Research Article Open Access
Development and Validation of an HPLC Method for Quantifying Dapiprazole in Bulk Preparations
Jaya Prasanthi K and Syama Sundar B*
Department of Chemistry, Acharya Nagarjuna University, Guntur, India
- *Corresponding Author:
- B. Syama Sundar
Department of Chemistry
Acharya Nagarjuna University
Nagarjuna Nagar, Guntur-522510, India
E-mail: profbsyamsundar@yahoo.co.in
Received date: July 16, 2012; Accepted date: August 24, 2012; Published date: August 30, 2012
Citation: Jaya Prasanthi K, Syama Sundar B (2012) Development and Validation of an HPLC Method for Quantifying Dapiprazole in Bulk Preparations. J Anal Bioanal Tech 3:143. doi: 10.4172/2155-9872.1000143
Copyright: © 2012 Jaya Prasanthi K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A simple, precise, rapid and accurate Reverse phase HPLC method was developed for the estimation of Dapiprazole in bulk form. A Thermo hypersil C-18 column (250 mm×4.6 mm 5μm) with mobile phase consisting of mixture of methanol: acetonitrile: and ortho-phosphoric acid in the ratio of 89: 9: 1 (v/v) at pH 5.8 adjusted with ortho-phosphoric acid. The flow rate was 0.8 mL/min and the effluents were monitored at 243 nm. The retention time was 3.725 min. The detector response was linear in the concentration of 20-120 μg/mL. The respective linear regression equation being y=1956.4x+3409. The limit of detection and limit of quantification was 0.5 μg/mL and 1.6 μg/mL respectively. The percentage assay of Dapiprazole was 99.94%. This method has been validated and shown to be specific, sensitive, precise, linear, accurate, rugged, robust and fast. Hence, this method can be useful for the routine determination of Dapiprazole in bulk drug and in its pharmaceutical dosage form.
Keywords
Dapiprazole; RP-HPLC; Bulk Drug
Introduction
Dapiprazole hydrochloride is an alpha-adrenergic blocking agent [1]. Chemically, it is 5,6,7,8-tetrahydro-3-[2-(4- o-tolyl-1-piperazinyl) ethyl]-s -triazolo[4,3-a] pyridine hydrochloride [2,3]. Dapiprazole hydrochloride has the empirical formula C19H27N5 .HCl and a molecular weight of 361.93. It is a sterile, white, lyophilized powder soluble in water. Dapiprazole hydrochloride ophthalmic solution is indicated in the treatment of iatrogenically induced mydriasis produced by adrenergic (phenylephrine) or parasympatholytic (tropicamide) agents [4,5]. The novel synthetic method has been developed by concerning the yields and mild reaction conditions to synthesize Dapiprazole starting from stable reactants such as ethyl 3-chloro propionate and o-tolyl piperzine, which appears as impurity in the final product. In vivo evaluation was performed for in ocular vehicle for long acting alfa adrenergic receptor blocking activity of dapiprazole hydrochloride [6]. Following topical instillation on the eye, it crosses the corneal epithelium reaching high concentrations in the ocular tissue and producing a prompt mitotic and hypotensive effect. The high concentration ratio between ciliary bodies and iris versus aqueous humor suggests a peculiar affinity for these structures containing adrenoceptors of the alpha type [7,8]. Also, a study was conducted to compare the cycloplegic and mydriatic effects of Paremyd™, a formulation of 0.25% tropicamide and 1% hydroxyamphetamine, to 0.5% tropicamide and 2.5% phenylephrine [9]. The influence of dapiprazole on pupil size was compared with brinzolamide and reported that pupil mydriasis at scotopic illumination levels was reduced by both drugs in a similar fashion [10]. The aim of this paper was to develop validate a simple and reliable HPLCUV method for the determination of Dapiprazole in bulk drug. This manuscript describes the development and validation, in accordance with International Conference on Harmonization (ICH) guidelines, of a rapid, economical, precise, and accurate stability-indicating isocratic reversed-phase HPLC method for analysis of Dapiprazole in bulk sample. To the best of our knowledge, literature survey reveals no chromatographic methods for the estimation of Dapiprazole in bulk samples. The availability of an HPLC method with high sensitivity and selectivity will be very useful for the determination of Dapiprazole in bulk and also in pharmaceutical formulations. The chemical structure of Dapiprazole is represented in Figure 1.
Experimental
Instrument
Quantitative HPLC was performed on liquid Chromatograph, Shimadzu LC 2010 dual λ detector equipped with automatic injector with injection volume 20 μL. The HPLC system was equipped with LC solution Software. The pH measurements were carried out with Elico, model LI 120, pH meter equipped with a combined glass-calomel electrode calibrated using standard buffer solutions of pH 4.0, 7.0 and 9.2.
Materials/reagents
Acetonitrile and methanol HPLC grade (Qualigens) and Water HPLC grade (Milli-Q), ortho-Phosphoric acid (Rankem). Dapiprazole working and reference sample is obtained as gift sample from M/s Bioleo Analytical Labs India Pvt. Ltd, Hyderabad.
HPLC conditions
The contents of the mobile phase were methanol: acetonitrile: and ortho-phosphoric acid in the ratio of 89: 9: 1 (v/v/v) at pH 5.8. They were filtered before use through a 0.45 μm membrane filter, and pumped from the respective solvent reservoirs to the column at a flow rate of 0.8 mL/min. The run time was set at 8.0 min and the column temperature was ambient. Prior to the injection of the drug solution, the column was equilibrated for at least 30 min with the mobile phase flowing through the system. The eluents were monitored at 243 nm.
Preparation of standard stock solution
A standard stock solution of the drug was prepared by dissolving 10 mg of Dapiprazole USP in 10 ml volumetric flask containing 5 ml of methanol, sonicated for about 15 min and then made up to 10 ml with mobile phase to get 1000 μg/ml of Dapiprazole.
Working standard solution
The primary standard solution was further diluted by taking 2 ml of the stock solution with 25 ml of mobile phase to get the concentration of 80 μg/mL.
Preparation of sample solution
A sample of the powder of Dapiprazole synthesized in in-house, equivalent to 10 mg of the active ingredient, was mixed with 5 ml of methanol in 10 ml volumetric flask. The mixture was allowed to stand for 30 minutes with intermittent sonication for complete solubility of the drug, and then filtered through a 0.45 μm membrane filter, followed by addition of mobile phase up 10 ml to obtain a stock solution of 1 mg/ml. The resultant solution was further diluted by taking 2 ml of the stock solution with 25 ml of mobile phase to get the concentration of 80 μg/mL.
Linearity
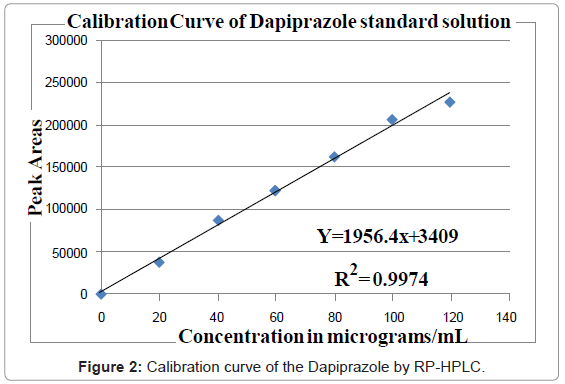
Aliquots of standard drug solution of Dapiprazole 2.5, 5.0 and 7.5 ml (80 μg/ml) were taken and transferred into series of 10 ml volumetric flasks and diluted up to 10 ml with mobile phase as dilute to get 20, 40 and 60 μg/ml. The working standard solution is directly used to get 80 μg/ml. Another set of aliquots of 1.0 and 1.2 ml (1000 μg/mL) were taken and transferred into series of 10 ml volumetric flasks and diluted up to 10 ml with mobile phase as diluents to get 100 and 120 μg/ml. Each of these drug solutions (20 μL) was injected three times into the column, and the peak areas and retention times were recorded. Evaluation was performed with UV detector at 243 nm and a Calibration graph was obtained by plotting peak area versus concentration of Dapiprazole (Figure 2).
Assay
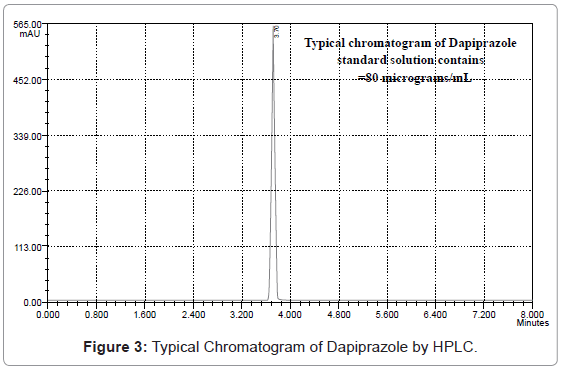
20 μL of sample solution was injected into the injector of liquid chromatograph. The retention time was found to be 3.724 minutes. The amount of drug present in bulk sample was calculated by comparing the peak area of the sample solution with that of the standard solution.
Recovery studies
Recovery is a useful way to assess how efficient an extraction procedure is the closer the recovery value is to 100%, the better the sensitivity will be. Recovery values have to be reproducible to prove that they are accurate. Accuracy was determined by recovery studies of Dapiprazole, known amount of standard was added to the preanalyzed sample and subjected to the proposed HPLC analysis. Results of recovery study are shown in Table 1. The study was done at three different concentration levels.
| Sample | % found by the proposed method | % Recovery* |
|---|---|---|
| 1. 2. 3. |
99.43 99.55 99.46 |
99.97 98.365 98.247 |
*Average of three different concentration levels
Table 1: Results of HPLC Assay and Recovery studies
Limit of Detection (LOD) and Limit of Quantification (LOQ)
The LOD is defined as the analyte concentration that gave a signal to noise (S/N) ratio of 3:1. The LOQ was defined as the analyte concentration that gave an S/N ratio of 5:1. The limit of detection (LOD) and limit of quantification (LOQ) for Dapiprazole were found to be 0.5 μg/mL and 1.6 μg/mL respectively. The signal to noise ratio is 3 for LOD and 10 for LOQ.
Results and Discussion
For developing the method, a systematic study on the effect of various factors was carried out by varying one parameter at a time and keeping all other conditions constant, that is, OFAT (One Factor at a Time) mode of study. Method development consists of selecting the appropriate detection wave length and stationary and mobile phases.
Proper wavelength was needed to determine maximum detector response. From the spectrum, it is clear that Dapiprazole absorbs maximum light between 235 nm to 245 nm. For the RP-HPLC analysis, the UV/Vis detector was fixed at 243 nm as maximum wave length (λmax) for determination of Dapiprazole. At this wavelength, we have observed a zero back ground noise and no absorption of light by mobile phase solvent system.
To explore the possibility of better separation a Cyano column was tested with the same mobile phase ternary mixture of methanol: acetonitrile: and ortho-phosphoric acid in the ratio of 89: 9: 1 (v/v/v) at pH 5.8. The retention times were long with polar columns. Retention of the analyte on the cyano columns was much weaker than on C18 columns, resulting in unacceptable k’ (capacity factor) value of the column (<1) for Dapiprazole. Further development trials have been performed with octadecyl columns of different types and configurations from different manufacturers. Under these chromatographic conditions, the analyte of interest has exhibited poor peak efficiencies (N) and peak symmetries (Tailing factor); and a partial resolution (Rs) between drug and mobile phase components. Finally Thermo hypersil C18 column (250 mm×4.6 mm 5 μm) column was selected based on the peak shape and the baseline separation from the other interfering peaks in the formulation sample.
Different mobile phases were tested to optimize analytical performance. The optimum composition of mobile phase was determined by comparing the influence of different binary mixtures such as acetonitrile- methanol and acetonitrile -water and methanolwater in different proportions. None of the solvent system in binary phase has given good peak shape and theoretical plates. It is well known that multiple-component mobile phases result in better separation efficiency than binary mobile phases, because with these solvent strength and selectivity can be varied simultaneously to obtain the retention times desired. A third component, o-phosphoric acid, was therefore included in the mobile phase and ternary mixtures of methanol: acetonitrile: and ortho-phosphoric acid in the ratio of 89: 9: 1 (v/v) at pH 5.8. The present mobile phase system has been finalized for the estimation of Dapiprazole in bulk drug in terms of peak symmetry, optimum resolution, reasonable run time, and acceptable k values.
System suitability
The system suitability tests were carried out on freshly prepared standard stock solution of Dapiprazole. The system was suitable for use, the tailing factors for Dapiprazole were 1.15 and USP theoretical plates were found to be significantly high around 21505 in number. Parameters that were studied to evaluate the suitability of the system are given in Table 2.
| Validation Parameter | Results |
|---|---|
| System Suitability Theoretical Plates (N) Tailing factor Retention time in minutes % Area |
21505 1.15 3.27 99.96 |
| LOD (μg/mL) LOQ (μg/mL) |
0.5 1.6 |
Table 2: Validation Summary.
Specificity
Specificity is the ability of a method to discriminate between the principal anlayte(s) of interest and additional excipients that are present in the sample. The degree of specificity testing varies depending on the method type and the stage of validation. The effect of wide range of intermediates and other precursors, generally used in in-house synthesis of Dapiprazole were investigated under optimized chromatographic conditions. Specificity is the ability to assess unequivocally the analyte in the presence of components which may be expected to be present. Typically these might include impurities, degradants, matrix, etc. The common reagents present in the synthetic route did not interfere with the elution or quantification of the method. Acceptance criteria for specificity, RSD should be less than 2%.
Linearity and range
The plot of peak area of each sample against respective concentration of Dapiprazole was found to be linear in the range of 20-120 μg/mL with correlation coefficient of 0.9999. Linear regression least square fit data obtained from the measurements are given in Table 3. The respective linear regression equation being y=1956.4x+3409. The regression characteristics, such as slope, intercept, and %RSD were calculated for this method and given in Table 3.
| Drug | Dapiprazole |
|---|---|
| Concentration range (μg/mL) | 20-120 |
| Slope (m) | 1956.4x |
| Intercept (b) | 3409 |
| Correlation coefficient | 0.9999 |
| % RSD | 0.566 |
Table 3: Linear Regression Data for Calibration curves.
Robustness
Robustness is a measure of the performance of a method when small, deliberate changes are made to the method conditions. The intent of this validation parameter is to identify the most critical method conditions to the successful performance of the method. The robustness of the method was assessed by deliberate alteration of the experimental conditions. One factor at a time was changed to study the effect. Variation of the detection wavelength by ± 2 nm (241 nm and 245 nm), the amount of acetonitrile in the mobile phase was varied by ± 10%), and mobile phase pH (± 2 pH-units) had no significant effect on the retention time and chromatographic response of the method, indicating that the method was robust. When the chromatographic conditions were deliberately altered, system suitability results remained within acceptance limits and selectivity for individual substance was not affected.
Ruggedness
Ruggedness is an older term that has been replaced by intermediate precision, according to the USP, is the degree of reproducibility of the results obtained under a variety of conditions, expressed as %RSD; which is a measure of how well the method performs under normal conditions form laboratory-to-laboratory, instrument-to-instrument, and analystto- analyst. A ruggedness test is performed as part of the validation of an analytical method. Ruggedness test was determined between two different analysts, instruments and columns. Small differences in areas and good constancy in retention times were observed. The high degrees of reproducibility of detector responses and retention times indicate that the method is fairly rugged.
From the typical chromatogram of Dapiprazole as shown in Figure 3, it was found that the retention time was 3.724 min. A mixture of methanol: acetonitrile: and ortho-phosphoric acid in the ratio of 89: 9:1 (v/v) at pH 5.8 as the mobile phase at a flow rate of 0.8 ml/min was found to be most suitable to obtain a peak well defined and free from tailing. In the present developed HPLC method, the standard and sample preparation required less time and no tedious extractions were involved. A good linear relationship (r2=0.9999) was observed between the concentration range of 20-120 μg/mL. Low values of standard deviation are indicative of the high precision of the method. The assay of Dapiprazole in bulk sample was found to be 99.94%. From the recovery studies it was found that about 99.06% of Dapiprazole was recovered which indicates high accuracy of the method. The absence of additional peaks in the chromatogram indicates non-interference of the common reagents and intermediates used in in-house synthesis of Dapiprazole in bulk drug production. This demonstrates that the developed HPLC method is simple, linear, accurate, sensitive and reproducible.
Thus, the developed method can be easily used for the routine quality control of Dapiprazole bulk sample within a short analysis time.
Conclusion
It can be seen from the results presented that the proposed procedure has good precision and accuracy. Results of the analysis of pharmaceutical formulations revealed that proposed methods are suitable for their analysis with virtually no interference of the usual intermediates and impurities formed during the in-house synthesis of Dapiprazole. This demonstrates that the developed HPLC method is simple, linear, accurate, sensitive and reproducible. Thus, the developed method can be easily used for the routine quality control of bulk forms of Dapiprazole within a short analysis time.
Acknowledgements
The authors are grateful to M/s M/s Bioleo Analytical Labs India Pvt. Ltd Hyderabad for the supply of Dapiprazole as a gift sample. One of us (KJP) is highly thankful to, Management of BCAS, Bapatla for giving her an opportunity to pursue this project under FDP. The authors also thankful to Q.S.Labs, Hyderabad for providing the necessary facilities to carry out the research work.
References
- Allinson RW, Gerber DS, Bieber S, Hodes BL (1990) Reversal of mydriasis by dapiprazole. Ann Ophthalmol 22: 131-133, 138.
- Allinson RW (1994) Rev Eyes: alpha blocker to reverse diagnostic mydriasis. Ocular Surgery News 19.
- Bonomi L, Marchini G (1987) The potential interest of alpha-adrenergic blocking agents in ophthalmology. Proceedings of second session of symposium 1-6.
- Bucci MG, D'Andrea D, Bettini A, De Gregorio M (1987) Dapiprazole for the reversal of mydriasis due to tropicamide. Glaucoma 9: 94-98.
- Buschmann W (1996) Dapiprazol - Erfahrungen in der Praxis. Augenspiegel 4: 14-18.
- Saettone MF, Alderigi C, Giannaccini B, Galli-angeli D (1989) Preparation and Evaluation of A Sustained-Release Ophthalmic Vehicle for Dapiprazole. Drug development and industrial pharmacy 15: 2621-2637.
- Valeri P, Palmery M, Severini G, Piccinelli D, Catanese B (1986) Ocular pharmacokinetics of dapiprazole. Pharmacol Res Commun 18: 1093-1105.
- Bianchi E, Segre G (1990) Ocular pharmacokinetics of radioactive dapiprazole after topical and parenteral administration. Euro J Pharmacol 183: 396.
- Than TP (1996) Comparison of the cycloplegic and mydriatic effects of 0.5% tropicamide and 2.5% phenylephrine to Paremyd and the counteracting effects of dapiprazole. Clinical Eye and Vision Care 8: 209-213.
- Marx-Gross S, Krummenauer F, Dick HB, Pfeiffer N (2005) Brimonidine versus dapiprazole: Influence on pupil size at various illumination levels. J Cataract Refract Surg 31: 1372-1376.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 18131
- [From(publication date):
August-2012 - Nov 17, 2025] - Breakdown by view type
- HTML page views : 13346
- PDF downloads : 4785