Research Article Open Access
Development of a Validated Stability-Indicating RP-HPLC Method for Dronedarone Hydrochloride in Pharmaceutical Formulation
Bhatt KK, Emanual Michael Patelia* and Ishani Amin
Department of Pharmaceutical Chemistry and Analysis, Indukaka Ipcowala College of Pharmacy, Gujarat, India
- *Corresponding Author:
- Emanual Michael Patelia
Department of Pharmaceutical Chemistry and Analysis
Indukaka Ipcowala College of Pharmacy
New Vallabh Vidyanagar–388121, Gujarat, India
E-mail: ricky.emanual@gmail.com
Received date: January 15, 2013; Accepted date: January 28, 2013; Published date: February 04, 2013
Citation: Bhatt KK, Patelia EM, Amin I (2013) Development of a Validated Stability- Indicating RP-HPLC Method for Dronedarone Hydrochloride in Pharmaceutical Formulation. J Anal Bioanal Tech 4:161. doi: 10.4172/2155-9872.1000161
Copyright: © 2013 Bhatt KK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A simple, precise, rapid, selective, and economic reversed phase high-performance liquid chromatography (RPHPLC) method has been established for estimation analysis of DRO. A Brownlee ODS C-18 column (250×4.6 mm i.d) chromatographic column equilibrated with mobile phase methanol-0.02 M KH2PO4 (80:20, v/v) (Final pH adjusted to 4 using Orthophosphoric acid) was used. Mobile phase flow rate was maintained at 1 ml/min and effluents were monitored at 289 nm. The sample was injected using a 20 μl fixed loop, and the total run time was 10 min. Experimental conditions such as pH of mobile phase, column saturation time, selection of wavelength, etc. were critically studied and the optimum conditions were selected. In RP-HPLC linear range was found to be 5-15 μg/ml and, mean recovery was found to be 99.99-100.03% and Rt of dronedarone was found to be 4.7 min. Degradation in acid, base, peroxide and thermal was found in range 8-20%. This stability indicating HPLC method is economic, sensitive, and less time consuming than other chromatographic procedures. It is a user-friendly and importance tool for analysis of dronedarone in tablet dosage forms.
Keywords
Stability indicating; HPLC; Brownlee ODS; Degradation; Tablet
Introduction
DRO (DRO; N-(2-Butyl-3-{4-[3-(dibutylamino) propoxy] benzoyl}-1-benzofuran-5-yl) methane sulfonamide hydrochloride; (Figure 1) is a widely used for the paroxysmal or persistent atrial fibrillation or atrial flutter [1,2].
Literature survey revealed that various analytical methods like spectrophotometric [3], HPLC [4] and LC-MS in plasma [5] have been reported for the determination of DRO and either individually or combination with some other drugs, but no stability indicating HPLC method was reported for estimation of DRO in dosage forms. Since the conditions that cause instability and result in degradation products of the API cannot be predicted initially, one has to subject the API to a variety of stress conditions. Trial and error are needed to find the proper combination of stress agent concentration and time to effect degradation, preferably in the 5-15% range. Depending on the API, not every stress agent may affect degradation, but each agent has to be evaluated to determine whether degradation occurs or not. Typical degradative conditions involve hydrolysis, photolysis, acid/base reactions, and temperature. Achieving 100% degradation would require too much effort and could be possibly cause secondary degradation. Secondary degradation products are the degradation products, which are not likely to be formed under normal storage conditions. Depending on the API, not all of the degradation conditions effect degradation, and after a reasonable effort (varying concentrations and time) to produce a degradation product with no success, one can move on to the next condition. The review of literature prompted us to develop an accurate, selective and precise estimation method for the estimation of dosage forms.
Experimental
Chemicals and materials
DRO were procured from Cadila pharmaceuticals, Ahmedabad. Methanol (HPLC Grade), Acetonitrile (HPLC Grade), Water (HPLC Grade), Hydrochloric acid (A. R. Grade), Potassium di-hydrogen Phosphate (A. R. Grade), Sodium hydroxide (A. R. Grade), Hydrogen peroxide (A. R. Grade) and Ortho phosphoric acid (A. R. Grade) were used as solvents to prepare the mobile phase. Dronedarone equivalent to 400 mg of DRO (Proprietary Name: MULTAQ) tablets were procured from local market.
Chromatographic conditions
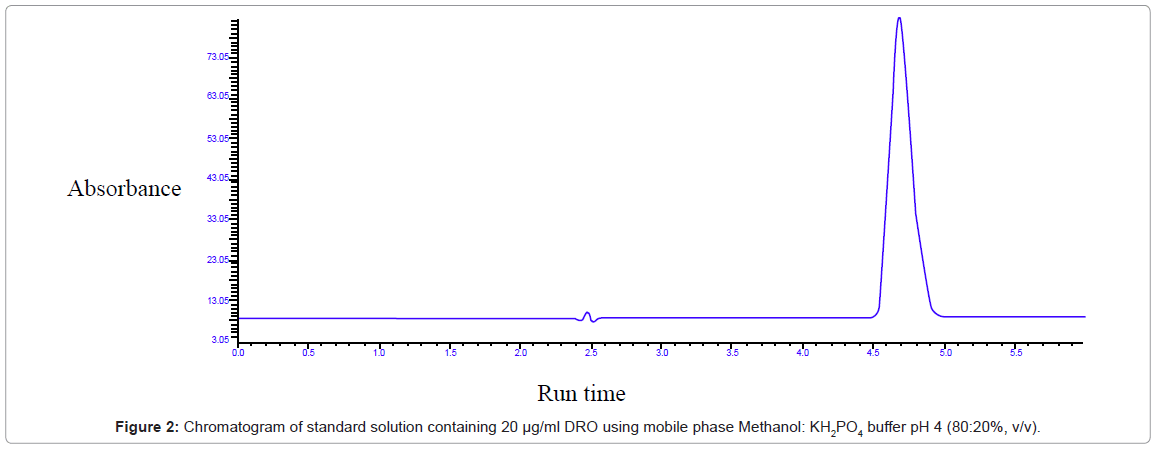
Proper selection of the HPLC method depends upon the nature of the sample (ionic, ionizable or neutral molecule), its molecular weight and solubility. The drug selected for the present study is polar in nature and hence either reversed phase or ion-pair or ionexchange chromatography can be used. Reversed phase HPLC was selected for the separations because of its simplicity and suitability. Analytical wavelength of 289 nm was selected for determination of DRO. The drug solution was prepared in methanol and mobile phase Methanol: 0.02 mM KH2PO4 buffer in (80:20, v/v) ratio, pH adjusted to 4 with orthophosphoric acid and same absorbance was obtained at this wavelength. So in the same mobile phase of 85:15 methanol: KH2PO4 buffer pH was adjusted to 7 but peak broadening was observed. Finally the mobile phase containing Methanol: 0.02 mM KH2PO4 buffer in (80:20, v/v) ratio, pH adjusted to 4 with orthophosphoric acid showed satisfactory result. At 1 mL/min flow rate DRO shows 4.7 min retention time and total time of analysis was 6 min.
Sample preparation
Forced degradation study
Acidic degradation: Weigh accurately tablet powdered equivalent to about 400 mg DRO and transferred into 10 ml volumetric flask. The solution was subjected to 2 ml of 2.0 N HCl for time period of 2 hr at 80°C in water bath. After exposure to the degradation condition, solution was cooled and 2.0 N NaOH. Drop wise 2.0 N HCl was added till pH was neutralized to pH 7. Add 2 ml of methanol and sonicate the solution for about 10 minutes with occasional shaking. Make the volume up to mark with methanol and mix. Filter the solution with 0.41 μ Whattman filter. The above solution was appropriately diluted with methanol to obtain the solution 1000 μg/ml. From this solution 1 ml was pippeted out in 10 ml volumetric flask and diluted with methanol to give solution of 100 μg/ml. From this solution 1 ml was pippeted out in 10 ml volumetric flask and diluted with methanol to give solution of 10 μg/ml. The solution was analysed by the proposed method and chromatogram was recorded.
Alkali degradation: Weigh accurately tablet powdered equivalent to about 10 mg DRO and transferred into 10 ml volumetric flask. The solution was subjected to 2 ml of 2.0 N NaOH for time period of 2 hr at 80°C in water bath. After exposure to the degradation condition solution was cooled and drop wise 2.0 N HCl was added till pH was neutralized to pH 7. Add 2 ml of methanol and sonicate the solution for about 10 mins with occasional shaking. Make the volume up to mark with methanol and mix. The above solution was appropriately diluted with methanol to obtain the solution 1000 μg/ml. From this solution 1 ml was pippeted out in 10 ml volumetric flask and diluted with methanol to give solution of 100 μg/ml. From this solution 1 ml was pippeted out in 10 ml volumetric flask and diluted with methanol to give solution of 10 μg/ml. The solution was analysed by the proposed method and chromatogram was recorded.
Peroxide degradation: Weigh accurately tablet powdered equivalent to about 10 mg DRO and transferred into 10 ml volumetric flask. The solutions were subjected to different strength of 6% Hydrogen peroxide for different time period of 2 hrs at 80°C in water bath. After exposure to the degradation condition solution was cooled and 2 ml of methanol and sonicate the solution for about 10 mins with occasional shaking. Make the volume up to mark with methanol and mix. Filter the solution with 0.41 μ Whattman filter. The above solution was appropriately diluted with methanol to obtain the solution 1000 μg/ ml. From this solution 1 ml was pippeted out in 10 ml volumetric flask and diluted with methanol to give solution of 100 μg/ml. From this solution 1 ml was pippeted out in 10 ml volumetric flask and diluted with methanol to give solution of 10 μg/ml. The solution was analysed by the proposed method and chromatogram was recorded.
Thermal degradation: Weigh accurately tablet powdered equivalent to about 10 mg DRO and transferred into 10 ml volumetric flask. Store it at 100°C for 8 hours in hot air oven. Cool it to room temperature. Add few ml of diluent to it, and sonicate the solution for about 20 mins with occasional shaking. Make the volume up to mark with methanol and mix. Filter the solution with 0.4 μ whattman filter. The above solution was appropriately diluted with methanol to obtain the solution 1000 μg/ml. From this solution 1 ml was pippeted out in 10 ml volumetric flask and diluted with methanol to give solution of 100 μg/ml. From this solution 1 ml was pippeted out in 10 ml volumetric flask and diluted with methanol to give solution of 10 μg/ml. The solution was analysed by the proposed method and chromatogram was recorded.
Preparation of standard solution: Stock solutions were prepared by weighing 10 mg DRO and transferring to 10 ml volumetric flasks containing a few ml of methanol. Volume was made up with methanol, which gave 1000 μg/ml of the drugs. Aliquots from the stock solutions were appropriately diluted with mobile phase to obtain working standard of 10 μg/ml of drug.
Method validation
The developed method was validated for linearity and range, specificity, accuracy, precision, limit of detection, limit of quantitation, robustness and solution stability as per ICH guidelines.
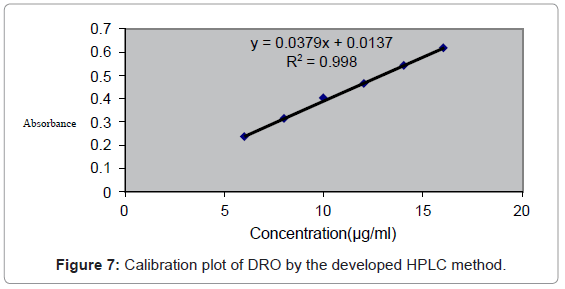
Linearity and range: The linearity was determined at six levels over the range of 5-15 μg/ml. Take absorbances of above linearity solution preparations at each concentration. Calculate mean absorbance at each concentration and plot a graph of mean absorbance (y-axis) versus concentration (x-axis). (n=6) calculate and record value of correlation coefficient (r), y-intercept and slope of regression line.
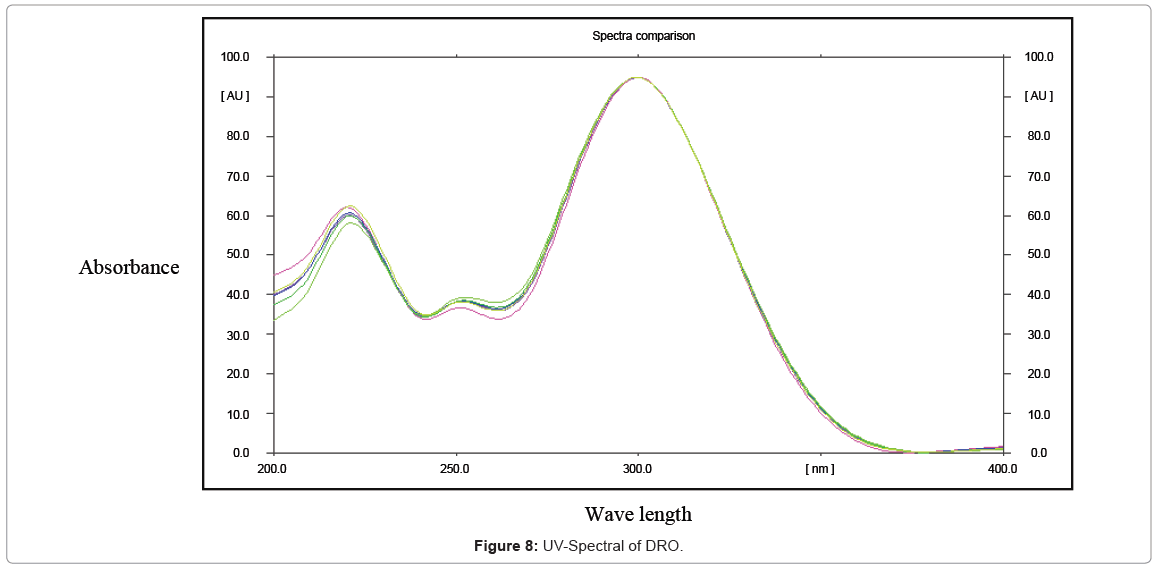
Specificity: The specificity of the method was ascertained by analyzing DRO in presence of excipients like talc, polyethylene glycol, lactose and micro-crystalline cellulose were used for tablet formulations. The bands of DRO were confirmed by comparing Rt values and respective spectra of sample with those of standards. The peak purity of DRO was assured by comparing the chromatogram.
Accuracy (% Recovery): The accuracy of the method was determined by calculating recoveries of DRO by method of standard additions. Known amount of DRO (0%, 50%, 100%, 150%) were added to a pre quantified sample solution, and the amount of DRO was estimated by measuring the peak areas and by fitting these values to the straight-line equation of calibration curve.
Method precision (Repeatability): Standard solutions of DRO (0.001, 0.01, 0.1, 1, 10 & 20 μg/ml) were prepared and spectrums were recorded. Absorbance was measured at 289 nm using methanol as a blank. The absorbances of the same concentration solution were measured six times and %RSD was calculated.
Intermediate precision (Reproducibility): Variation of results of three different concentrations (0.001, 1 and 20 μg/ml) within the same day (intra-day), variation of results between days (inter-day) was analyzed. Intra-day precision was determined by analyzing DRO for three times in the same day. Inter-day precision was determined by analyzing DRO daily for three days.
Limits of Detection (LOD) and Limits of Quantitation (LOQ): The limit of detection (LOD) is defined as the lowest concentration of an analyte that can reliably be differentiated from background levels. Limit of quantification (LOQ) of an individual analytical procedure is the lowest amount of analyte that can be quantitatively determined with suitable precision and accuracy. LOD and LOQ were calculated using following equation as per ICH guidelines. LOD=3.3×σ/S; LOQ=10×σ/S; Where σ is the standard deviation of y-intercepts of regression lines and S is the slope of the calibration curve.
Robustness: Robustness of the method was performed by variation in mobile phase ratio, flow rate and pH.
Solution stability: The sample preparations were analyzed by HPLC at regular intervals for 24 hrs as per test procedure.
Results and Discussion
Method development and optimization of chromatographic conditions
When methanol and water was used as mobile phase drug eluted late and had broadening. When acetonitrile and water was used drug peak merged with solvent front. So buffer was used. When 90:10 ratio of methanol: KH2PO4 buffer pH 4 was tried drug peak was near to solvent peak. So ratio of 85:15 methanol: KH2PO4 buffer pH 4 was tried it gave satisfactory result but when stability study was carried out the degradant peak merged with solvent peak (Figure 2).
Accelerated degradation
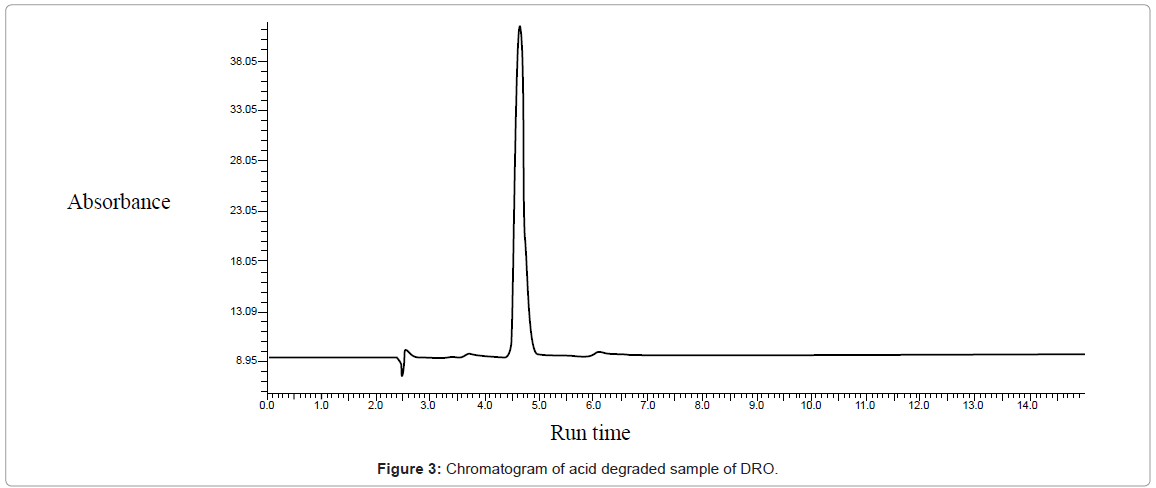
Acid hydrolysis: Initially 0.1 N HCl was used at 80°C for 2 hours. But no degradation was obtained. Concentration of HCl was increased to 1 N but still no degradation was obtained so, concentration was increased to 2 N HCl at 80°C for 2 Hours and slight degradation was obtained as shown in figure 3.
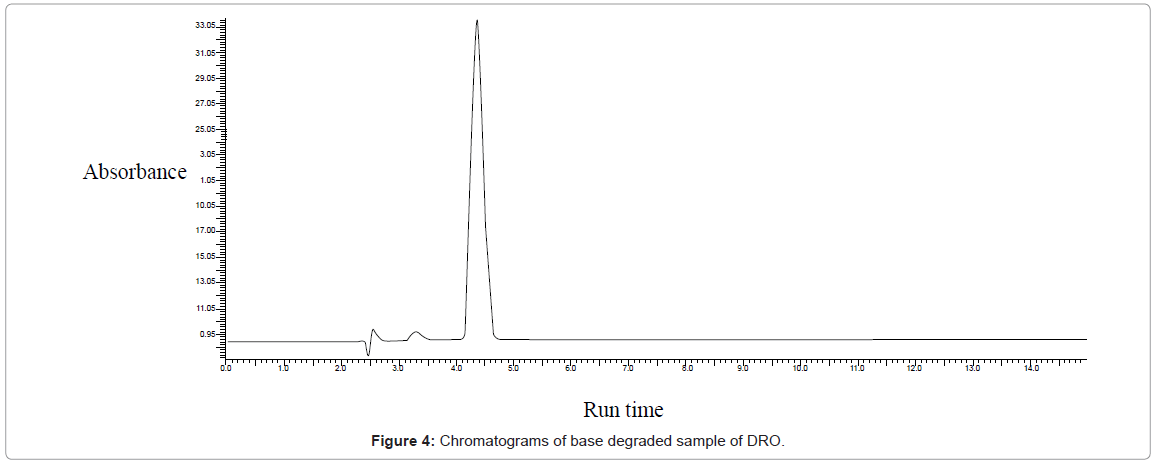
Base hydrolysis: Initially 0.1 M NaOH was used at 80°C for 2 hours. But no degradation was obtained. Concentration of NaOH was increased to 1 N but still no degradation was obtained so concentration was increased to 2 N NaOH at 80°C for 2 Hours and slight degradation was obtained as shown in figure 4.
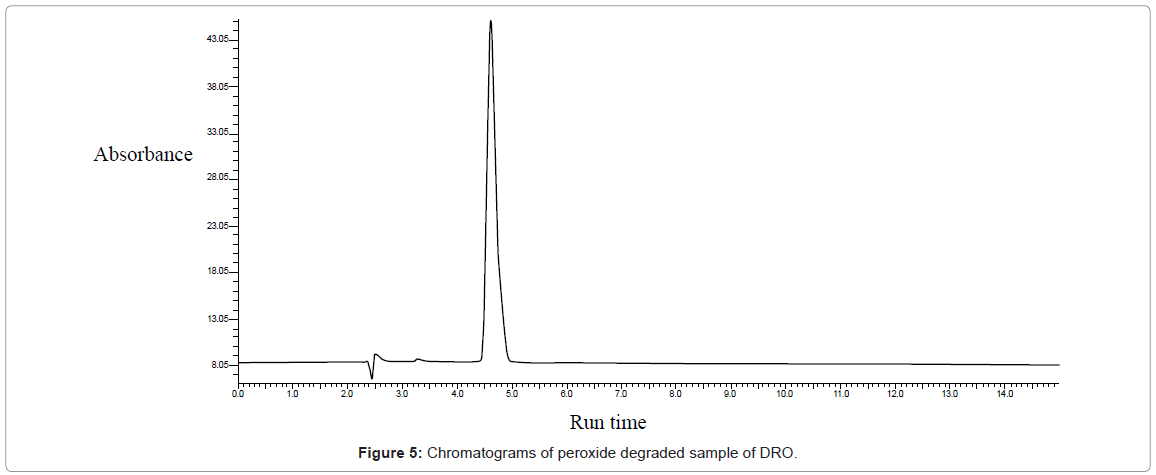
Peroxide degradation: 3% hydrogen peroxide did not show significant degradation when refluxed at 80°C for 2 hours. So concentration was increased to 6%. Slight degradation was obtained with 6% hydrogen peroxide at 80°C for 2 Hours (Figure 5).
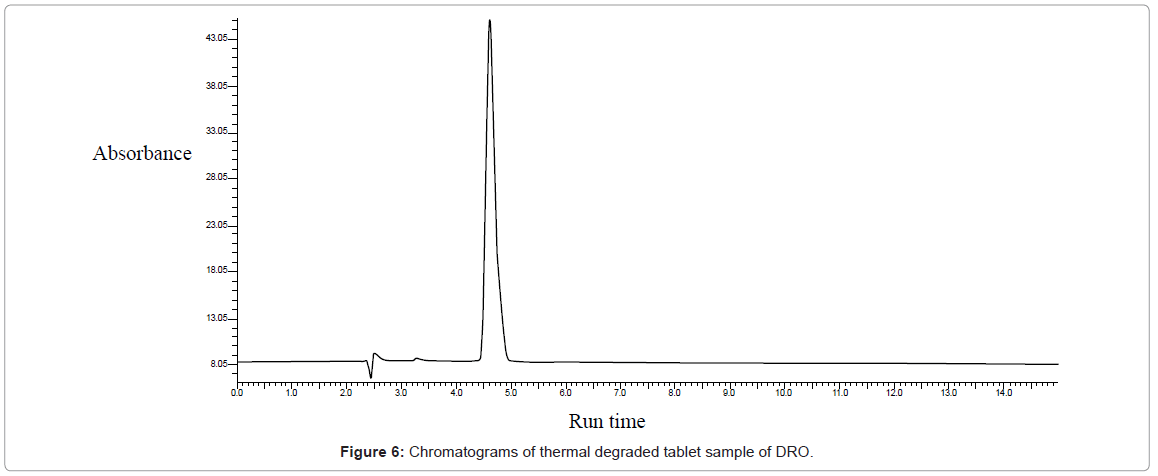
Thermal degradation (Figure 6): Degradation was obtained when sample was kept in oven at 100°C for 8 hours. The retention time of degradation products and % degradation are shown in table 1.
| Conditions | Time (min) | Area (10 μg/ml) | Degradation (%) | Retention time of degradation products | |
|---|---|---|---|---|---|
| DRO | DRO After Degradation | ||||
| Acidic (HCl) | 15 | 500191.55 | 415954.95 | 16.91 | 3.7, 6.2 |
| Basic (NaOH) | 15 | 409586.98 | 18.12 | 3.5 | |
| Oxidative | 15 | 42586.89 | 14.86 | 3.7 | |
| Thermal | 15 | 459827.45 | 8.1 | 3.4 | |
Table 1: Forced degradation study of DRO for the proposed method.
Validation of the method
Linearity: The linearity was determined at six levels over the range of 5-15 μg/ml. The calibration curve for DRO was prepared by plotting area versus concentration. The following equations for straight line were obtained for DRO.
Specificity: The specificity of the method was ascertained by analyzing DRO in presence of excipients like talc, polyethylene glycol, lactose and micro-crystalline cellulose were used for tablet formulations. The peaks of DRO were confirmed by comparing Rt values and respective spectra of sample with those of standards. The peak purity of DRO was assured by comparing the chromatogram (Table 2).
| Sample | Correlation of centre and Slope spectra | |
|---|---|---|
| r (s, m) | r (m, e) | |
| DRO | 0.991 | 0.998 |
| DRO formulation | 0.997 | 0.997 |
Table 2: Peak purity correlation results of DRO in two formulations at peak start, middle and end using PDA detector (WATERS HPLC, MODEL NO. 1752).
Accuracy: Accuracy was determined by calculating the recovery. The method was found to be accurate with % recovery 99.99%-100.03% for DRO (Table 3).
| Level | Amount of drug added (μg/ml) | Amount of drug taken (μg/ml) | Amount of drug found (μg/ml) | % Recovery | Mean recovery (%) (n=3) | %RSD |
|---|---|---|---|---|---|---|
| DRO | DRO | DRO | DRO | DRO | DRO | |
| 0 % | 0 | 4.8 | 4.76 | 99.16 | 99.71 | 0.82 |
| 0 | 4.8 | 4.81 | 100.20 | |||
| 0 | 4.8 | 4.79 | 99.79 | |||
| 50 % | 2.4 | 4.8 | 7.13 | 100.4 | 100.03 | 0.65 |
| 2.4 | 4.8 | 7.13 | 100.4 | |||
| 2.4 | 4.8 | 7.05 | 99.29 | |||
| 100% | 4.8 | 4.8 | 9.63 | 99.79 | 100.02 | 0.41 |
| 4.8 | 4.8 | 9.7 | 100.5 | |||
| 4.8 | 4.8 | 9.63 | 99.79 | |||
| 150% | 7.2 | 4.8 | 12 | 100.5 | 99.99 | 0.49 |
| 7.2 | 4.8 | 11.91 | 99.83 | |||
| 7.2 | 4.8 | 11.89 | 99.66 |
Table 3: Results from accuracy study.
Precision:
a) Repeatability
Repeatability data are shown in table 5. The % RSD is <2 for DRO which indicate that the method is precise.
| Parameters | DRO |
|---|---|
| Range | 0.001-20 μg/ml |
| Retention time (min) | 4.7 |
| Tailing factor | 1.19 |
| Resolution | 2.4 |
| Theoretical Plates | 3921.39 |
| Accuracy (%) | 99.99-100.03 |
| Precision (%RSD) | |
| Intra-day (n=3) | 0.16-0.92 |
| Inter-day (n=3) | 0.21-0.54 |
| Instrument precision (%RSD) | 0.19-0.54 |
| Specificity | Specific |
Table 4: Summary of validation parameters of developed HPLC method.
| Parameter | Method condition | % RSD of peak area |
|---|---|---|
| DRO | ||
| Flow rate | 0.9 ml/min | 0.78 |
| 1.1 ml/min | 0.93 | |
| 1.0 ml/min | 0.37 | |
| pH of Mobile phase | 4.05 | 0.47 |
| 3.95 | 0.56 | |
| 4 | 0.67 |
Table 5: Results from the robustness study of method.
b) Intra and inter day precision
Variation of results within the same day (intra-day), variation of results between days (inter-day) was analyzed. For intra-day (n=3) % RSD was found to be 0.16–0.92 and % RSD for inter-day (n=3) was 0.21-0.54 for DRO (Table 5). The % RSD is <2 for DRO which indicate that the method is precise (Table 4).
Limits of Detection (LOD) and Limits of Quantitation (LOQ): Under the experimental conditions used, the lowest amount of drug that could be detected (LOD) for DRO was found to be 0.19 μg/ml. The limit of quantification (LOQ) for DRO was found to be 1.19 μg/ml, with an RSD <2%.
Robustness: Acceptable %RSD values obtained after making small deliberate changes in the developed
Stability indicating HPLC method indicates that the method is robust for the intended purpose (Table 5).
Solution stability: The sample preparations were analyzed by HPLC system at regular intervals for 24 hrs as per test procedure. The method is also rugged as there was no change in absorbance up to 24 hours of preparation of solution in Methanol (Figure 7).
Method application
The proposed, developed and validated method was successfully applied to analysis of DRO in their marketed formulation. There was no interference of excipients commonly found in tablets as described in specificity studies. The assay results obtained were satisfactory, accurate, and precise as indicated by the good recovery and acceptable standard deviation values (Table 6). The good performance of the method indicates that it can be used for the determination of DRO in pharmaceutical formulation (Figure 8).
| Tablet | Component | Actual Concentration (μg/ml) DRO | Amount Obtained (μg/ml) DRO | % of drug found ± RSD (n=5) |
|---|---|---|---|---|
| Brand A (MULTAQ) | DRO | 4.8 | 4.78 | 99.58 ± 0.87 |
n=number of determinations
Table 6: Results from analysis of Dronederone in the tablet dosage form.
Conclusion
This developed and validated method for analysis of DRO in pharmaceutical preparations is very rapid, accurate, and precise. The method was successfully applied for determination of DRO in its pharmaceutical tablet formulation. Moreover it has advantages of short run time and the possibility of analysis of a large number of samples, both of which significantly reduce the analysis time per sample. Hence this method can be conveniently used for routine quality control analysis of DRO in its pharmaceutical formulation.
Acknowledgements
The authors are thankful to Cadila Pharmaceuticals Ltd., Ahmedabad for providing gift sample of DRO. The authors are very thankful to Sophisticated Instrumentation Center for Applied Research & Testing (SICART), Vallabh Vidyanagar, India), for providing necessary facilities to carryout research work. The authors are also thankful to Indukaka Ipcowala College of Pharmacy (IICP) for providing laboratories facilities.
References
- www.chemspider.com/imageview.aspx
- www.rxlist.com/multaq.drug.htm
- Patel A, Akhtar J, Sharma C (2012) Spectrophotometric Estimation of Dronedarone in Pure Drug and Pharmaceutical Formulation. Asian J Bio and Phar Res 2: 266-271.
- Bolderman RW, Hermans JJ, Maessen JG (2009) Determination of the class III antiarrhythmic drugs dronedarone and amiodarone, and their principal metabolites in plasma and myocardium by high-performance liquid chromatography and UV-detection. J Chromatogr B Analyt Technol Biomed Life Sci 877: 1727-1731.
- Xie C, Yang S, Zhong D, Dai X, Chen X (2011) Simultaneous determination of dronedarone and its active metabolite debutyldronedarone in human plasma by liquid chromatography- Tandem Mass Spectrophotometry: application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 879: 3071-3075.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14786
- [From(publication date):
February-2013 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10129
- PDF downloads : 4657