Research Article Open Access
Development and Validation of a Stability-Indicating HPLC Method for the Simultaneous Determination of Salbutamol Sulphate and Theophylline in Pharmaceutical Dosage Forms
Mukesh Maithani* and Ranjit SinghSchool of Pharmaceutical Sciences, Shobhit University, NH-58, Modipuram, Meerut, Uttar Pradesh (250 110), India
- *Corresponding Author:
- Dr. Mukesh Maithani
School of Pharmaceutical Sciences
Shobhit University,NH-58, Modipuram, Meerut
Uttar Pradesh (250 110), India
Tel:+91-9758860810
E-mail: mukeshmaithani@gmail.com
Received date: December 27, 2010; Accepted date: February 09, 2011; Published date: February 17, 2011
Citation: Maithani M, Singh R (2011) Development and Validation of a Stability- Indicating HPLC Method for the Simultaneous Determination of Salbutamol Sulphate and Theophylline in Pharmaceutical Dosage Forms. J Anal Bioanal Tech 1:116. doi: 10.4172/2155-9872.1000116
Copyright: © 2011 Maithani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The study describes development and subsequent validation of a stability indicating reverse-phase high- performance liquid chromatography method for the simultaneous estimation of salbutamol sulphate and theophylline in tablet dosage forms. A reversed-phase phenomenax C-18 column (250 mm × 8 mm i.d., particle size 10 μm) column with mobile phase consisting of acetonitrile and phosphate buffer 65:35 (v/v) (pH 4.2 ± 0.02, adjusted with triethylamine) was used. The flow rate was 1.2 mL min -1 and effluents were monitored at 235 nm. The retention times (t R ) of salbutamol sulphate and theophylline were found to be 5.33 min and 13.36 min, respectively. The method was validated in terms of linearity, range, specificity, accuracy, precision, limit of detection (LOD) and limit of quantitation (LOQ). The linearity for both the drugs was found in the range of 2-64 μg mL -1 . The % recoveries of salbutamol sulphate and theophylline were found to be 99.41 and 101.11, respectively. The utility of the procedure is verified by its application to marketed formulations that were subjected to accelerated degradation studies. The method distinctly separated the drug and degradation products even in actual samples. The products formed in marketed tablet dosage forms are similar to those formed during stress studies.
Keywords
Salbutamol sulphate; Theophylline; Stability-indicating method; RP-HPLC; Tablet dosage forms
Introduction
Salbutamol sulphate (SS) chemically, 4-[2-(tert-butylamino)-1- hydroxyethyl] - 2- (hydroxymethyl) phenol; sulfuric acid is a short acting β2-adrenergic receptor agonist used for the relief of broncho-spasm in conditions such as asthma and chronic obstructive pulmonary disease. Theophylline (TP) chemically, 1, 3-dimethyl-7H-purine-2, 6-dione also known as dimethylxanthine, is a methylxanthine drug used in respiratory diseases such as COPD and asthma [1-4]. The structures of SS and TP are shown in Figure 1. Numerous UV, HPLC, GC and HPTLC based methods have been reported for estimation of these drugs alone as well as in combination with other drugs in pharmaceutical dosage forms [5-24]. But no stability-indicating assay method has been reported for the simultaneous determination of SS and TP in the presence of their degradants using the ICH approach of stress testing. Therefore, the present work was aimed to develop a simple, rapid, precise, and accurate isocratic reversed-phase stability indicating HPLC method for simultaneous determination of SS and TP in the tablet dosage form. Stress testing under various conditions like hydrolysis (i.e., acid, base, and water), oxidation, heat, and photolytic degradation was carried out as per ICH guidelines [25-28]. Validation of developed analytical method was carried out as per ICH guidelines [29].
Experimental
Chemicals and reagents
Reference standards of SS and TP were procured as gift samples from Torrent Pharmaceutical, Gandhinagar, India. HPLC grade acetonitrile, water and triethylamine were obtained from Rankem, RFCL Limited, New Delhi, India. Potassium dihydrogen orthophosphate AR and ortho phosphoric acid AR grade were procured from Central Drug House (P) Limited, New Delhi, India.
Instrumentation
The HPLC (PerkinElmer series 200) instrument equipped with a model series 200 pump, vacuum degasser, rheodyne injector with a 20 µL loop, UV-Visible detector and C-18 column was used.
Chromatographic conditions
The isocratic mobile phase was consisted of acetonitrile and phosphate buffer 65:35 (v/v) (pH 4.2 ± 0.02, adjusted with triethylamine). The mobile phase was sonicated for 30 min and filtered through a 0.45 µ membrane filter paper. Flow rate of mobile phase was 1.2 mL min-1. The variable wavelength UV-visible detector was set at 235 nm. All analyses were performed at ambient temperature.
Preparation of standard stock solution
50 mg SS and 50 mg TP were accurately weighed and transferred to 100 mL volumetric flasks separately and dissolved in the mobile phase to give stock solutions of 500 µg mL-1 each of SS and TP.
Preparation of sample solution
Twenty tablets (Theo Asthalin Forte, Cipla Pharmaceuticals, India) were weighed and powdered finely. Tablet powder equivalent to 4 mg of SS and 200 mg of TP was transferred to a 100 mL volumetric flask and dissolved in 50 mL of mobile phase. The solution was ultrasonicated for 30 min and filtered through 0.45 micron membrane filter. The solutions were further diluted with mobile phase to obtain concentration of 2 µg mL-1of SS and 100 µg mL-1 of TP and were subjected to HPLC analysis as described earlier. From the peak area of SS and TP, the amount of drugs in samples was computed.
Forced degradation studies
From the previously mentioned stock solutions of standard drug and sample, 5 mL of aliquots were diluted separately up to 10 mL with 3% H2O2 (v/v), distilled water, 0.1 M HCl, and 0.1 M NaOH to achieve a concentration of 50 µg mL-1 and 100 µg mL-1 each of SS and TP, respectively. Solutions in water, 0.1M HCl, and 0.1M NaOH were heated at 80°C for 24 h. For oxidative degradation, drugs were stored at room temperature (RT) in 3% H2O2 (v/v) for 48 h. Degradation was also carried out in solid state by exposing pure drugs and drug product to dry heat at 80°C for 48 h. Photolytic studies were carried out by exposing a thin layer of solid SS and TP and their packaged (blister strip) and loose (removed from the blister pack) tablets placed in a Petri-dish as well as the solutions of drugs and samples in 0.1 M HCl, 0.1 M NaOH, and water to light in the photostability chamber for 30 days. Suitable controls were maintained under dark conditions. Samples were withdrawn initially and subsequently at prefixed time intervals. Samples were neutralized by either acid or alkali and were diluted with mobile phase to yield starting concentrations of 50 µg mL-1 and 100 µg mL-1 each of SS and TP, respectively. Appropriate blanks were injected before analysis of forced degraded samples.
Method Validation
Specificity
Specificity was tested against standard compounds and against potential interferences in the presence of placebo. No interference was detected at the retention time of SS and TP in sample solution.
Linearity
Linearity is studied to determine the range over which analyte response is a linear function of concentration. This study was performed by preparing standard solutions at seven different concentrations and analyses were performed in triplicate. The responses were measured as peak area. The calibration curves were obtained by plotting peak area against concentration.
Precision
The precision of an analytical method is the closeness of replicate results obtained from analysis of the same homogeneous sample. Precision was considered at two levels, i.e. repeatability and intermediate precision, in accordance with ICH recommendations. Repeatability, or intra-day precision, was determined by performing nine analyses at three concentrations on the same day. Intermediate precision was determined by analyzing the same sample in the same way on different days. Results from determination of repeatability and intermediate precision were expressed as SD and RSD.
Accuracy
The accuracy of an analytical method is the closeness of results obtained by that method to the true value for the sample. It is expressed as recovery (%), which is determined by the standard addition method. Samples were spiked with 80, 100, and 120% of the standard and analyzed. The experiment was performed in triplicate. Recovery (%) and RSD (%) were calculated for each concentration.
Limits of detection and limit of quantitation
The LOD and LOQ were separately determined on the basis of standard calibration curve. The residual standard deviation of the regression line or the standard deviation of y-intercepts of regression lines was used to calculate LOD and LOQ. Following formulae were used; LOD= 3.3×D/S and LOQ= 10×D/S, where, D is the standard deviation of the y-intercepts of regression line and S is the slope of the calibration curve.
Results and Discussion
Method development
Several mobile phase compositions were tried to resolve the peaks of SS and TP. The optimum mobile phase containing acetonitrile and phosphate buffer 65:35 (v/v) , (pH 4.2 ± 0.02, adjusted with triethylamine) was selected because it could resolve the peaks of SS (tR = 3.13±0.03 min) and TP (tR = 4.58±0.05 min) with a resolution factor of 7.51. Quantification was achieved with UV detection at 235 nm on the basis of peak area at 1.2 mL min-1 flow rate.
Method validation
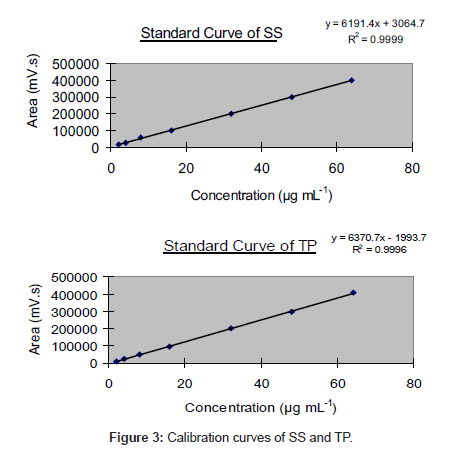
Linearity and range: Seven different concentrations (2, 4, 8, 16, 32, 48 and 64 µg mL-1) of the mixture of two drugs were prepared for linearity studies. A typical HPLC chromatogram obtained during simultaneous determination of SS and TP is given in Figure 2. The calibration curves obtained by plotting peak area against concentration showed linear relationship over a concentration range of 2-64 µg mL-1 for both the drugs. The linear regression equations for SS and TP were found to be y = 6191.4x + 3064.7 and y = 6370.7x - 1993.7, respectively. The regression coefficient values (R2) were found to be 0.9999 and 0.9996, respectively indicating a high degree of linearity. Calibration curves of SS and TP are shown in Figure 3. Regression characteristics of the proposed HPLC method are given in Table 1.
| Linearity experiment | SS | TP |
|---|---|---|
| Range (µg mL-1) | 2 – 64 | 2 – 64 |
| Regression coefficient (R2) | 0.9999 | 0.9996 |
| Slope | 6161.4 | 6370.7 |
| Intercept | 3064.7 | 1993.7 |
Table 1: Regression characteristics of the proposed HPLC method.
Figure 2: HPLC chromatogram obtained during simultaneous determination of SS and TP. Chromatographic conditions: Phenomenex C18 column (250 mm× 8 mm i.d., particle size 10 μm); flow rate 1.2 mL min-1; mobile phase acetonitrile and phosphate buffer 65:35 (v/v) (pH 4.2 ± 0.02, adjusted with triethylamine) and UV detection at 235 nm.
Specificity: The specificity studies proved the absence of interference, since none of the peaks appeared at the retention time of SS and TP. The interaction study in standard solution was also carried out by comparing peak of each drug individually and in drug mixture.
Precision: From the standard stock solutions, mixed standards containing SS and TP were prepared. Standard solutions (n=3) were injected using a universal rheodyne injector with injection volume of 20 µL. The intra-day and inter-day precisions were assessed by analyzing standard solutions. The % RSD was found to be between 0.42 and 0.38 for both the drugs. The lower values of % RSD indicate that the method is precise.
Accuracy: Recovery studies were carried out by applying the standard addition method. Known amounts of standard SS and TP corresponding to 80%, 100%, and 120% of the label claim were added to sample of tablet dosage form separately. The average % recoveries for SS and TP in marketed formulation were found to be 99.41 and 101.11, respectively. The results revealed that there was no interference of excipients. The results of accuracy are shown in Table 2.
| Drug | % simulated dosage nominal | % Mean (n=3) | ±SD | RSD (%) |
|---|---|---|---|---|
| SS TP SS TP SS TP |
80 80 100 100 120 120 |
99.59 101.95 99.19 101.51 99.09 99.87 |
0.92 0.15 0.25 0.82 0.31 0.35 |
0.18 0.12 0.40 0.81 0.56 0.42 |
Table 2: Percent recovery data.
Limit of detection (LOD) and limit of quantitation (LOQ): The limit of detection and limit of quantification were found to be 0.40 and 0.75 µg mL-1 for SS and 0.55 and 0.80 µg mL-1 for TP. The values indicate that the method is sensitive.
Degradation studies
Acidic conditions: Both the drugs were found to be labile to acid hydrolysis in 0.1M HCl at 80°C. It was observed that SS gradually degraded on heating at 80°C in 0.1M HCl for 24 h, forming degradation products showing retention time 7.62 and 12.32 min. TP showed higher degradation as compared to SS. At the end of 12 h, around 25% fall in TP peak area was observed. After refluxing for 24 h, drug was degraded by 60% with corresponding increase in concentration of the degradation products. The degradation products showed tR of 9.03, 10.08, and 11.11 min Figure 4A.
Figure 4: HPLC chromatogram of SS and TP obtained from degradation studies: acid hydrolysis (0.1 M HCl, 80°C, 24 h) (A); alkaline hydrolysis (0.1 M NaOH, 80°C, 24 h) (B); neutral hydrolysis (water, 80°C, 24 h) (C); oxidative degradation (3% H2O2, RT, 48 h) (D); thermal degradation (dry heat, 80°C, 48 h) (E); and photolytic degradation (photostability chamber, 30 d) (F). Chromatographic conditions: Phenomenex C18 column (250 mm × 8 mm i.d., particle size 10 μm); flow rate 1.2 mL min-1; mobile phase acetonitrile and phosphate buffer 65:35 (v/v) (pH 4.2 ± 0.02, adjusted with triethylamine) and UV detection at 235 nm.
Degradation in alkali: SS was found to be highly labile to alkaline hydrolysis. Around 60% degradation of the drug was observed in 0.1M NaOH at 80°C within 2 h. The degradation peaks appeared at tR 7.62 and 12.32 min whereas mild degradation was seen in TP in alkaline condition. It was observed that around 10-12% of the drug degraded on heating it in 0.1M NaOH for 24 h at 80°C. Two peaks were generated at 9.0 and 11.11min in the chromatogram Figure 4B.
Neutral (water) conditions: In neutral condition, SS was found to be relatively stable. Upon heating the drug solution in water at 80°C for 24 h, only minor degradation product at tR 7.62min was formed. On further heating up to 48 h, there was no rise in the proportion of the already degraded peaks. On the other hand, 10-15% degradation of TP was seen after heating for 24 h at 80°C with the generation of three minor peaks at around 6.52, 10.08, and 11.11min Figure 4C.
Oxidative conditions: SS was found to be relatively stable following exposure to oxidative condition (3% H2O2 at RT for 48 h) resulting in 4-5% degradation while TP was found to degrade more than 25%. Mild degradation was seen in SS with appearance of single peak at 7.62 min whereas the degradation products of TP appeared at tR 9.08 and 11.11 min Figure 4D.
Photolytic conditions: Mild decomposition was seen on exposure of SS and TP solid drug powder and their tablets to light in the photo stability chamber. The photolytic exposure (30 days) of SS in 0.1M HCl and 0.1M NaOH resulted in 45% and 12% degradation, respectively. On the other hand standard TP and its tablet were found to be more stable under acidic photolytic stress conditions, resulting in 25% decomposition. SS and TP API and the pharmaceutical tablets were found to be sufficiently stable under neutral photolytic degradation conditions. The major degradation peaks of TP appeared at tR 9.08 and 11.11 min while degradation peak was found at 7.62 min for the SS Figure 4F.
Analysis of marketed formulation
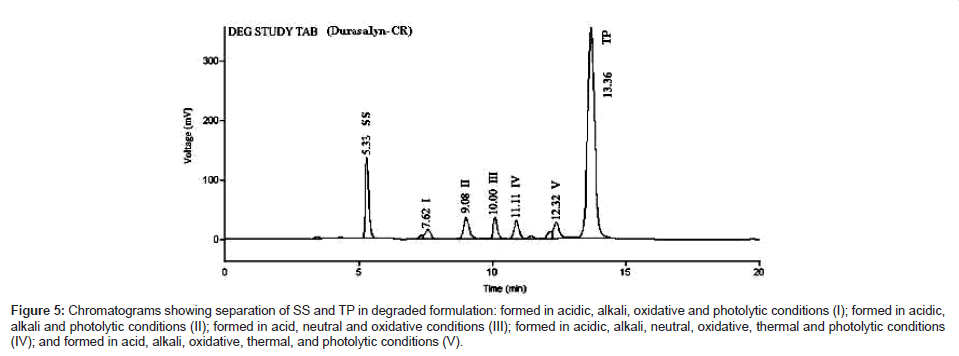
The developed method was successfully applied to analyze SS and TP in marketed tablet formulations. A clear separation of the drugs and degradation products was achieved in tablet with no interference from excipients Figure 5. Analysis of marketed tablets (Theo Asthalin Forte, Cipla Pharmaceuticals, India) was carried out using optimized mobile phase and HPLC conditions. The average % drug content of tablets obtained by the proposed method for SS and TP were found to be 99.85 and 100.75 respectively, which showed that the estimation of dosage forms was accurate within the acceptance level of 95% to 105%. The results are given in the Table 3.
Figure 5: Chromatograms showing separation of SS and TP in degraded formulation: formed in acidic, alkali, oxidative and photolytic conditions (I); formed in acidic, alkali and photolytic conditions (II); formed in acid, neutral and oxidative conditions (III); formed in acidic, alkali, neutral, oxidative, thermal and photolytic conditions (IV); and formed in acid, alkali, oxidative, thermal, and photolytic conditions (V).
| Drug | Label claim (mg/tablet) |
Quantity found (mg/tablet) (n = 3) |
RSD (%) | % Assay |
|---|---|---|---|---|
| Salbutamol sulphate | 4 | 39.94 | 0.29 | 99.85% |
| Theophylline | 200 | 201.50 | 0.38 | 100.75% |
Table 3: Analysis of marketed tablets.
System suitability parameters
For system suitability parameters, seven replicate injections of mixed standard solution were injected and parameters such as the resolution, capacity factor, tailing factor, theoretical plate, retention volume and asymmetry factor of the peaks were calculated. The results are shown in Table 4.
| Parameters | SS | TP |
|---|---|---|
| Retention time (min) | 3.13±0.03 | 4.58±0.05 |
| Resolution | 7.51 | |
| Capacity factor | 0.23 | 0.41 |
| Tailing factor | 1.2 | 1.1 |
| Theoretical plates | 15250 | 18001 |
Table 4: System suitability data.
Conclusions
The present study envisages the stability behavior of SS and TP individually and in combination as per the ICH guidelines. TP was found to be more susceptible under stress conditions in comparison to SS. The method was found to be accurate and precise with good and consistent recoveries at all levels studied. The good % recovery in tablet forms suggests that the excipients present in the dosage forms have no interference in the determination. The %RSD was also less than 2% showing high degree of precision of the proposed method. In addition, simple isocratic elution and easy extraction procedure offered rapid and cost-effective analysis of SS and TP. The proposed method can be used for routine analysis of SS and TP in combined dosage form. It can be also used in the quality control in bulk manufacturing.
Acknowledgements
The authors are grateful to Torrent Pharmaceutical (Gandhinagar, India) for providing gift samples of SS and TP.
References
- The Indian Pharmacopoeia (2007), The Controller of Publication, New Delhi, 390, 1735
- The British Pharmacopoeia (2008), The Stationary Office, London, 1518, 2116.
- The United States Pharmacopeia (2008), The United State Pharmacopoeial Convention, 3374.
- Current Index of Medical Specialties (2010), Medimedia Health Private Ltd. Bangalore, 144.
- Supriya S, Sheetal M, Kadam, VJ (2009) Validated stability indicating HPLC method for estimation of theophylline from a novel microsphere formulation. Asian J Pharm 3: 13-17.
- Ramakrishna VSN, Vishwottam NK, Manoj S, Koteshwara M, Devender RA (2007) A simple and rapid HPLC/UV method for the simultaneous quantification of theophylline and etofylline in human plasma. J Chrom B 848: 271-276.
- Nikola L, Dragica Z, Olgica S, Suzana , Igor K, et al. (2003) Development and validation of the HPLC method for the determination of theophylline serum concentration. Bul Chems Tech Maced. 22: 97-104.
- Ghulam M, Mahmood A, Muhammad AM, Muhammad WA (2009) A new reverse phase HPLC method with fluorescent detection for the determination of salbutamol sulfate in human plasma. Bull. Chem. Soc. Ethiop. 23: 1-8.
- Marcia SB, Marcia CV, Heloisa P, Rodolfo O, Jose NR, et al. (2002) Simultaneous determination of caffeine, theobromine, and theophylline by high performance liquid chromatography. J Chromat Sci 40: 45-48.
- Pai PNS, Rao GK, Murthy MS, Agarwal A, Puranik S (2009) Simultaneous determination of salbutamol sulphate and bromhexine hydrochloride in tablets by reverse phase liquid chromatography. Indian J Pharm Sci 71: 53-55.
- Basu M, Pathak B (1990) Estimation of salbutamol sulphate in pharmaceutical formulations. Indian Drugs 28:109-110.
- Bhatia NM, Jain DK, Trivedi P (1998) Simultaneous analysis of salbutamol sulfate and bromhexine hydrochloride from solid dosage form using multiwavelength UV-spectrophotometry. Indian Drugs 35: 566-569.
- Geetha N, Baggi TR (1989) Improved spectrophotometric method for the determination of salbutamol sulphate with 3-methyl benzothiazolin-2-one hydrazone. Microchem J 39: 137-144.
- Habib IHI, Hassouna MEM, Zaki GA (2005) Simultaneous spectrophotometric determination of salbutamol and bromhexin in tablets. Farmaco 60: 249-254.
- Hotchkiss SA, Caldwell J (1987) High-performance liquid chromatographic assay for theophylline and its major metabolites in human urine. J Chromatogr 423: 179-188.
- Kawakatsu K, Nishimura K, Kawai M, Chikuma M (1989) Separation and determination of theophylline from paraxanthine in human serum by reversedphase high-performance liquid chromatography. J Pharm Bio Anal 7: 965-973.
- Nirogi RV, Kandikere VN, Shukla M, Mudigonda K, Ajjala DR (2007) A simple and rapid HPLC/UV method for the simultaneous quantification of theophylline and etofylline in human plasma. J Chromat B Analyt Technol Biomed Life Sci 848: 271-276.
- Rao GR, Raghuveer S, Khadgapathi P (1987) High Performance liquid chromatographic determination of salbutomol sulfate and bromhexine hydrochloride in its combined dosage forms. Indian Drugs 25: 15-20.
- Ray S, Bandopadhyay A (1990) Reversed phase high performance liquid chromatographic determination of salbutamol sulphate in pharmaceutical formulations. Indian Drugs 27: 313-316.
- Reddy MN, Sankar DG, Rao GD, Sreedhar K (1991) Spectrophotometric determination of salbutamol and terbutaline. East Pharm 34: 127-128.
- Satinsky D, Karlicek R, Svaboda A (2002) Using on-line solid phase extraction for flow injection spectrophotometric determination of salbutamol. Anal Chim Acta 455: 103-109.
- Schack JA, Waxler SH (1949) An ultraviolet spectrophotometric method for the determination of theophylline and theobromine in blood and tissues. J Pharmacol Exp Ther 97: 283-291.
- Singh RA, Kumar D, Agarwal AK (2005) Simultaneous estimation of cetirizine hydrochloride and salbutamol sulphate in pharmaceutical dosage forms by reverse phase high performance liquid chromatography. Pharma Rev 3: 144- 146.
- Talwar N, Singhai AK, Shakya AK, Saraf S, Jain NK (1991) Different spectrophotometric determination of salbutamol sulphate in tablets. Indian Drugs 28: 244-245.ICH, Q1A (1993) Stability testing of new drug substances and products in Proceedings of the international conference on harmonization. Geneva, Switzerland.
- ICH, Q1A (1993) Stability testing of new drug substances and products in Proceedings of the international conference on harmonization. Geneva, Switzerland.
- ICH, Q2B (1996) Harmonised tripartite guideline, Validation of analytical procedure Methodology, International conference on harmonization.Geneva, Switzerland.
- ICH (2002) Guidance on analytical method validation, International convention on quality for the pharmaceutical industry. Toronto, Canada.
- ICH, Q1B (1996) Stability testing: photostability testing of new drug substances and Products. In International Conference on Harmonization. IFPMA, Geneva, Switzerland.
- ICH (1996) Validation of analytical procedures methodology ICH harmonized tripartite guidelines.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 20418
- [From(publication date):
January-2011 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 14944
- PDF downloads : 5474