, Houssami N (2001) Randomized clinical trials inoncology: Understanding and attitudes predict willingness to participate. JClin Oncol 19:3554-3561.
- KillienM, Bigby JA, Champion V, Fernandez-Repollet E, Jackson RD, et al. (2000)Involving minority and underrepresented women in clinical trials: the NationalCenters of Excellence in Women’s Health. J Womens Health Gend Based Med9:1061-1070.
- Siminoff LA, Zhang A, ColabianchiN, Sturn CM, Shen Q (2000) Factors that predict the referral of breast cancerpatients onto clinical trials by their surgeons and medical oncologists. J ClinOncol18:1203-1211.
- Pinto HA, McCaskill-StevensW, Wolfe P, Marcus AC (2000) Physician perspectives on increasing minorities incancer clinical trials: an Eastern Cooperative Oncology Group (ECOG) Initiative. Ann Epidemiol10:S78-S84.
- Ashton CM, Haidet P,Paterniti DA, Collins TC, Gordon HS, et al. (2003) Racial and ethnicdisparities in the use of health services: bias, preferences, or poorcommunication? J Gen Intern Med 18:146-152.
- Murthy VH,Krumholz HM, Gross CP (2004) Participation in cancer clinical trials: race-,sex-, and age-based disparities. JAMA291:2720-2726.
- Swanson GM, Ward AJ (1995) Recruitingminorities into clinical trials: toward a participant-friendly system. J NtlCancer Inst 87:1747-1759.
- Meropol NJ, Weinfurt KP,Burnett CB, Balshem A, Benson AB 3rd, et al. (2003) Perceptions ofpatients and physicians regarding phase I cancer clinical trials: implicationsfor physician-patient communication. J Clin Oncol21:2589-2596.
- DaughertyC, Ratain MJ, Grochowski E, Stocking C, Kodish E, et al. (1995) Perceptions ofcancer patients and their physicians involved in phase I trials. J Clin Oncol13:1062-1072.
- TomamichelM, Jaime H, Degrate A, de Jong J, Pagani O, et al. (2000) Proposing phase I studies: patients',relatives', nurses' and specialists' perceptions. Ann Oncol11:289-294.
- KaiserHF, Caffry J (1965) Alpha factor analysis. Psychometrika 30:1-14.
- HarmanH H (1976) Modern Factor Analysis. (3rd edn), University of Chicago Press,Chicago, IL.
- MayerLS, Younger MS (1976) Estimation of standardized coefficients. J Natl Cancer Inst71:154-157.
- MenardS (2002). Applied logistic regression analysis. Sage University Papers Serieson Quantitative Applications in the Social Sciences, 07-106.( 2nd edn),Sage,Thousand Oaks, CA.
- SPSS Incorporated (2011) PAWS version 19. SPSS Incorporated, Chicago, IL.
- Nguyen TT, Somkin CP, Ma Y(2005) Participation of Asian-American women in cancer chemopreventionresearch: physician perspectives. Cancer104:3006-3014.
- Ho J, Pond GR,Newman C, Maclean M, Chen EX, et al. (2006) Barriers in phase I cancer clinicaltrials referrals and enrollment: five-year experience at the Princess MargaretHospital. BMC Cancer6:263.
- AvinsAL, Goldberg H (2007) Creating a culture of research. Contemp Clin Trials28:557-562.
- GiulianoAR, Mokuau N, Hughes C, Tortolero-Luna G, Risendal B, et al. (2000) Participation of minorities in cancerresearch: the influence of structural, cultural and linguistic factors. AnnEpidemiol10:S22-S34.
- Jenkins V, Farewell D, BattL, Maughan T, Branston L, et al. (2010) The attitudes of 1066 patients withcancer towards participation in randomized clinical trials. Br J Cancer103:1801-1807.
- Liaison Committee on MedicalEducation (2012) Functions and structure of a medical school: standards foraccreditation of medical education programs leading to the MD degree. Liaison Committee on Medical Education,Washington, DC.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 15896
- [From(publication date):
August-2012 - Oct 18, 2025] - Breakdown by view type
- HTML page views : 11177
- PDF downloads : 4719