Research Article Open Access
Differences in Expression of Fatty Acid Synthase among Histological Subtypes of Intraductal Papillary Mucinous Neoplasm of the Pancreas – An Experience of Immune Histochemical Analysis
Hiroshi Maekawa1*, Hajime Orita1, Tomoaki Ito1, Mutsumi Sakurada1, Tomoyuki Kushida1, Koichi Sato1 and Ryo Wada2
1Department of Surgery, Juntendo University School of Medicine, Shizuoka Hospital, Shizuoka, Japan
2Department of Pathology, Juntendo University School of Medicine, Shizuoka hospital, Shizuoka, Japan
- *Corresponding Author:
- Hiroshi Maekawa
Department of Surgery
Juntendo University School of Medicine
Shizuoka Hospital, Shizuoka, Japan
Tel: +81 55.948.3111
Fax: +81 55.946.0514
E-mail: hmaekawa0201@gmail.com
Received date: June 14, 2013; Accepted date: September 30, 2013; Published date: October 10, 2013
Citation: Maekawa H, Orita H, Ito T, Sakurada M, Kushida T, et al. (2013) Differences in Expression of Fatty Acid Synthase among Histological Subtypes of Intraductal Papillary Mucinous Neoplasm of the Pancreas – An Experience of Immune Histochemical Analysis. J Gastroint Dig Syst 3:144. doi:10.4172/2161-069X.1000144
Copyright: © 2013 Maekawa H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background: Fatty acid synthase plays an important role in tumorigenesis. We investigated the expression of fattyacid synthase in cases of intraductal papillary mucinous neoplasm of the pancreas, thought to be one of the precancerous lesions of pancreatic cancer, using immunohistochemistry. We also examined whether there are any differences between histological subtypes in intraductal papillary mucinous neoplasm of the pancreas. Methods: Eight cases of intraductal papillary mucinous neoplasm were examined using immunohistochemistry. Fatty acid synthase and Ki-67 expressions were investigated, and positive cellular rates were compared with histological subtypes of intra-ductal papillary mucinous neoplasm. Results: Pathologically, cases of the gastrictype were represented as low-grade dysplasia; however, cases of the intestinal or pancreatobiliary type were classified as intermediate- or high-grade dysplasia. Concerning fatty acid synthase expression, positive cellular rates of the gastric type were lower than those in cases of the intestinal or pancreatobiliary type. The positive cellular rates of Ki-67 expression were similar to those of fatty acid synthase expression. Conclusion: Although this study included only a small sample number, fatty acid synthase expression differed between the gastric and other subtypes in intraductal papillary mucinous neoplasm of the pancreas.
Keywords
Fatty acid Synthase; Immunohistochemistry; IPMN
Introduction
Fatty Acid Synthase (FAS) is usually expressed in hormonesensitive cells and cells showing high-level lipid metabolism in animals [1]. FAS increases the de novo biosynthesis of fatty acids, which contributes to cellular activities [2] including energy metabolism, building cellular membranes, and intracellular second messengers [3,4]. FAS expression is suppressed in normal cells because serum fatty acids are usually rich in the diet. In cancer cells and preneoplastic lesions, FAS expression has been found to be up-regulated. It has been suggested that FAS contributes to tumorigenesis [5,6] because it’s over expression has been demonstrated in precancerous lesions and early cancers using immunohistochemical staining [6-8]. IPMN is considered to be one of the precancerous lesions of pancreatic ductal adenocarcinoma. In the 2010 WHO classification, IPMNs are classified as showing low-, intermediate-, or high-grade dysplasia on the basis of the degree of architectural and cytological atypia identified [9]. Previously, Walter et al. [10] reported that FAS tissue expression in 30 cases of IPMN was correlated with the histologic grade. However, differences in FAS expression among histological subtypes of IPMN, such as the gastric, intestinal, and pancreatobiliary types have not been fully discussed. In this report, we examined the differences of FAS expression among subtypes of IPMN resected in our department using immunohistochemistry.
Methods
Eight cases of IPMN resected in our department between 2001 and 2012 were examined. Clinical data such as the gender, age, recurrence, and prognosis were obtained from clinical records. Pathological diagnoses were performed under H.E. staining and immunohistochemistry according to the 2010 WHO classification [9]. The subtypes of IPMN were determined according to immunohistochemical staining using MUC1, MUC2, and 45M1 mouse monoclonal antibody obtained from LEICA MICROSYSTEMSNEWCASTLE Ltd. (Newcastle, UK).
We investigated the expressions of FAS and Ki-67 in tissue sections cut from resected specimens formalin-fixed and paraffin-embedded with immunohistochemical staining.
Immunohistochemical procedure for FAS expression
We used 4-μm-thick tissue sections cut from specimens formalinfixed and paraffin-embedded. Sections were deparaffinized by treatment with xylene for 3 min twice and were rehydrated by passage through 100% ethanol for 30 sec (x3). Antigen retrieval was performed by microwaving slides in 10 mM sodium citrate buffer (T.R.S.) obtained from DAKO (Carpinteria, CA, USA) for 8 min. Endogenous peroxidase activity was quenched by incubating slides for 5 min in 3% H2O2. Slides were then washed in tris-buffered saline -0.05% Tween 20 (TBST) for 5 min twice and blocked with a solution containing 5% goat serum for 15 min at room temperature. Samples were incubated with the primary antibody for 60 min at room temperature. Anti-human FAS rabbit IgG was obtained from IMMUNO-BIOCHEMICAL LABORATORIES Co., Ltd. (Fujioka, Gunma, Japan). Slides were washed with TBST for 5 min (x3) and incubated with the secondary antibody, anti-rabbit IgG (Envison+/HRP from DAKO), for 60 min at room temperature. Slides were washed in TBST for 5 min (x3) and incubated with diaminobenzidine solution containing 20 mg/ml DAB-4HCl in 0.01M PB and 30% H2O2 until colorarion developed. Slides were then stained with hematoxylin, dehydrated, and mounted with cover slips.
Immunohistochemical procedure for Ki-67 expression
An immunoperoxidase assay for Ki-67 analysis was performed using a commercially available kit obtained from ROCHE DIAGNOSTICS Co., Ltd. (Tokyo, Japan) in the same manner as that described in the immunohistochemical procedure for FAS analysis. Monoclonal mouse anti-human Ki-67 antigens were obtained from DAKO (Carpinteria, CA, USA).
Scoring of immune reactivity
Cellular FAS expression was classified into positive and negative. We classified FAS staining of IPMN cells relative to the staining of adipose cells using the following criteria. Scoring of cellular FASpositivity was determined as follows: negative for not stained or stained less than adipose cells, positive for stained the same as or more than adipose cells.
Percentages of FAS-positive cells were evaluated in high power magnification chosen at random. Data show the average of 10 series of these counts. Quantification of Ki-67-positive cells was performed in the same manner.
Ethics
Informed consent was obtained from all patients included in this study. The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki. This study was approved by the Ethical Committee of our hospital.
Results
Clinical features of IPMN cases
Clinical features of the 8 cases are presented in Table 1. Five were male and 3 were female. The average age was 70 years old. Six cases were treated with pancreatoduodenectomy, and one case underwent total pancreatectomy. The remaining case underwent spleen-preserving distal pancreatectomy. No patient showed recurrence of the disease or pancreatic ductal adenocarcinoma during the postoperative follow-up.
| Gender | Age (years old) | Serum CA19-9 level (IU/l) | Diabetes mellitus | Surgical procedure | |
|---|---|---|---|---|---|
| Case 1 | F | 67 | 12.7 | + | PD |
| Case 2 | M | 70 | 27.4 | - | PD |
| Case 3 | M | 62 | 2 | - | TP |
| Case 4 | F | 70 | 73 | + | PD |
| Case 5 | F | 71 | 31 | + | PD |
| Case 6 | M | 68 | 7 | - | PD |
| Case 7 | M | 83 | 2 | - | PD |
| Case 8 | M | 69 | 14 | - | SPDP |
PD: pancreatoduodenectomy, TP: total pancreatectomy
SPDP: Spleen-Preserving Distal Pancreatectomy
Table 1: Clinical features of the 8 IPMN cases.
Pathological features of IPMN cases
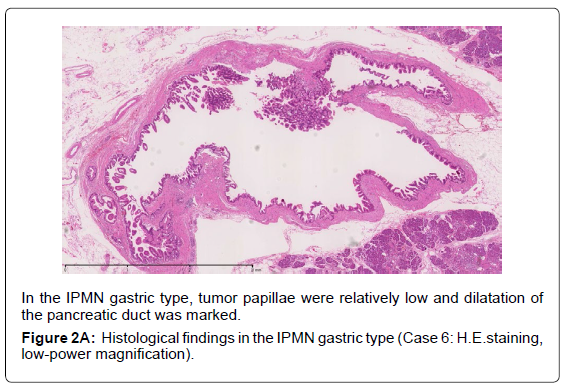
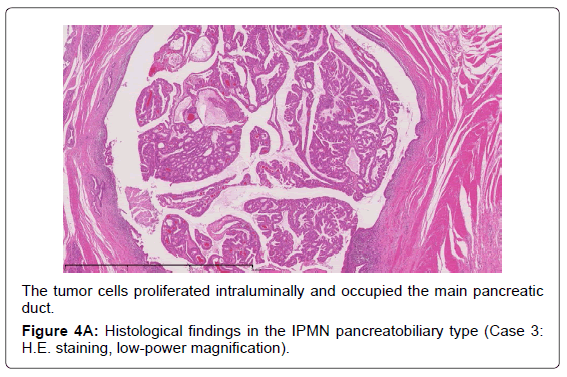
Histopathological features of the 8 cases are shown in Table 2. Five cases had the main duct type, two had the mixed type, and the last one had the branch duct type. The cases are shown with the histological subtypes and dominant type of dysplasia based on the WHO classification. Five were classified as the gastric type, and all corresponded to low-grade dysplasia. Two were the pancreatobiliary type, which corresponded to high-grade dysplasia, and the last one was the intestinal type, corresponding to intermediate-grade dysplasia. We encountered no cases of the oncocytic type of IPMN. FAS expression was detected in all cases.
| Gross appearance | Histological subtypes | Degree of dysplasia | FAS- positivity (%) | Ki-67- positivity (%) | |
|---|---|---|---|---|---|
| Case 1 | MD | Gastric | IPMN-low | 5.3 | 7.2 |
| Case 2 | BD | Gastric | IPMN-low | 7.6 | 0.9 |
| Case 3 | MD | Pancreatobiliary | IPMN-high | 59 | 12.6 |
| Case 4 | MD | Intestinal | IPMN-intermediate | 18.7 | 23.4 |
| Case 5 | MD | Gastric | IPMN-low | 10 | 2.4 |
| Case 6 | Mixed | Gastric | IPMN-low | 5 | 0.8 |
| Case 7 | Mixed | Pancreatobiliary | IPMN-high | 60 | 40.9 |
| Case 8 | MD | Gastric | IPMN-low | 6.4 | 0.8 |
MD: Main Duct type, BD: Branch Duct type
Table 2: Histopathological features of the 6 IPMN cases.
Difference in FAS or Ki-67 expression in the subtypes of IPMN
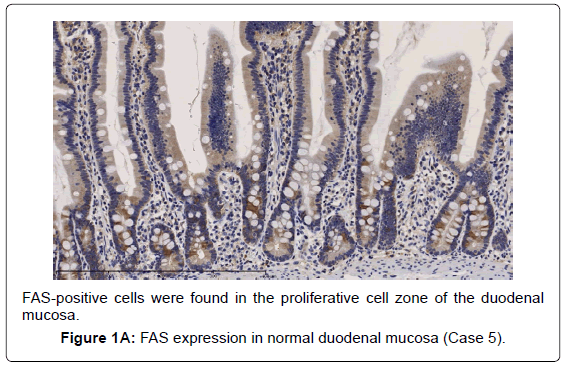
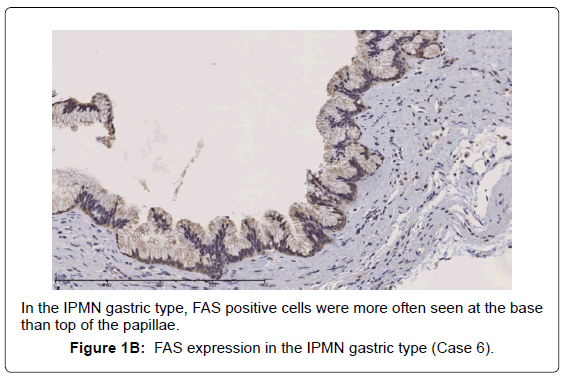
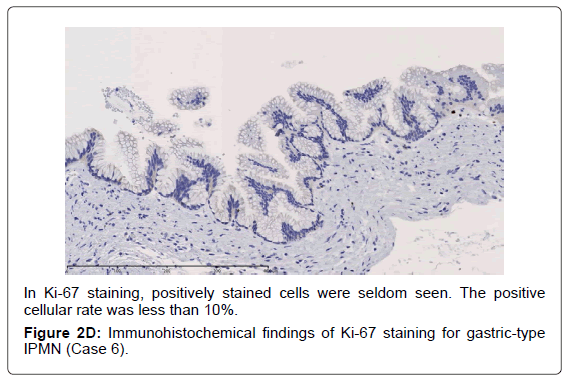
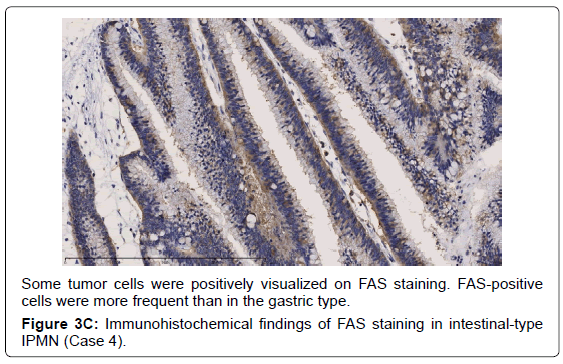
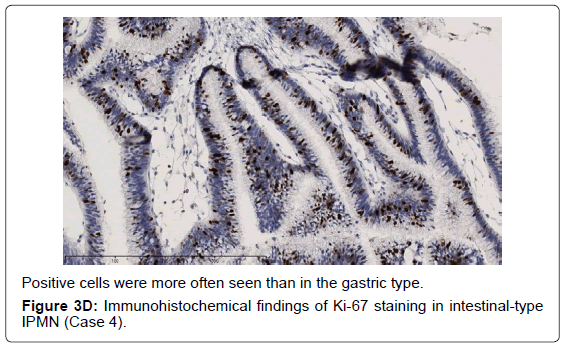
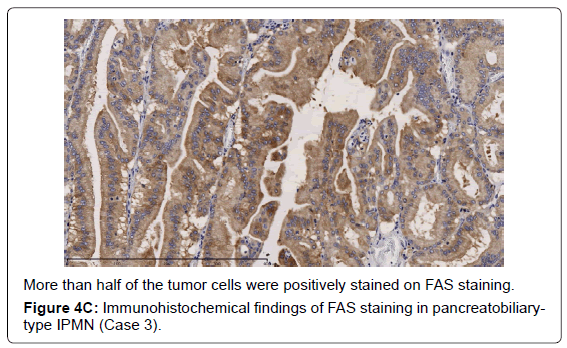
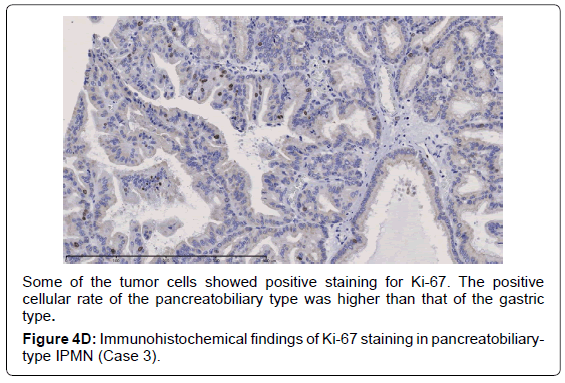
In the gastric-type, FAS expression in tumor tissue was similar to that of normal duodenal mucosa. In duodenal mucosa, FAS-positive cells were found in the proliferative cell zone (Figure 1A). In the gastric type, FAS-positive cells were more often seen at the base than top of the papillae (Figure 1B). In FAS expression, positive cellular rates of the gastric type were lower than those of the intestinal or pancreatobiliary type (Table 2). In Ki-67 expression, positive cellular rates of the gastric type were also lower than those of other sub types, similar to FAS expression (Table 2) (Figures 2-4). We divided these eight cases into two groups: the gastric type and others (non-gastric type). Then, we compared FAS- and Ki-67-positivity of the gastric type and the others (non-gastric types) statistically. The positivity of the gastric type was lower than that of the non-gastric type (Table 3).
| Gastric type | Non-gastric type | p-value | |
|---|---|---|---|
| FAS-positivity | 6.9 | 45.9 | 0.0357 |
| Ki-67-positivity | 2.4 | 25.6 | 0.0358 |
Statistical method: Mann-Whitney U test
Table 3: Comparison of gastric with non-gastric type for FAS- and Ki-67-positivity.
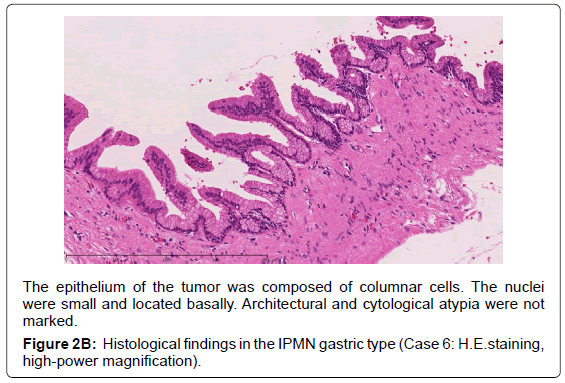
The epithelium of the tumor was composed of columnar cells. The nuclei two groups: the gastric type and others (non-gastric type). Then, we
were small and located basally. Architectural and cytological atypia were not
marked.
Figure 2B: Histological findings in the IPMN gastric type (Case 6: H.E.staining,
high-power magnification).
Discussion
Recently, it was suggested that FAS plays an important role in tumor growth, and FAS expression has been associated with cancer progression, a high risk of recurrence, and shorter survival in several kinds of cancer [11,12]. This means that FAS expression is an indicator of a poor prognosis in several kinds of cancer [13,14].
Some previous studies reported that FAS over expression is also detected in precancerous lesions or carcinoma in situ [5-8]. These results show that FAS over expression is an early event in cancer development.
IPMN has been considered to be one of the precancerous lesions of pancreatic ductal adenocarcinoma [15]. In cases of IPMN, concomitant pancreatic ductal adenocarcinoma is detected synchronously or metachronously at a relatively high incidence [16-18]. Approximately 30% of resected IPMNs have an associated invasive carcinoma, which can be uni- or multifocal. Most invasive carcinomas arise in association with main-duct type IPMNs with high- grade dysplasia [9].
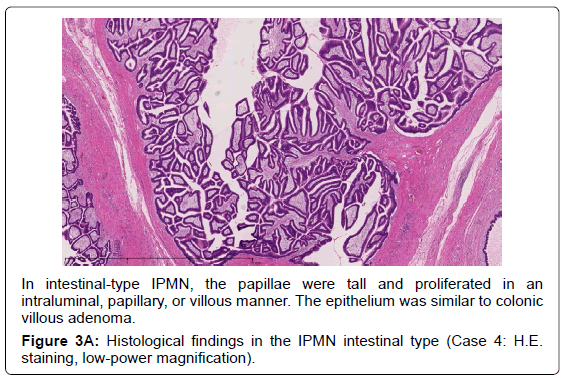
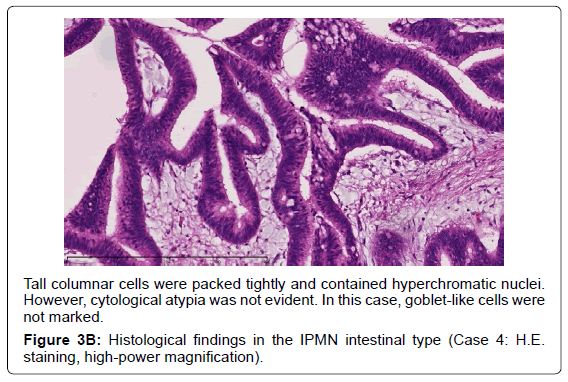
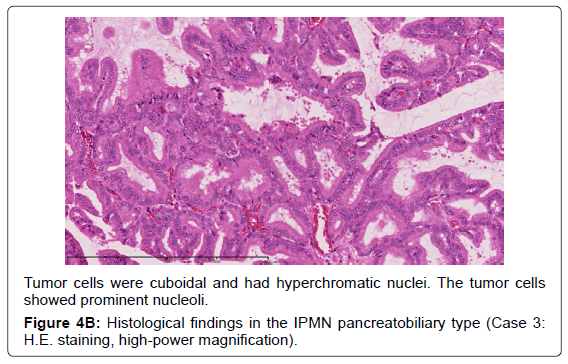
Adsay et al. proposed that IPMNs could be sub classified according to the direction of differentiation using immunohistochemistry in 2004 [19]. In the 2010 WHO classification, four subtypes of IPMN were also defined [9]. The gastric typeis flat or has thick, finger-like papillae lined with epithelia resembling gastric foveolar epithelia with abundant apical mucin and basally located nuclei [20]. The gastric type is considered to be a common precursor of the other types of IPMN [19]. The intestinal type is characterized by long villous papillae resembling the epithelium of colorectal villous adenomas. The intestinal type is usually classified with intermediate- or high-grade dysplasia [9,20]. The pancreatobiliary type is characterized as forming thin, branching papillae with high-grade dysplasia. The neoplastic cells are cuboidal, with round, hyperchromatic nuclei, prominent nucleoli, moderately amphophilic cytoplasm, and have a less mucinous appearance [9].
In this investigation, regarding FAS and Ki-67 expression, the gastric type showed a lower positivity than the other subtypes. These results suggest that the gastric type grew slowly. The gastric type is not considered to be a candidate for pancreatic ductal carcinoma. However, the intestinal or pancreatobiliary type might be regarded as a candidate for pancreatic ductal adenocarcinoma because they showed higher rates of FAS or Ki-67 expression.
We need to accumulate more reports on FAS expression in IPMN in order to clarify the exact significance of FAS expression.
Conclusion
Although this study included only a small sample number, fatty acid synthase expressions were different among the IPMN subtypes. FAS expression showed a difference based on tumor aggressiveness between the gastric type and other subtypes.
References
- Kusakabe T, Maeda M, Hoshi N, Sugino T, Watanabe K, et al. (2000) Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J Histochem Cytochem 48: 613-622.
- Kuhajda FP (2006) Fatty acid synthase and cancer: new application of an old pathway. Cancer Res 66: 5977-5980.
- Liu H, Liu JY, Wu X, Zhang JT (2010) Biochemistry, molecular biology, and pharmacology of fatty acid synthase, an emerging therapeutic target and diagnosis/prognosis marker. Int J Biochem Mol Biol 1: 69-89.
- Mashima T, Seimiya H, Tsuruo T (2009) De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer 100: 1369-1372.
- Piyathilake CJ, Frost AR, Manne U, Bell WC, Weiss H, et al. (2000) The expression of fatty acid synthase (FASE) is an early event in the development and progression of squamous cell carcinoma of the lung. Hum Pathol 31: 1068-1073.
- Kusakabe T, Nashimoto A, Honma K, Suzuki T (2002) Fatty acid synthase is highly expressed in carcinoma, adenoma and in regenerative epithelium and intestinal metaplasia of the stomach. Histopathology 40: 71-79.
- Nemoto T, Terashima S, Kogure M, Hoshino Y, Kusakabe T, et al. (2001) Overexpression of fatty acid synthase in oesophageal squamous cell dysplasia and carcinoma. Pathobiology 69: 297-303.
- Kearney KE, Pretlow TG, Pretlow TP (2009) Increased expression of fatty acid synthase in human aberrant crypt foci: possible target for colorectal cancer prevention. Int J Cancer 125: 249-252.
- Bosman FT, Carneiro F, Hruban RH, Thiese ND (2010) WHO classification of tumors of the digestive system. (4thedn) Lyon IARC 280-313.
- Walter K, Hong SM, Nyhan S, Canto M, Fedarko N, et al. (2009) Serum fatty acid synthase as a marker of pancreatic neoplasia. Cancer Epidemiol Biomarkers Prev 18: 2380-2385.
- Liu H, Liu Y, Zhang JT (2008) A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol Cancer Ther 7: 263-270.
- Yang Y, Liu H, Li Z, Zhao Z, Yip-Schneider M, et al. (2011) Role of fatty acid synthase in gemcitabine and radiation resistance of pancreatic cancers. Int J Biochem Mol Biol 2: 89-98.
- Gansler TS, Hardman W 3rd, Hunt DA, Schaffel S, Hennigar RA (1997) Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol 28: 686-692.
- Alo PL, Amini M, Piro F, Pizzuti L, Sebastiani V, et al. (2007) Immunohistochemical expression and prognostic significance of fatty acid synthase in pancreatic carcinoma. Anticancer Res 27: 2523-2527.
- Scarlett CJ, Salisbury EL, Biankin AV, Kench J (2011) Precursor lesions in pancreatic cancer: morphological and molecular pathology. Pathology 43: 183-200.
- Yamaguchi K, Ohuchida J, Ohtsuka T, Nakano K, Tanaka M (2002) Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology 2: 484-490.
- Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, et al. (2004) Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg 239: 788-797.
- Uehara H, Nakaizumi A, Ishikawa O, Iishi H, Tatsumi K, et al. (2008) Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut 57: 1561-1565.
- Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, et al. (2004) Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an "intestinal" pathway of carcinogenesis in the pancreas. Am J SurgPathol 28: 839-848.
- Furukawa T, Klöppel G, VolkanAdsay N, Albores-Saavedra J, Fukushima N, et al. (2005) Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch 447: 794-799.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15166
- [From(publication date):
October-2013 - Dec 06, 2025] - Breakdown by view type
- HTML page views : 10435
- PDF downloads : 4731