Research Article Open Access
Direct Actions of Granulocyte-Colony Stimulating Factor on Human Neuronal and Monocytic Cell Lines
Amanda Pennington1,2, Vasyl Sava1, Shijie Song1,4, Niketa Patel3,4 and Juan Sanchez-Ramos1,2,4*
1Departments of Neurology, University of South Florida, 13220 Laurel Drive, Tampa, Florida, USA
2Molecular Pharmacology and Physiology, University of South Florida, 13220 Laurel Drive, Tampa, Florida, USA
3Molecular Medicine, University of South Florida, 13220 Laurel Drive, Tampa, Florida, USA
4James Haley VA Medical Center, University of South Florida, 13220 Laurel Drive, Tampa, Florida, USA
- Corresponding Author:
- Sanchez-Ramos J
Department of Neurology
University of South Florida
13220 Laurel Drive, Tampa, Florida 33612
E-mail: jsramos@health.usf.edu
Received date: July 19, 2013; Accepted date: August 16, 2013; Published date: August 23, 2013
Citation: Pennington A, Sava V, Song S, Patel N, Sanchez-Ramos J (2013) Direct Actions of Granulocyte-Colony Stimulating Factor on Human Neuronal and Monocytic Cell Lines. J Alzheimers Dis Parkinsonism 3:121. doi: 10.4172/2161-0460.1000121
Copyright: © 2013 Pennington A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Introduction: Granulocyte colony stimulating factor (G-CSF) administration produces beneficial effects in rodent models of stroke, trauma and neurodegenerative diseases by acting on both bone marrow-derived and neuronal cells. The aim of the study was to elucidate cellular mechanism(s) of G-CSF action by direct application to neuronal and monocytic cell lines.
Method: Cell culture models of monocytes (THP-1) and neurons (SH-SY5Y) cells were incubated with G-CSF. The following parameters were measured: G-CSF receptor binding kinetics; DNA synthesis; signal transduction, in particular expression of alternatively spliced protein kinase C (PKCδVIII) and the anti-apoptotic protein Bcl-2; changes in adhesiveness and migratory properties induced by G-CSF in the monocytic cells.
Results: G-CSF receptor binding kinetics in the two lines differed, with Kd in the neuronal line being significantly higher than that of the monocytic cells. Despite higher affinity of G-CSF for receptors on the monocytic cells, G-CSF treatment increased Bcl-2 expression in the neuronal line at lower concentrations than that required in the monocytic cell line. G-CSF did not increase either cellular adhesiveness or migration through a semi-permeable membrane, whereas monocyte chemotactic protein (MCP-1) significantly improved migration.
Conclusions: The cellular and molecular responses to G-CSF treatment of monocytic cells suggest that neither changes in adhesiveness nor migratory capacity are responsible for the beneficial effects of G-CSF administration in models of neurologic diseases. G-CSF induction of anti-apoptotic signaling in neurons is an important component of its neuroprotective effects in models of brain injury.
Keywords
Neurons; Neuroprotection; Apoptosis; Bcl 2; Cytokines
Introduction
G-CSF (granulocyte colony stimulating factor, Neupogen™) is a therapeutic hematopoietic cytokine commonly used for treatment of neutropenia and to increase generation of hematopoietic stem/ progenitor cells in bone marrow donors [1,2]. G-CSF is naturally synthesized by endothelium, macrophages, bone marrow stromal cells, fibroblasts and a number of other immune cells [3-5]. Interestingly, G-CSF also exerts neurotrophic and neuro-protective effects and has been identified, along with its receptor (G-CSFR), in neurogenic zones of the hippocampus (HC), the sub-ventricular zone and the olfactory bulb [6]. G-CSF and G-CSFR are also both expressed by neurons in several other areas of the brain including pyramidal cells in cortical layers (specifically II and V), entorhinal cortex, Purkinje cells of the cerebellum, and in cerebellar nuclei in rats [6]. The G-CSF receptor is also fairly ubiquitous and can be found on the cell surfaces of endothelial cells, lymphocytes, monocytes, platelets, and neutrophils [7-10].
Mice bred to have a deficiency in G-CSF were reported to have problems with memory formation and development of motor skills [11]. More specifically, the hippocampus from these mice exhibited deficits in the induction of long term potentiation in the CA1 region, decreased neuronal precursor cells in the dentate gyrus (DG) and decreased dendritic complexity in neurons in the DG and CA1 region of the HC [11]. The defects seen in G-CSF deficient mice support the designation of G-CSF as a neurotrophic factor, playing a role in neurogenesis and the maintenance of structural and functional integrity of the hippocampal formation [11]. In combination with cognitive training, G-CSF can also significantly improve spatial learning and new neuron survival in the hippocampus [12].
A significant decrease of G-CSF plasma levels was found in patients with early Alzheimer’s Disease (AD) in comparison to healthy age matched controls [13]. In addition, the authors reported a significant inverse correlation between G-CSF plasma levels and A-β1-42 levels in the cerebrospinal fluid of AD patients. These observations, coupled with evidence that bone marrow-derived cells contribute to the microgliosis in AD, led to studies of the disease-modifying impact of G-CSF in animal models of AD [14-16].
Treatment of transgenic (Tg) APP/PS1 AD mice with subcutaneous G-CSF for two weeks resulted in significant reduction of total β-amyloid (A-β) deposits in both hippocampus (HC) and entorhinal cortex (EC) [16]. G-CSF treated Tg mice also exhibited a significant increase in microgliosis in both HC and the EC. A correlation was observed between decreased A-β deposition and improvement in cognitive function as well as a suggestion of increased neurogenesis [16]. The mechanism of action of G-CSF responsible for the cognitive changes (performance in the radial arm water maze) in Tg mice is not known. However, it is clear that G-CSF has the ability to act on cells in the peripheral immune system, such as on hematopoietic stem progenitor cells and monocytes [17], as well directly upon CNS neurons [6]. It is not easy in animal studies to determine the extent to which the disease-modifying and beneficial effects on behavior are mediated by stimulation of hematopoiesis, with increased numbers of circulating monocytes and increased infiltration of these cells into brain, and/or from the direct actions of G-CSF on neurons and neural stem/progenitor cells in hippocampus.
The objective of the present study was to study the cellular and molecular effects of G-CSF in two distinct human cell lines. One cell line was chosen to model neurons (SH-SY5Y) and the other to model monocytes (THP-1). Human cell lines were chosen because G-CSF is currently under investigation in subjects with stroke, traumatic brain injury, Alzheimer’s Disease and Amyotrophic Lateral Sclerosis (ALS). The parameters of cellular functions chosen for study here included a) G-CSF receptor binding kinetics, b) cellular proliferation and c) signal transduction, in particular expression of alternatively spliced protein kinase C (PKC) and the anti-apoptotic protein Bcl2. Finally, migration and adhesiveness of the monocytic cells were investigated in vitro to determine if G-CSF contributes to mobilization and infiltration of circulating monocytes into brain.
Materials and Methods
Cells and cell lines
Two cell lines have been chosen for the in vitro studies. One is a human monocyte cell line that is representative of the peripheral immune system, THP-1, which grows in suspension. The other cell line is a human neuroblastoma cell line SH-SY5Y, which is a mixed cell line although only adherent cells were utilized [18]. Both cell lines are known to express the G-CSFR and the THP-1 cell line is also known to express the receptor, CCR2 [6,19]. Both cell lines were purchased from ATCC (Manassas, VA). THP-1 cell line was cultured in RPMI- 1640 medium supplemented with 10% fetal bovine serum (FBS) plus 1% penicillin-streptomycin (ATCC, Manassas, VA) in a humidified atmosphere of 5% CO2 at 37°C. SH-SY5Y cell line was cultured in 1:1 EMEM/F12 medium with 10% FBS plus 1% penicillin/streptomycin in a humidified atmosphere of 5% CO2 at 37°C.
Proliferation assay for human monocytic and human neuronal cell lines in vitro
THP-1 cells (or SH-SY5Y cells) were cultured in 24 well plates (3×105 cells/well, 6-8 replicates/concentration of G-CSF) for 24 hours then G-CSF was added to THP-1 cell line in basal media of RPMI-1640 (or EMEM/F12) + 10% FBS. 24 hours after G-CSF addition 37,000 Bq/ ml (500 pmol/ml) [3H]-thymidine (Perkin-Elmer Life and Analytical Sciences, Inc., MA, USA) was added to the cultures for 4h, then the cells were harvested, and [3H]-thymidine incorporated into DNA was determined. The specific activity of [3H]-thymidine was 74 Bq/pmol. Counting was performed on Beckman Coulter LS6500 scintillation counter. Uptake was normalized based on counts per minute (CPM) over total protein (μg).
Adhesion assay for human monocytic cell line
THP-1 cells are a monocyte cell line which are not typically very adhesive; when PMA (phorbol myristate acetate) is added to these cells they become more macrophage-like and hence more adhesive [20]. THP-1 cells were cultured in a 24 well plate pre-coated with fibronectin (10 ng/mL) and were treated with either basal media alone (RPMI-1640 medium + 2% FBS), G-CSF (250 ng/ml) in basal media, PMA (200 nM) in basal media, or G-CSF+ PMA in basal media for 24h. Media was then discarded and unattached cells were gently rinsed off with sterile 1X PBS. A total of 5 fields per well were counted at 20x magnification under bright field microscopy (IX2 inverted microscope; Olympus, Tokyo, Japan).
Migration assay for human monocytic cell line in vitro
THP-1 cells were harvested then brought up in DMEM media + 10% FBS. CCR-2 antagonist, RS 504393, (Tocris Biosci, Inc, Minneapolis, MN) was used to inhibit the effect of recombinant human MCP-1 (R&D Systems, Inc., Minneapolis, MN, 279-MC) on the chemotaxis of human monocytes. Cells were pre-treated with CCR2 antagonist in DMEM + 10% FBS prior to start of migration assay.
RS-504393 is a CCR-2 antagonist that shows modest inhibitory activity in a chemotaxis assay to MCP-1. It inhibits CCR-2 by occupying the CCR-2 binding site that includes acidic residue Glu291 and specifically inhibits MCP-1 and MCP-3 signaling. This compound is not a chemotaxis agonist and does not stimulate post receptor signaling of any kind [21]. It also binds significantly to a-adrenergic receptors [22].
A reusable modified Boyden 48-well chamber (Neuroprobe, Gaithersburg, MD) with a 5μm polycarbonate fibronectin coated filter was utilized. The top wells of chamber contained 55 μL (˜40,000 cells) of THP-1 cell line in DMEM media + 10% FBS and the bottom wells contained 27 μL of potential chemoattractant diluted in DMEM media +10% FBS. The plate was then incubated for 2 hours in humidified incubator @ 37°C in 5% CO2. The number of monocytes, which migrated to the bottom side of the filter, was quantified using Vectashield Hardset mounting media (Vector Labs, Burlingame, CA) containing 40-6-diamidino-2-phenylindole (DAPI). All (20 x) images of DAPI-labeled monocytes (5 images/well, 6 wells per concentration) were acquired using an Olympus BX60 microscope with an attached digital camera system (DP-70, Olympus, Tokyo, Japan), and the digital image was routed into a windows PC for quantitative analysis using Image J (NIH, Bethesda, MD).
G-CSF receptor parameters (KD, Bmax) assessed with [125I]- G-CSF on both human monocytic and human neuronal cell lines
[125I]-G-CSF (specific activity 46.55 TB q/mmol) was purchased from Perkin Elmer (Waltham, Massachusetts). Binding of [125I]-G-CSF was measured in previously described method with some modifications [17]. Cells were incubated in 180 μL of binding medium containing 0.2% BSA, 5 mM MgSO4 and 50 mM Hepes, pH 7.2 at a concentration of 1.5×107cells/ml and the appropriate concentration of [125I]-G-CSF. Incubations were carried out at room temperature with periodic shaking to ensure continuous mixing of cells and radioactive ligand. The incubation time of 2 hrs was estimated from preliminary experiments as being found sufficient to reach equilibrium. Nonspecific binding of [125I]-G-CSF was measured by incubations in the presence of a 100- fold molar excess of unlabeled G-CSF. At the end of the incubation, bound and free radio ligands were discriminated by using separating oil according to previously published method [23]. Namely, 80 μL aliquots were sampled from the incubation mixture, each aliquot was layered on 300 μL of separating oil placed in 500 μL polyethylene tubes and centrifuged for 5 min at 6000 rpm. The separating oil consisted of 1.5 parts dibutyl phthalate and 1 part bis (2-ethylhexyl) phthalate (Aldrich-Sigma, St. Louis, MO). Bound and free ligand activities were counted after cutting tubes in two pieces and placing top and tip into separate scintillation vials. Counting was performed on Beckman Coulter LS6500 scintillation counter. Glacial acetic acid was employed to solubilize the pellet [24]. The specific binding was determined from the amount of bound [125I]-G-CSF blocked by competition with excess unlabeled G-CSF. The parameters of saturation binding experiments including dissociation constant (kd), maximum binding capacity (Bmax) and binding cooperativity (h) were calculated with GraphPad Prism 5 software (La Jolla, CA) by using nonlinear regression analysis.
Signal transduction triggered by G-CSF (eg measurement of PKCδVIII and Bcl2 with Western blot)
THP-1 cells or SH-SY5Y cells 3×106 cells in 5 ml media in a 25 cm2 flask were treated with or without 100 ng/ml G-CSF for 24 h. Whole cell lysates (60 mg) were separated on 10% polyacrylamide gel electrophoresis-SDS (PAGE-SDS). Proteins were electrophoretically transferred to nitrocellulose membranes, blocked with Tris buffered saline- 0.1% Tween 20 containing 5% non-fat dried milk, washed, and incubated with a polyclonal antibody against either Bcl2 (Cell Signaling) or PKCδVIII-specific polyclonal antibody (Patel laboratory) [25]. The house-keeping gene GAPDH was used as an internal standard. Following incubation with anti-rabbit IgG-HRP, enhanced chemiluminesence (Pierce™) was used for detection and the gels were analyzed using UN-SCAN-IT™ software (Silk Scientific, Inc.).
Statistical analysis
In the case of western blot analysis, two-tailed unpaired t-test was utilized, and significance was determined after 3 or more experiments. The remaining in vitro data analyses were performed using one-way or two-way ANOVA. All data was presented as mean ± SD. All statistical analysis of data was done via PRISM4 or PRISM5 statistical analysis software (GraphPad Prism, La Jolla, CA). All comparisons were considered significant at level of P<0.05.
Results
G-CSF receptor binding kinetics in the neuronal cell line differs from that in monocytic line
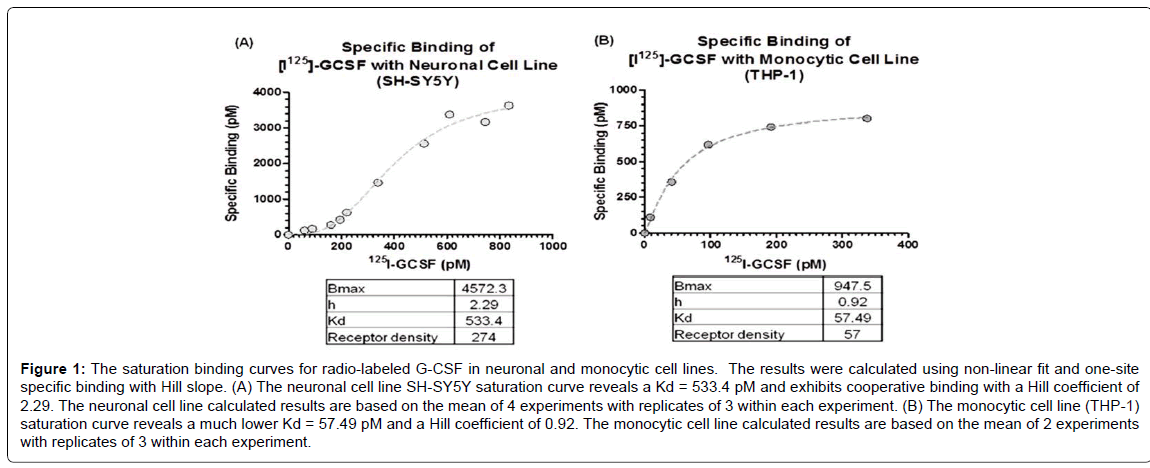
Increasing concentrations of radio-labeled G-CSF (alone or in the presence of 100 fold excess “cold” G-CSF) were added to the cell cultures to generate a saturation curve. Specific binding of [125I]- G-CSF to receptors expressed in monocytic cells (THP-1 cells) and the neuronal cell line (SH-SY5Y) revealed significant differences in Kd, Bmax and number of receptors per cell (Figure 1). The Kd for monocytes was 10 fold lower (greater affinity) than that in neurons. The relationship between specific binding and concentration of the ligand was not linear in the neuronal cultures and exhibited a Hill coefficient of 2.29, indicating substantial cooperative binding of the ligand to receptors expressed in neurons compared to the monocytes which had a Hill coefficient of 0.92. Although there is a greater binding affinity in the monocytes in comparison to the neurons, there are a 5-fold greater number of receptors per cell in the neurons in comparison to the monocytes.
G-CSF treatment increases expression of anti-apoptotic proteins Bcl2 and PKCδVIII in neurons and monocytes
Binding of G-CSF to its receptor triggers receptor homodimerization and activation of complex signal transduction and anti-apoptotic pathways which increase expression of STAT3, Bcl-2, and decrease expression of Bax [26-29]. To evaluate the biological response of G-CSF, we measured the anti-apoptotic proteins Bcl2 and protein kinase C delta VIII (PKCδVIII). PKCδ is a serine/threonine kinase mediating cellular growth, differentiation and apoptosis. PKCδ is alternatively spliced to generate isoforms with distinct functions in apoptosis. Human PKCδVIII and its mouse homolog PKCδII have been shown to function as anti-apoptotic proteins [25,30].
G-CSF was added to the cell cultures and incubated for 24 hours. Whole cell lysates were immunoblotted for Bcl2 and PKCδVIII. Despite the much lower binding affinity of G-CSF for receptors borne on neurons compared to binding affinity in monocytic cells, the expression of the anti-apoptotic protein Bcl2 and PKCδVIII, was much greater at equivalent concentrations (100 ng/mL or 5.3 nM) in the human neuronal cell line (Figure 2). G-CSF, however, also increased the expression of PKCδVIII and Bcl2 in the monocytic line at a higher concentration (200 ng/mL or 10.6 nM).
G-CSF increases DNA synthesis in both human monocytes and neurons
G-CSF is known to stimulate DNA synthesis and proliferation of hematopoietic stem/progenitor cells and neural stem/progenitor cells. DNA synthesis was assessed by measuring 3H-thymidine incorporation in the neuronal and monocytic cell lines. Addition of G-CSF to THP-1 cells and to SH-SY5Y resulted in a concentration-dependent increase in DNA synthesis that was similar in both cell lines (Figures 3A and 3B).
Figure 1: The saturation binding curves for radio-labeled G-CSF in neuronal and monocytic cell lines. The results were calculated using non-linear fit and one-site specific binding with Hill slope. (A) The neuronal cell line SH-SY5Y saturation curve reveals a Kd = 533.4 pM and exhibits cooperative binding with a Hill coefficient of 2.29. The neuronal cell line calculated results are based on the mean of 4 experiments with replicates of 3 within each experiment. (B) The monocytic cell line (THP-1) saturation curve reveals a much lower Kd = 57.49 pM and a Hill coefficient of 0.92. The monocytic cell line calculated results are based on the mean of 2 experiments with replicates of 3 within each experiment.
Figure 2: Western blot of PKCdVIII and Bcl2 expression in human neuronal (SH-SY5Y) and monocytic cell lines (THP-1) incubated with G-CSF for 24h (100 ng/ml or 200 ng/ml). Both PKCdVIII and Bcl2 expression are increased in the neuronal cells (100 ng/ml; 5.3 nM), and in the monocytic cells (200 ng/ml; 10.6 nM). The above graphs (B) and (D) show PKCdVIII or Bcl2 percent densitometric units normalized to GAPDH, and represent three separate experiments. The results were analyzed with two-tailed StudentâÂ?Â?s t-test. *** p<0.0001.
Figure 3: G-CSF increased DNA synthesis of human monocytes (THP-1) and human CNS neurons (SH-SY5Y). (A) THP-1 cells treated with concentrations of G-CSF 25, 50 and 100 ng/mL had a significant increase in thymidine incorporation (DNA synthesis) (One-way ANOVA P<0.0001). (B) SH-SY5Y cells treated with concentrations of G-CSF 25, 50, and 100 ng/mL had a significant increase in thymidine incorporation (DNA synthesis) (One-way ANOVA P<0.0001).
Figure 4: MCP-1, but not G-CSF served as a chemoattractant for human monocytes (THP-1 cell line). (A) Migration of monocytes was not elicited by G-CSF at various concentrations (G-CSF added to lower wells of migration assay) (n=6 wells per concentration). (B)MCP-1 stimulated cell migration in a concentration-dependent manner through a 5 µm fibronectin coated filter over a 2 h period (One-way ANOVA p<0.0001).
Effect of G-CSF and MCP-1 on migration of human monocytes
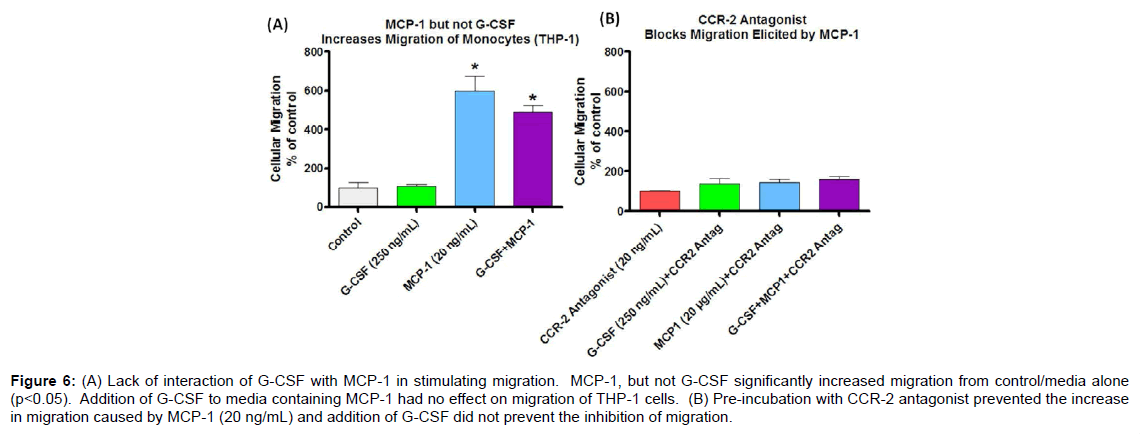
To assess whether G-CSF effects on circulating monocytes is responsible for increased infiltration of monocytes into brain, where they contribute to the increase in total microglia observed in earlier studies [16], an in vitro assessment of migratory capacity of the monocytes was undertaken. MCP-1 is a known monocyte chemoattractant and therefore was used as a standard to compare to G-CSF to examine its potential as a chemoattractant. At MCP-1 concentrations 5, 10, and 20 ng/mL there was a significant increase in migration of THP-1 monocytes (Figure 4B) across the fibronectin coated filter, although at various concentrations of G-CSF no chemoattractant effect was found (Figure 4A). Also G-CSF in combination with MCP-1 did not result in a synergistic effect on migration of THP-1 cells (Figure 6).
Effect of CCR2 antagonist on migration of human monocytes
Increased migration of monocytes triggered by MCP-1 was antagonized by pre-incubation with a CCR2 antagonist (20 μg/ ml) for 30 min (Figure 5). Although G-CSF alone did not act as a chemoattractant it was of interest to see if G-CSF could overcome the antagonism of MCP-1 caused by CCR2 antagonist. As shown in Figure 6 there was a lack of interaction between G-CSF and MCP-1in regards to migration.
Effect of G-CSF on adhesion on human monocytes
When PMA (phorbol myristate acetate) is added to THP-1 cells they become more macrophage-like and hence significantly more adhesive [20]. If G-CSF could improve adhesion this would lead to enhanced diapedesis and therefore facilitates infiltration into the brain. Unfortunately, G-CSF did not significantly improve adhesion of THP-1 cells at various concentrations and did not further improve adhesion caused by PMA (Figures 7A and 7B).
Discussion
Both monocytic and neuronal cell lines expressed G-CSF receptors, but pharmacokinetic parameters of radio-labeled ligand binding and subsequent biological responses were markedly different. The Kd for binding of the ligand with the neuronal G-CSF receptors was 10 fold greater (i.e. 10 fold lesser affinity) than binding with monocyte receptors and the number of receptors per cell was 5 fold greater in the neuronal line. Moreover, neuronal binding exhibited significant cooperativity, indicated by Hill coefficient of 2.29 compared to 0.92 in the monocytic cell line. Cooperative binding refers to enhanced binding of a ligand to a macromolecule when there are already other ligands present on the same macromolecule. The relevance of cooperative binding to the differential effects of G-CSF in neurons and monocytes is not clear, but may reflect the fact that G-CSF signaling in neuronal populations is predominantly of an autocrine nature. Both the G-CSF ligand and its receptor are expressed in neurons, so autocrine signaling and cooperative binding may trigger a well-coordinated response in a population of neurons.
Figure 6: (A) Lack of interaction of G-CSF with MCP-1 in stimulating migration. MCP-1, but not G-CSF significantly increased migration from control/media alone (p<0.05). Addition of G-CSF to media containing MCP-1 had no effect on migration of THP-1 cells. (B) Pre-incubation with CCR-2 antagonist prevented the increase in migration caused by MCP-1 (20 ng/mL) and addition of G-CSF did not prevent the inhibition of migration.
Figure 7: (A) THP-1 cells were treated with a range of G-CSF concentrations in a 24 well plate pre-coated with fibronectin. G-CSF did not significantly improve adhesion of THP-1 cells. PMA was used as a positive control. (B) Addition of G-CSF to PMA did not increase or decrease cellular adhesiveness elicited by PMA.
Another important distinction between the monocytic and neuronal lines was found in signal transduction triggered by G-CSF. The expression of the anti-apoptotic proteins PKCδVIII and Bcl2 were substantially increased in the neuronal cells, but not in the monocytic cells treated with equal, relatively high concentrations (5.3 nM). It is paradoxical that neurons exhibit a greater anti-apoptotic response to G-CSF than monocytes, despite the much greater affinity of monocyte G-CSF receptors for its ligand. Differences in G-CSF signal transduction observed here may be attributed to variations in the structure of G-CSF receptor or variations in the signaling portion of the receptor in neurons and monocytes. It is known that spliced transcript variants of this gene occur in the part of the G-CSF receptor gene (CSF3R) encoding the intracellular domain of the G-CSFR [31,32]. It is not known whether variations in the intracellular domain distinguish the neuronal from monocytic G-CSF receptors, a possibility that merits further investigation.
An explanation for the differences between monocytes and neurons also might be related to the capacity of monocytes to be replenished, in vivo, by progenitors in the bone marrow. Mature neurons can be replaced only in limited neurogenic niches such as the sub-ventricular zone of the hippocampus. Therefore the survival of differentiated, mature neurons may be more dependent on the capacity to overexpress anti-apoptotic proteins than monocytes. However, the neuronal line used here (SH-SY5Y) maintains mitotic competency and increases DNA synthesis in response to G-CSF treatment just as well as the monocytes.
Although G-CSF did not appear to directly alter migratory capacity or cellular adhesiveness of the monocytic cell line, administration in humans is known to increase absolute levels of circulating monocytes in addition to total neutrophil count and hematopoietic stem cells (CD34 cells) [33]. This corresponds to the in vitro data where proliferation in a human monocytic cell line was increased with G-CSF treatment. The mechanism of infiltration of a monocyte population from blood to brain, differentiation into microglia and peri- amyloid plaque accumulation in AD brains is not completely understood, but it is known that cell signaling by monocyte chemoattractant protein (MCP-1) plays a key role. Addition of MCP-1 to monocytes, but not G-CSF, enhanced their migration through micropores in a cell culture migration system. G-CSF treatment did not result in an improvement in adhesive properties, which would be helpful to monocytes undergoing diapedesis through the endothelial cells of the BBB and into the brain. If G-CSF does in fact enhance monocyte infiltration into the brain it does not appear to do so by improved migratory properties, such as chemoattraction or adhesion, and therefore it would more likely be caused by overall increase in total peripheral monocytes or circulating hematopoietic stem cells, which may result in increased migration by “mass effect.” In other words, more monocytes in the peripheral circulation may result in a greater extent of brain microgliosis in the setting of neuroinflammation.
During the inflammatory process, monocyte chemotactic protein (MCP-1) mediates the recruitment of CCR-2 expressing monocytes into the brain [34] as well as activation of macrophages [35]. MCP-1 is mostly expressed in the perivascular space and brain parenchyma during neuroinflammation [36-40]. CCR-2 is not only expressed by monocytes but also by both neurons and astrocytes within the brain [41,42]. CCR-2 is also expressed in microvascular endothelial cells in both mice and humans [43].
Many pro-inflammatory molecules can trigger MCP-1 release, including Aβ amyloid peptides, in both monocytes as well as several cells within the brain [22]. MC-1 is up-regulated early in brains of transgenic mice that develop AD-like pathology [34,44-47]. Elevated levels of MCP-1 have been observed in AD patients both in microglia associated with mature senile plaques (45) and in microvessels isolated from brain cortices [48].
The strategy of blocking the MC-1/CCR2 interaction has been proposed as being effective in preventing macrophage-induced tissue damage. Loss of MCP-1 function is enough to impair monocyte trafficking in several inflammatory models [22]. Mice bred to have the CCR-2 receptor knocked-out (CCR-2-/-) exhibited impairment in modulating recruitment of monocytes to areas of inflammation [49]. It also was reported that cross breeding Tg2576 AD mice with Tg mice deficient in CC-2 (TgCC-2-/-) resulted in decreased infiltration of microglia, increased plaque load and accelerated disease progression [46]. Another method to inhibit the MCP-1/CCR-2 interaction is through pharmacological methods such as inhibition of the CCR-2 receptor or through antibodies to MCP-1 directly. Anti-bodies to MCP-1 significantly inhibit the migration of microglia and monocytes in response to Aβ-stimulated macrophage supernatant in vitro [50,51].
A limitation of the present study is its reliance on immortalized human cell lines that differ from mature neurons and monocytes in vivo. Some observations reported here result from the immortal nature of the cells. For example, the finding of increased DNA synthesis and proliferation induced by G-CSF treatment of the neuronal line is unlikely to occur in mature differentiated neurons in vivo. Nevertheless, there is evidence that G-CSF stimulates proliferation of neural stem/ progenitor cells of the sub-granular zone of the hippocampus both in vitro and in vivo [16]. A major advantage to studying these two cellular phenotypes in a cell culture system is the ability to isolate and control the micro-environment for each cell population.
In summary, G-CSF bound to its receptor in the neuronal cell line with a 10 fold lower affinity than in the monocytic line, but the number of G-CSF receptors in the neuronal line was 5 fold higher than in the monocytic cells. Importantly, neuronal binding exhibited significant cooperativity, reflecting the fact that G-CSF signaling in neuronal populations is predominantly of an autocrine nature. Since both the G-CSF ligand and its receptor are expressed in authentic neurons in vivo, so autocrine signaling and cooperative binding may trigger a well-coordinated response, such as anti-apoptosis, in a population of neurons. In fact, intracellular signaling triggered by G-CSF resulted in generation of two anti-apoptotic molecules at lower concentrations than that required in monocytes. Since G-CSF did not alter adhesiveness and migration of monocytic cells, it is unlikely that increased infiltration of monocytes into brain is a result of intrinsic change in monocyte behavior, but might simply be a result increased numbers of circulating monocytes in the context of an inflammatory signaling cascade specific to the neurologic disorder being studied. Anti-apoptotic signaling in neurons triggered by G-CSF, as demonstrated here can be inferred to play a significant role in mediating neuroprotective effects in vivo models of neurologic disease. This conclusion does not negate the importance of interactions with other immune cells and cytokines (immunomodulatory actions), and effects on other neural cells within the brain such as microglia and astrocytes.
Acknowledgements
Research supported by VA Merit Grant to JSR. The authors have no conflict of interest, have not required the use of animals for the present project and have exclusively relied on human cell lines obtained from ATCC. We have abided by the statement of ethical standards for manuscripts submitted to the Journal of Alzheimer’s Disease and Parkinsonism.
References
- Dale D (2003) Current management of chemotherapy-induced neutropenia: the role of colony-stimulating factors. Semin Oncol 30: 3-9.
- Pusic I, DiPersio JF (2008) The use of growth factors in hematopoietic stem cell transplantation. Curr Pharm Des 14: 1950-1961.
- Demetri GD, Griffin JD (1991) Granulocyte colony-stimulating factor and its receptor. Blood 78: 2791-2808.
- Malipiero UV, Frei K, Fontana A (1990) Production of hemopoietic colony-stimulating factors by astrocytes. J Immunol 144: 3816-3821.
- Wells JA, de Vos AM (1996) Hematopoietic receptor complexes. Annu Rev Biochem 65: 609-634.
- Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, et al. (2005) The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest 115: 2083-2098.
- Bussolino F, Wang JM, Defilippi P, Turrini F, Sanavio F, et al. (1989) Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature 337: 471-473.
- Morikawa K, Morikawa S, Nakamura M, Miyawaki T (2002) Characterization of granulocyte colony-stimulating factor receptor expressed on human lymphocytes. Br J Haematol 118: 296-304.
- Shimoda K, Okamura S, Harada N, Kondo S, Okamura T, et al. (1993) Identification of a functional receptor for granulocyte colony-stimulating factor on platelets. J Clin Invest 91: 1310-1313.
- Hanazono Y, Hosoi T, Kuwaki T, Matsuki S, Miyazono K, et al. (1990) Structural analysis of the receptors for granulocyte colony-stimulating factor on neutrophils. Exp Hematol 18: 1097-1103.
- Diederich K, Sevimli S, D1örr H, Kösters E, Hoppen M, et al. (2009) The role of granulocyte-colony stimulating factor (G-CSF) in the healthy brain: a characterization of G-CSF-deficient mice. J Neurosci 29: 11572-11581.
- Diederich K, Schäbitz WR, Kuhnert K, Hellström N, Sachser N, et al. (2009) Synergetic effects of granulocyte-colony stimulating factor and cognitive training on spatial learning and survival of newborn hippocampal neurons. PLoS One 4: e5303.
- Laske C, Stellos K, Stransky E, Leyhe T, Gawaz M (2009) Decreased plasma levels of granulocyte-colony stimulating factor (G-CSF) in patients with early Alzheimer's disease. J Alzheimers Dis 17: 115-123.
- Malm TM, Koistinaho M, Pärepalo M, Vatanen T, Ooka A, et al. (2005) Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis 18: 134-142.
- Tsai KJ, Tsai YC, Shen CK (2007) G-CSF rescues the memory impairment of animal models of Alzheimer's disease. J Exp Med 204: 1273-1280.
- Sanchez-Ramos J, Song S, Sava V, Catlow B, Lin X, et al. (2009) Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer's mice. Neuroscience 163: 55-72.
- Kondo S, Okamura S, Asano Y, Harada M, Niho Y (1991) Human granulocyte colony-stimulating factor receptors in acute myelogenous leukemia. Eur J Haematol 46: 223-230.
- Xie HR, Hu LS, Li GY (2010) SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson's disease. Chin Med J (Engl) 123: 1086-1092.
- Chen N, Bürli RW, Neira S, Hungate R, Zhang D, et al. (2008) Discovery of a potent and selective c-Kit inhibitor for the treatment of inflammatory diseases. Bioorg Med Chem Lett 18: 4137-4141.
- Shapira L, Takashiba S, Kalmar JR, Van Dyke TE, Barak V, et al. (1993) Rapid fluorometric quantification of monocyte attachment in tissue culture wells. J Immunol Methods 165: 93-98.
- Mirzadegan T, Diehl F, Ebi B, Bhakta S, Polsky I, et al. (2000) Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: binding to a common chemokine receptor motif within the helical bundle. J Biol Chem 275: 25562-25571.
- Dawson J, Miltz W, Mir AK, Wiessner C (2003) Targeting monocyte chemoattractant protein-1 signalling in disease. Expert Opin Ther Targets 7: 35-48.
- Dower SK, Hefeneider SH, Alpert AR, Urdal DL (1985) Quantitative measurement of human interleukin 2 receptor levels with intact and detergent-solubilized human T-cells. Mol Immunol 22: 937-947.
- Jordan WC, Spiehler V, Haendiges R, Helman EZ (1974) Evaluation of alternative counting methods for radioimmunoassay of hepatitis-associated antigen (HB-Ag). Clin Chem 20: 733-737.
- Jiang K, Apostolatos AH, Ghansah T, Watson JE, Vickers T, et al. (2008) Identification of a novel antiapoptotic human protein kinase C delta isoform, PKCdeltaVIII in NT2 cells. Biochemistry 47: 787-797
- Tian SS, Lamb P, Seidel HM, Stein RB, Rosen J (1994) Rapid activation of the STAT3 transcription factor by granulocyte colony-stimulating factor. Blood 84: 1760-1764.
- Hunter MG, and Avalos BR (1998) Phosphatidylinositol 3'-kinase and SH2-containing inositol phosphatase (SHIP) are recruited by distinct positive and negative growth-regulatory domains in the granulocyte colony-stimulating factor receptor. J Immunol 160: 4979-4987.
- Dong F, Larner AC (2000) Activation of Akt kinase by granulocyte colony-stimulating factor (G-CSF): evidence for the role of a tyrosine kinase activity distinct from the Janus kinases. Blood 95: 1656-1662.
- Yata K, Matchett GA, Tsubokawa T, Tang J, Kanamaru K, et al. (2007) Granulocyte-colony stimulating factor inhibits apoptotic neuron loss after neonatal hypoxia-ischemia in rats. Brain Res 1145: 227-238.
- Patel NA, Song SS, Cooper DR (2006) PKCdelta alternatively spliced isoforms modulate cellular apoptosis in retinoic acid-induced differentiation of human NT2 cells and mouse embryonic stem cells. Gene Expr 13: 73-84.
- Dong F, van Paassen M, van Buitenen C, Hoefsloot LH, Löwenberg B, et al. (1995) A point mutation in the granulocyte colony-stimulating factor receptor (G-CSF-R) gene in a case of acute myeloid leukemia results in the overexpression of a novel G-CSF-R isoform. Blood 85: 902-911.
- Germeshausen M, Skokowa J, Ballmaier M, Zeidler C, Welte K (2008) G-CSF receptor mutations in patients with congenital neutropenia. Curr Opin Hematol 15: 332-337.
- Sanchez-Ramos J, Cimino C, Avila R, Rowe A, Chen R, et al. (2012) Pilot study of granulocyte-colony stimulating factor for treatment of Alzheimer's disease. J Alzheimers Dis 31: 843-855.
- Yamamoto M, Horiba M, Buescher JL, Huang D, Gendelman HE, et al. (2005) Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am J Pathol 166: 1475-1485.
- Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ (1989) Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med 169: 1485-1490.
- Mastroianni CM, Lancella L, Mengoni F, Lichtner M, Santopadre P, et al. (1998) Chemokine profiles in the cerebrospinal fluid (CSF) during the course of pyogenic and tuberculous meningitis. Clin Exp Immunol 114: 210-214.
- Losy J, Zaremba J (2001) Monocyte chemoattractant protein-1 is increased in the cerebrospinal fluid of patients with ischemic stroke. Stroke 32: 2695-2696.
- Sindern E, Niederkinkhaus Y, Henschel M, Ossege LM, Patzold T, et al. (2001) Differential release of beta-chemokines in serum and CSF of patients with relapsing-remitting multiple sclerosis. Acta Neurol Scand 104: 88-91.
- Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, et al. (2003) Overexpression of monocyte chemoattractant protein 1 in the brain exacerbates ischemic brain injury and is associated with recruitment of inflammatory cells. J Cereb Blood Flow Metab 23: 748-755.
- Sørensen TL, Ransohoff RM, Strieter RM, Sellebjerg F (2004) Chemokine CCL2 and chemokine receptor CCR2 in early active multiple sclerosis. Eur J Neurol 11: 445-449.
- Banisadr G, Quéraud-Lesaux F, Boutterin MC, Pélaprat D, Zalc B, et al. (2002) Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem 81: 257-269.
- Gillard SE, Lu M, Mastracci RM, Miller RJ (2002) Expression of functional chemokine receptors by rat cerebellar neurons. J Neuroimmunol 124: 16-28.
- Dzenko KA, Andjelkovic AV, Kuziel WA, Pachter JS (2001) The chemokine receptor CCR2 mediates the binding and internalization of monocyte chemoattractant protein-1 along brain microvessels. J Neurosci 21: 9214-9223.
- Janelsins MC, Mastrangelo MA, Oddo S, LaFerla FM, Federoff HJ, et al. (2005) Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer's disease mice. J Neuroinflammation 2: 23.
- Ishizuka K, Kimura T, Igata-yi R, Katsuragi S, Takamatsu J, et al. (1997) Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer's disease. Psychiatry Clin Neurosci 51: 135-138.
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, et al. (2007) Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 13: 432-438.
- Sly LM, Krzesicki RF, Brashler JR, Buhl AE, McKinley DD, et al. (2001) Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer's disease. Brain Res Bull 56: 581-588.
- Grammas P, Ovase R (2001) Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol Aging 22: 837-842.
- Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV Jr, et al. (1997) Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 100: 2552-2561.
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, et al. (2003) CD36 mediates the innate host response to beta-amyloid. J Exp Med 197: 1657-1666.
- Meda L, Bernasconi S, Bonaiuto C, Sozzani S, Zhou D, et al. (1996) Beta-amyloid (25-35) peptide and IFN-gamma synergistically induce the production of the chemotactic cytokine MCP-1/JE in monocytes and microglial cells. J Immunol 157: 1213-1218.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 14965
- [From(publication date):
August-2013 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 10304
- PDF downloads : 4661