Research Article Open Access
Development and Validation of Analytical Method by RP-HPLC for Quantification of Alpha-Mangostin Encapsulated in PLGA Microspheres
Aimen Abdo Elsaid Ali, Muhammad Taher, Helaluddin ABM and Farahidah Mohamed*
Department of Pharmaceutical Technology, Kulliyyah of Pharmacy, International Islamic University Malaysia, Malaysia
- *Corresponding Author:
- Farahidah Mohamed
Department of Pharmaceutical Technology
Kulliyyah of Pharmacy
International Islamic University Malaysia
Jalan Istana, Bandar Indera Mahkota
25200 Kuantan, Pahang, Malaysia
E-mail: farahidah@iium.edu.my
Received date: September 21, 2012; Accepted date: November 26, 2012; Published date: December 03, 2012
Citation: Ali AAE, Taher M, Helaluddin ABM, Mohamed F (2012) Development and Validation of Analytical Method by RP-HPLC for Quantification of Alpha- Mangostin Encapsulated in PLGA Microspheres. J Anal Bioanal Tech 3:155. doi: 10.4172/2155-9872.1000155
Copyright: © 2012 Ali AAE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited..
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A simple, rapid, precise and highly accurate RP-HPLC method was developed and validated for determination of alpha-mangostin content extracted from PLGA-microspheres. Method was developed using a silica-based deactivated C-18 column (4.6×100 mm, 3 μm) with a mobile phase of 70-80 % v/v acetonitrile (A) and 0.1% v/v orthophosphoric acid (B), with the following pre-determined timed-gradient program: 70% (A) isocratic for 6 min, 70-75% (A) in 1.2 min, 75-80% (A) in 0.4 min, 80% (A) isocratic for 2.4 min, 80-70% (A) in 0.4 min, finally 70% (A) isocratic for 5 min, with a flow rate of 1 mL/min, detected at 320 nm by a UV detector. Linearity was obtained over the range of 1-200 μg/mL with r2=0.9995. The precision was achieved based on repeatability and intermediate precision with RSD of 0.13-0.6% and 0.57-1.2%, respectively. Percent recovery of 100.55% to 103.82% with RSD 0.086 - 0.15 implied high accuracy of the method. Limit of detection and limit of quantitation were 0.038 and 0.121 μg/ml, respectively suggesting good sensitivity of the method. The method is envisaged to be effectively used for routine quality control assay for encapsulated alpha-mangostin in PLGA microspheres.
Keywords
Microencapsulation; Xanthone; Garcinia malaccensis
Introduction
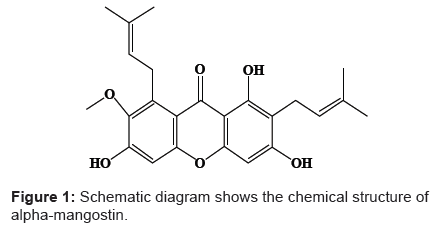
Alpha-mangostin is a biosynthetic di-prenylated tetra-oxygenated xanthone derivatives [1] which was firstly isolated from the fruit rind of Garcinia mangostana GM by Schmid in 1855 [2]. Its chemical structure of 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)- 9H-xanthen-9-one (Figure 1) was elucidated by Yates et al. [3]. Alphamangostin represented the major active constituent among the detected xanthones and therefore, it has been considered as analytical marker for the quality control of GM products [4,5]. Recently, alpha-mangostin has been commonly employed as ingredient in nutritional supplements, herbal cosmetics [4] and some topical pharmaceutical preparations [6] owing to its previously investigated multi-pharmacological activities that were previously reviewed by Pedraza-Chaverri et al. [7]. It is well known that alpha-mangostin exhibited a broad spectrum of biological effects including anti-oxidant [8], anti-inflammatory [9], anti-allergic [10], analgesic [11], neuro-protective [12], anti-mycobacterial [1], antifungal [13], anti-bacterial [14] and anti-proliferative [15,16] activities. In addition, recent studies have indicated that alpha-mangostin had anti-metastatic effect against various cancer cell lines [17-19]. Nevertheless, the results of pharmacokinetic study of pure alphamangostin revealed its low oral bioavailability attributed to the first pass metabolism beside its non-selective distribution into the rat tissue [20]. As such, we had previously encapsulated the alpha-mangostin into poly (lactic-co-glycolic acid) PLGA nanospheres in order to enhance its bioavailability through selective targeting of the compound into specific tissue. The selectivity is envisaged to be achieved based on “enhanced permeability and retention” (EPR) effect duly caused by its size that passively distributed and retained within porous vascular structure of tumor sites. In addition, the drug delivery employing PLGA co-polymer that was fabricated into microspheres had long been used clinically. Zoladex® and Lupron® were among the popular drugs. The carrier system was also employed here due to its ability to provide controlled-release of drugs apart from its biodegradability that do not warrant second surgery to remove the carrier. Although biodegradable PLGA microparticles have been widely employed as delivery vehicles for various macromolecules such as protein [21], peptides [22] and plasmid DNA [23], numerous small molecules were encapsulated in order to enhance their therapeutic activities and to minimize their adverse effects [24].
As part of routine characterization, encapsulation efficiency of alpha-mangostin was required to be determined and can only be accomplished following complete dissolution of the polymeric microsphere. Previously described method [4] for the assay of pure alpha-mangostin using methanol as solvent could not be applied to quantify alpha-mangostin from the PLGA microspheres. This was due to poor solubilising property of the methanol to completely dissolve the polymeric PLGA. Another study had employed acetonitrile as solvent to solubilise xanthone and 3-methoxyxanthone encapsulated-PLGA nanocapsules but there was no report on the analytical validation [25]. Further analytical methods were established and validated using LC-MS/MS detector in order to measure the alpha-mangostin concentration in human plasma [26] and in rat plasma [20]. However, up to date there is no official method to quantify the alpha-mangostin extracted from PLGA-nanospheres. We had therefore, optimized, developed and validated a new analytical technique using similar RPHPLC and had found more rapid quantification using this technique compared to the previous study.
Experimental
Chemicals and reagents
PLGA microspheres loaded with alpha-mangostin were prepared using solvent extraction method as previously described [23]. Acetonitrile and water (HPLC grade) were purchased from Merck (Germany). Phosphate buffered saline (PBS) composed of 0.01 M disodium phosphate, 0.002 M monopotassium phosphate, 0.138 M sodium chloride and 0.008 M potassium chloride, was obtained from Sigma-Aldrich (UK). Orthophosphoric acid was supplied by BDH chemicals (England). Alpha-mangostin was isolated from the stem bark of Garcinia malaccensis as reported by Taher et al. [27]. The purity of the isolated alpha-mangostin was approximately 94% as identified by HPLC and compared to the reference standard alpha-mangostin of purity 96.5% which was purchased from ChromaDex (Irvin, CA, USA).
Instrumentation and chromatographic condition
The HPLC system (Agilent, series 1200, USA) used consisted of a quaternary pump (G1311A), autosampler (G1329A), solvent degasser (G1322A), and UV detector (G1314B). The quantification wavelength of alpha-mangostin was set at 320 nm.
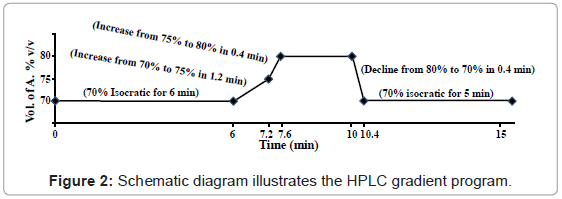
The chromatographic separation was performed at ambient temperature using Hypersil® BDS C18 column (4.6×100 mm, 3 μm) with C18 guard column. The mobile phase consisted of acetonitrile (A) and 0.1% (v/v) orthophosphoric acid diluted in water (B) was delivered at flow rate of 1.0 mL/min by applying the following programmed gradient elution: 70% (A) isocratic for 6 min, 70-75% (A) in 1.2 min, 75-80% (A) in 0.4 min, 80% (A) isocratic for 2.4 min, 80-70% (A) in 0.4 min, finally 70% (A) isocratic for 5 min as post-run for re-conditioning (Figure 2). Sample injection volume was adjusted to 10 μl. All solutions of mobile phase were freshly prepared, filtered through 0.45 μm Nylon filter under vacuum and degassed by sonication for 20 min prior to use. The results were analysed using ChemStation software.
Preparation of sample
For determination of alpha-mangostin content from PLGAmicrospheres, an appropriate amount of the lyophilized microspheres were completely dissolved in acetonitrile, followed by precipitation of the polymer with 30% v/v 0.01 M PBS (pH 7.4). Following centrifugation at 4000 rpm for 10 min, the supernatant containing the extracted alpha-mangostin was filtered through 0.2 μm PTFE microfilter prior to analysis using a corrective and validated RP-HPLC method. Concentration of the extracted alpha-mangostin was calculated based on a linear regression equation of the calibration curve.
Preparation of standards and calibration curve
The standard stock solution of alpha-mangostin was prepared by dissolving an accurately weighed reference compound in a mixture of acetonitrile and PBS (70:30, v/v) to obtain a solution with final concentration of 1 mg/mL. Serial dilution was performed thereafter to get calibration standard solutions of 1, 2, 4, 6, 8, 10, 20, 40, 60, 80, 100 and 200 μg/mL. All standard solutions were injected in triplicate and the linearity was assessed based on calibration equation which was calculated from plotting the mean peak areas versus corresponding concentrations. The linear regression equation was computed based on the method of least squares using Excel software.
Precision
The repeatability of measurement was evaluated by analysing the standard solution of alpha-mangostin at three concentration levels (20, 60 and 80 μg/mL). Each concentration level was repeated five times within the same day in order to determine the intra-day precision. With respect to inter-day precision (or intermediate precision), the same procedure was duplicated on two different days. The precision was estimated according to the relative standard deviation.
Accuracy
The recovery studies were conducted at three different levels of standard alpha-mangostin added to the samples solution with known content of alpha-mangostin. The samples were prepared according to the “preparation of sample” and the extracted alpha-mangostin was spiked with 10, 20 and 30 μg of reference standard. Three determinations were performed for each concentration level and the accuracy of the method was expressed as the percent recovery and grand mean recovery.
Limit of detection (LOD) and Limit of quantitation (LOQ)
In conformity with the “International Conference on Harmonization of Technical Requirement for the Registration of pharmaceuticals for Human Use” [28], the approach based on the standard deviation of the response and the slope was applied here in order to assess both the detection (LOD) and quantitation (LOQ) limits using the following equations:
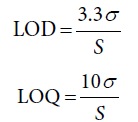
σ represents standard deviation of the response whereas S represents slope of the calibration curve.
Results and Discussion
Extraction of alpha-mangostin from plga-microspheres
The influence of different organic solvents on the extraction of alpha-mangostin from the pericarps of Garcinia mangostana had been studied previously by Cheok et al. [29]. They reported that the yield of phenolic compounds in which alpha-mangostin represented the major active substance was directly proportional to the dielectric constant of the employed solvents. The study found that methanol which exhibited the highest charge insulating activity with dielectric constant of 32.7 was considered as the selective solvent for the extraction of alphamangostin. However, extraction of alpha-mangostin that dispersed within the entanglement of PLGA co-polymer microspheres appeared not possible by methanol probably owing to its disability to solubilise the co-polymer. Therefore, in the present study we used acetonitrile to simultaneously dissolve all microspheres constituents, followed by addition of an aqueous PBS to precipitate PLGA co-polymer. The latter was removed by centrifugation at high speed. Similar solvent was previously applied to extract the xanthone and 3-methoxyxanthone from PLGA nanoparticles [25]. The extreme solvation power of acetonitrile (37.5) may attribute to its higher dielectric constant value when compared to that of methanol (32.7). Overall, a mixture of acetonitrile and PBS (70:30 v/v) was employed as the solvent throughout the validation procedure, including during extraction procedure of alpha-mangostin from PLGA microspheres.
Validation procedure
We had employed 320 nm as the detection wavelength based on previous independent study [15]. Similar mobile elution system consisted of acetonitrile and 0.1% v/v orthophosphoric acid in water was used in the present method as previously described by Pothitirat et al. [5]. However, the gradient elution programme was adjusted in accordance with the length of chromatographic column. It is well documented that efficient and fast chromatographic separation of any compound can be achieved by reduction in the particle size of HPLC’s column packing material. Hence, for rapid quantification of the extracted alpha-mangostin, we used short analytical column (100 mm) packed with silica beads of 3 μm in diameter.
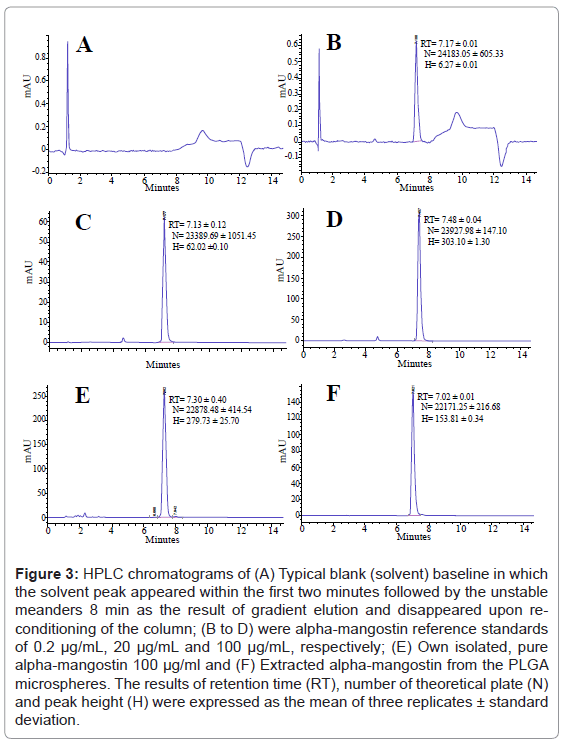
As shown in figure 3, it can be clearly observed that all HPLC chromatograms of alpha-mangostin in the following forms: standard 0.2 (B), 20 (C) and 100 (D) μg/ml, isolated pure 100 μg/ml (E) and extracted from PLGA microspheres (F) were eluted within 15 min and their relevant peaks appeared at approximately similar retention time RT of (7.17 ± 0.01), (7.13 ± 0.12), (7.19 ± 0.31), (7.48 ± 0.04), and (7.02 ± 0.34) min, respectively. The retention times were the mean of three replicates ± standard deviation. Previous analytical HPLC study had been established by Pothitirat and co-workers [5] to quantify the extracted alpha-mangostin from the pericarps of GM. Their chromatographic separation was carried out on a long analytical column (250 mm) packed with silica beads of 5 μm in diameter. They reported that alpha-mangostin was eluted at retention time of 16.23 min. In comparison, our chromatographic separation time appeared tremendously shorter i.e. 7.12 min as compared to 16.23 min which attributed to the length and the size of silica beads. On the other hand, Ji X et al. [30] and Yodhnu et al. [31] had also validated an analytical HPLC method for determination the concentration of alpha-mangostin in the fruit rind of GM using isocratic mobile phase composed of 0.2% formic acid and acetonitrile. Although their chromatographic separation was performed on a slightly longer column (125 mm) as compared to ours (100 mm), their retention time was slightly faster (6.2 min) than ours. It could be due to different mixture of solvent used.
Figure 3: HPLC chromatograms of (A) Typical blank (solvent) baseline in which the solvent peak appeared within the first two minutes followed by the unstable meanders 8 min as the result of gradient elution and disappeared upon reconditioning of the column; (B to D) were alpha-mangostin reference standards of 0.2 μg/mL, 20 μg/mL and 100 μg/mL, respectively; (E) Own isolated, pure alpha-mangostin 100 μg/ml and (F) Extracted alpha-mangostin from the PLGA microspheres. The results of retention time (RT), number of theoretical plate (N) and peak height (H) were expressed as the mean of three replicates ± standard deviation.
The solvent that composed of a mixture of acetonitrile and PBS (70:30, v/v) was employed as the control (A). The solvent peak appeared within the first two minute of the elution. It can also be seen that the baseline of control involved a negligible meanders that arise as the result of gradient elution. These peaks disappeared through re-conditioning of the column. It is noteworthy that the peak of 100 μg/ml standard alpha-mangostin (D) was slightly higher (H ~303.1 mA) than the peak of our isolated pure compound (E) at similar concentration (H ~279.7 mA), confirming purity of our isolated alpha-mangostin (94%) that closely match the purity of the standard (96.5%).
The retention time of our study (7.12 min) was the mean of 290 runs with RSD of less than 0.96%, suggesting high repeatability and faster quantification of alpha-mangostin than previously reported study of Pothitirat et al. [5]. The use of the short column packed with fine silica particle of 3 μm resulted in rapid quantification of alpha-mangostin as well as relatively large theoretical plate number (N) i.e. higher than ~22171 as illustrated in figure 3. Generally, N more than 2000 coincides with acceptable system suitability although we did not measure the corresponding tailing factor due to limitation of the software used.
The proposed analytical method was validated with respect to its linearity, precision, accuracy, LOD and LOQ as recommended by ICH guideline [28].
Linearity
The linearity of the method was found be linear within the range of 1-200 μg/ml with regression equation of “Y=37.5X+25.98” that showed a good correlation coefficient of 0.9995. In addition, we had also plotted lower range concentration of 0.2-0.8 μg/ml with regression equation of “Y=38.507X-0.0333” and found the good correlation was maintain although at very low concentration with coefficient 0.997.
Precision
Intra-day precision was determined by performing a five replicates of three concentration levels of standard alpha-mangostin on the same day. Similar procedures were duplicated on two different days in order to evaluate the inter-day (intermediate) precision. The results as summarised in table 1 showed acceptable repeatability measurements with relative standard deviation not exceeded 2%. It is worth noting that the %RSD values of intermediate precision were relatively large which suggest instability of alpha-mangostin in the solvent mixture of PBS and acetonitrile when left over 48 h prior to the subsequent test, but the value was still satisfactory i.e. %RSD less than 2.
| Concentration (μg/ml) | Repeatability | Intermediate precision | ||
|---|---|---|---|---|
| % of amount found* (n=5) | % RSD | % of amount found# (n=10) | % RSD | |
| 20 | 100.84 ± 0.135 | 0.134 | 101.75 ± 1.296 | 1.273 |
| 60 | 103.93 ± 0.622 | 0.598 | 104.35 ± 0.599 | 0.574 |
| 80 | 102.93 ± 0.255 | 0.248 | 103.73 ± 1.135 | 1.095 |
*Expressed as the mean of five replicates in a day ± SD
#Expressed as the mean of five replicates per day over two days ± SD
Table 1: Indicator for precision based on repeatability performed within the same day of measurement and intermediate precision of alpha-mangostin using the prospective HPLC method.
Accuracy
The extracted alpha-mangostin samples from PLGA-based microspheres were confirmed by spiking their peaks areas with three concentration levels of standard alpha-mangostin. The percentage of recovery was found to be within acceptable limit i.e. 100.6 % to 103.8% with relative standard deviation of less than 0.2% as illustrated in table 2 which implies high accuracy of the method. The calculated LOD and LOQ values were found to be 0.038 and 0.121 μg/ml, respectively indicating that the method is highly sensitive.
| Amount present in the MS formula (μg/ml)# | Added amount of reference standard alpha- mangostin (μg) | Detected amount (μg/ml)# | Percent Recovery (%) | % RSD |
|---|---|---|---|---|
| 35.66 ± 0.22 | 10 | 45.91 ± 0.019 | 100.55 | 0.086 |
| 20 | 56.59 ± 0.047 | 101.67 | 0.106 | |
| 30 | 68.17 ± 0.082 | 103.82 | 0.152 | |
| Grand mean of Recovery (%) | 102.01 (1.630) | |||
#Mean of three determinations at each level ± standard deviation. MS refer to microspheres
Table 2: Results of recovery study of alpha-mangostin using the prospective HPLC method.
Conclusion
The method depicted rapid, sensitive and high precision and accuracy technique to quantify extracted alpha-mangostin from PLGA microspheres. It should be useful as a routine quality control analysis for such dosage form.
Acknowledgements
The authors are grateful to International Islamic University Malaysia (IIUM) for financial support granted for this research (Grant ID: EDW B1002-350).
References
- Arunrattiyakorn P, Suksamrarn S, Suwannasai N, Kanzaki H (2011) Microbial metabolism of α-mangostin isolated from Garcinia mangostana L. Phytochemistry 72: 730-734.
- Schmid W (1855) Ueber das Mangostin. Justus Liebigs Annalen der Chemie 93: 83-88.
- Yates P and Stout GH (1958) The Structure of Mangostin1. J Am Chem Soc 80: 1691-1700.
- Pedraza-Chaverrà J, Reyes-Fermín LM, Nolasco-Amaya EG, Orozco-Ibarra M, Medina-Campos ON, et al. (2009) ROS scavenging capacity and neuroprotective effect of alpha-mangostin against 3-nitropropionic acid in cerebellar granule neurons. Exp Toxicol Pathol 61: 491-501.
- Pothitirat W and Gritsanapan W (2009) HPLC quantitative analysis method for the determination of α-mangostin in mangosteen fruit rind extract. Thai J Agri Sci 42: 7-12.
- Jujun P, Pootakham K, Pongpaibul Y, Tharavichitkul P, Ampasavate C (2009) HPLC Determination of Mangostin and Its Application to Storage Stability Study. Chiang Mai University J Nat Sci 8: 43-53.
- Rassameemasmaung S, Sirikulsathean A, Amornchat C, Maungmingsook P, Rojanapanthu P, et al. (2008) Topical application of Garcinia mangostana L. pericarp gel as an adjunct to periodontal treatment. Complement Ther Med 16: 262-267.
- Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas JM (2008) Medicinal properties of mangosteen (Garcinia mangostana). Food Chem Toxicol 46: 3227-3239.
- Chae HS, Oh SR, Lee HK, Joo SH, Chin YW (2012) Mangosteen xanthones, α-and g-mangostins, inhibit allergic mediators in bone marrow-derived mast cell. Food Chemistry 134: 397-400.
- Jang HY, Kwon OK, Oh SR, Lee HK, Ahn KS, et al. (2012) Mangosteen xanthones mitigate ovalbumin-induced airway inflammation in a mouse model of asthma. Food Chem Toxicol 50: 4042-4050.
- Cui J, Hu W, Cai Z, Liu Y, Li S, et al. (2010) New medicinal properties of mangostins: analgesic activity and pharmacological characterization of active ingredients from the fruit hull of Garcinia mangostana L. Pharmacol Biochem Behav 95: 166-172.
- Wang Y, Xia Z, Xu JR, Wang YX, Hou LN, et al. (2012) A-mangostin, a polyphenolic xanthone derivative from mangosteen, attenuates β-amyloid oligomers-induced neurotoxicity by inhibiting amyloid aggregation. Neuropharmacology 62: 871-881.
- Kaomongkolgit R, Jamdee K, Chaisomboon N (2009) Antifungal activity of alpha-mangostin against Candida albicans. J Oral Sci 51: 401-406.
- Koh JJ, Qiu S, Zou H, Lakshminarayanan R, Li J, et al. (2012) Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim Biophys Acta.
- Aisha AF, Abu-Salah KM, Ismail Z, Majid A (2012) In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthones extract. BMC Complement Altern Med 12: 104.
- Yoo JH, Kang K, Jho EH, Chin YW, Kim J, Nho CW (2011) α-and g-Mangostin Inhibit the Proliferation of Colon Cancer Cells via β-Catenin Gene Regulation in Wnt/cGMP Signalling. Food Chemistry 129: 1559-1566.
- Shih YW, Chien ST, Chen PS, Lee JH, Wu SH, Yin LT (2010) α-mangostin suppresses phorbol 12-myristate 13-acetate-induced MMP-2/MMP-9 expressions via alphavbeta3 integrin/FAK/ERK and NF-kappaB signaling pathway in human lung adenocarcinoma A549 cells. Cell Biochem Biophys 58: 31-44
- Shibata MA, Iinuma M, Morimoto J, Kurose H, Akamatsu K, et al. (2011) α-Mangostin extracted from the pericarp of the mangosteen (Garcinia mangostana Linn) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation. BMC Medicine 9: 69.
- Wang JJ, Sanderson BJ, Zhang W (2012) Significant anti-invasive activities of α-mangostin from the mangosteen pericarp on two human skin cancer cell lines. Anticancer Res 32: 3805-3816.
- Li L, Brunner I, Han AR, Hamburger M, Kinghorn AD, et al. (2011) Pharmacokinetics of α-mangostin in rats after intravenous and oral application. Mol Nutr Food Res 55 Suppl 1: S67-S74.
- Elsaid Ali A, Bakhtiar MT, Mohamed F Microencapsulation of alpha-mangostin into PLGA microspheres and optimization using response surface methodology (RSM) intended for pulmonary delivery (unpublished mansucript).
- Cilurzo F, Selmin F, Liberti V, Montanari L (2005) Thermal characterization of poly (lactide-co-glycolide) microspheres containing bupivacaine base polymorphs. Journal of thermal analysis and calorimetry 79: 9-12.
- Mohamed F, van der Walle CF (2008) Engineering biodegradable polyester particles with specific drug targeting and drug release properties. J Pharm Sci 97: 71-87.
- Oettinger CW, D'souza MJ (2012) Microencapsulated drug delivery: a new approach to pro-inflammatory cytokine inhibition. J Microencapsul 29: 455-462.
- Teixeira M, Alonso MJ, Pinto MM, Barbosa CM (2005) Development and characterization of PLGA nanospheres and nanocapsules containing xanthone and 3-methoxyxanthone. Eur J Pharm Biopharm 59: 491-500.
- Kondo M, Zhang L, Ji H, Kou Y, Ou B (2009) Bioavailability and antioxidant effects of a xanthone-rich Mangosteen (Garcinia mangostana) product in humans. J Agric Food Chem 57: 8788-8792.
- Taher M, Susanti D, Rezali MF, Zohri FS, Ichwan SJ, et al. (2012) Apoptosis, antimicrobial and antioxidant activities of phytochemicals from Garcinia malaccensis Hk.f. Asian Pac J Trop Med 5: 136-141.
- Baber N (1994) International conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use. Br J Clin Pharmacol 37: 401-404.
- Cheok CY, Chin NL, Yusof YA, Law CL (2012) Extraction of Total Phenolic Content from Garcinia mangostana Linn. hull. I. Effects of Solvents and UV–Vis Spectrophotometer Absorbance Method. Food and Bioprocess Technology 5: 2928-2933.
- Ji X, Avula B, Khan IA (2007) Quantitative and qualitative determination of six xanthones in Garcinia mangostana L. by LC-PDA and LC-ESI-MS. J Pharm Biomed Anal 43: 1270-1276.
- Yodhnu S, Sirikatitham A, Wattanapiromsakul C (2009) Validation of LC for the determination of alpha-mangostin in mangosteen peel extract: a tool for quality assessment of Garcinia mangostana L. J Chromatogr Sci 47: 185-189.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16768
- [From(publication date):
December-2012 - Dec 22, 2024] - Breakdown by view type
- HTML page views : 12167
- PDF downloads : 4601