Research Article Open Access
Development and Validation of a Liquid Chromatography Method for the Analysis of Paromomycin Sulfate and its Impurities
Ying Liu*, Shaorui Hou, Liping Wang and Shuxian YangHenan Provincial Institute for Food and Drug Control, Zhengzhou 450003, China
- *Corresponding Author:
- Ying Liu
Henan Provincial Institute for Food and Drug Control
Zhengzhou, China
Tel: 0086037163388265
Fax: 0086037163388265
E-mail: ying_leaf@263.net
Received date: September 21, 2010; Accepted date: September 29, 2010; Published date: October 01, 2010
Citation: Liu Y, Hou S, Wang L, Yang S (2010) Development and Validation of a Liquid Chromatography Method for the Analysis of Paromomycin Sulfate and its Impurities. J Anal Bioanal Tech 1:102. doi: J Anal Bioanal Techniques 2010, 1:102
Copyright: © 2010 Liu Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A rapid and simple method for the separation and quanti fi cation of paromomycin sulfate and its impurities by HPLC coupled with evaporative light scattering detection (ELSD) was developed. The chromatographic conditions included the use of a GRACE Alltima C 18 column (250mm×4.6mm, 5 μ m) maintained at 30°C and a mobile phase of 0.2 M tri fl uoroacetic acid water–acetonitrile (96:4, v/v) at a fl ow rate of 0.6 mL/min. The in fl uence of gas pressure and temperature of the drift tube in the detector on the detection response was also investigated. A system suitability test to check the quality of the separation was speci fi ed. The method showed good repeatability, linearity and robustness. It also enabled the simultaneous determination of the inorganic counter ions (sulfates).
Keywords
Paromomycin sulfate; Impurity; HPLC; ELSD
Introduction
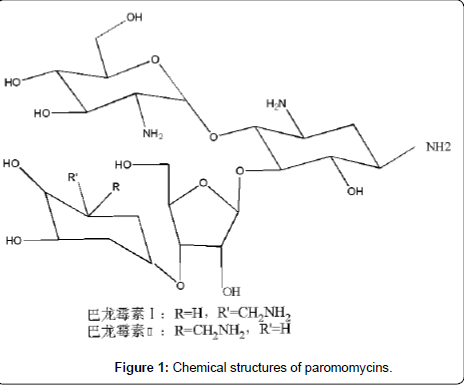
Paromomycin (PARO, synonym: aminosidine, Figure 1), a broad spectrum aminoglycoside antibiotic first isolated in 1956 from the fermentation of streptomyces [1], shows activity against both grampositive and gram-negative bacteria as well as some protozoa and cestodes. Although it is not used as an antibiotic any more, paromomycin has been approved since 2007 in India as an effective, well tolerated and affordable treatment for visceral leishmaniasis (VL) [2]. Recently it is being developed for use against multi-drug resistant strains of tuberculosis [3-4] and for the treatment of intestinal cryptosporidiosis in AIDS patients [5-8].
Various methods have been developed for the analysis of paromomycin, which include capillary zone electrophoresis [9] , capillary electrophoresis [10] , reverse-phase ion-pairing HPLC coupled with pulsed amperometric detection [11], LC-MS [12,13], the use of silver-coated gold nanoparticles as concentrating probes and matrices for surface-assisted laser desorption/ionization mass spectrometry [14], and gas-liquid chromatography. Aminoglycosides can be oxidized and therefore directly detected by amperometry. Pulsed amperometric detection (PAD) has broad linear range and very low detection limits for aminoglycoside antibiotics [15]. Like other aminoglycosides, paromomycin has no ultraviolet (UV) absorbing chromophores, and therefore the derivatization of the amino groups is required for high-performance liquid chromatography (HPLC) analyses with UV detection [16]. A HPLC method with fluorescence detection is also developed with post-column derivatization with ortho-phthalaldehyde (OPA) and 2-mercaptoethanol. Pre-column labeling with OPA is not used because of the low stability of the derivative [17]. Because of the thermal instability of aminoglycosides, the analysis of aminoglycosides by GC or gas-chromatography/mass spectrometry (GC/MS) is difficult [18]. The analysis of paromomycin by evaporative light-scattering detection (ELSD) has not been reported previously. ELSD has been applied as a universal detection method [19,20] when coupled with liquid chromatography. Different aminoglycosides generally produce the same response with ELSD detection. Therefore, the impurities can be easily quantified [21]. The present study developed and validated a specific, accurate and relative sensitive HPLC-ELSD method for determining paromomycin sulfate and its impurities. This method also enables the simultaneous determination of the inorganic counter ions (sulfates).
Experimental
Reagents and samples
HPLC grade trifluoroacetic acid (TFA) was purchased from Tedia (America). HPLC grade methanol and acetonitrile were purchased from Merck (Darmstadt, Germany). Paromomycin RS (batch no. 130371- 200305) and Neamine RS (Batch No. 85-1106) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products, China. Paromomycin samples (Batch No. 20050409-004, 20050412-005, and 20050420-007) were obtained from Kaifeng Pharmaceutical Company. All other chemicals and solvents were of the highest grade available.
Instruments and chromatographic conditions
The liquid chromatography system consists of a Waters 2695 liquid delivery unit quipped with a Waters 2424 evaporative lightscattering detector (Waters, USA). Another liquid chromatography system consists of a Waters 2695 liquid delivery unit equipped with a Alltech 2000 evaporative light-scattering detector (Alltech, USA). Empower chromatography manager (Waters, USA) was used for data processing.
The chromatographic separation was carried out using a GRACE Alltima C18 (250mm×4.6mm I.D., 5μm) column. The mobile-phase was 0.2 M trifluoroacetic acid water – acetonitrile (96:4). The flow rate was 0.6 mL/min, and the column temperature was maintained at 35°C. The nebulizing gas was high purity nitrogen. For Waters 2424 detector, a low temperature evaporative light scattering detector, the shift tube temperature was 60°C and the gas pressure was 30 psi.
Method Development and Validation
Selection of ion-pairing agents
Most of the mobile phases for the separation and detection of aminoglycoside antibiotics by HPLC-ELSD are based on TFAmethanol/ acetonitrile system [20-22]. Therefore, we selected TFA as the ion-pairing agent. Increasing of the concentration of the ionpairing agent resulted in the increase of the resolution between paromomycin and its nearest impurity. However, the HPLC system pressure was increased, and the high acid concentration shortened the column life. After testing a series of TFA concentrations, the optimal concentration was determined to be 0.2 M.
Selection of organic solvent in mobile phase
After selecting TFA as the ion-pairing agent, we tested methanol as the organic phase. However, paromomycin and the nearest impurity could not be separated efficiently. When acetonitrile was used as the organic phase, paromomycin and the nearest impurity were separated well. The final mobile phase was determined as 0.2 M trifluoroacetic acid water– acetonitrile (96:4).
Optimization of ELSD detection
The eluting mobile phase was nebulized and volatilized in the drift tube in ELSD. The temperature and the gas pressure were of critical importance to insure complete vaporization of the solvents. The nature of the gas used for the nebulization had been shown to play a key role in heat transfer and detection sensitivity [22]. The influence of the gas pressure and the drift tube temperature on paromomycin response was studied.
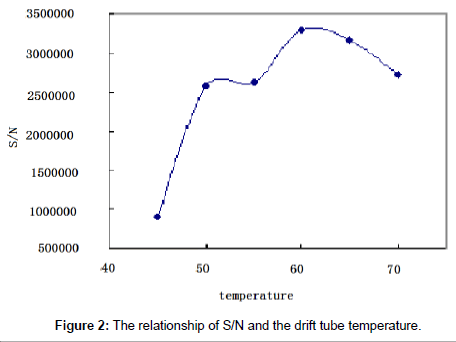
The drift tube temperature: Two sample solutions (500 and 2.25 μg/ml) were tested and software optimization was applied to maximize the peak areas and the signal-to-noise ratio. The optimal drift tube temperature was determined to be at 60°C (Figure 2).
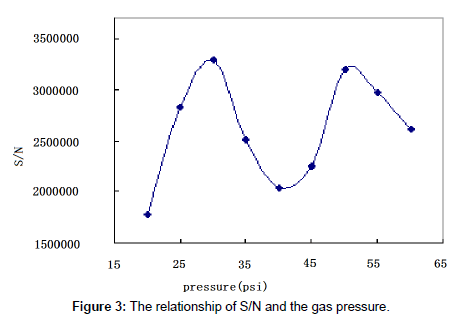
The gas pressure: In ELSD it is ideal to obtain the highest peak response with the lowest gas pressure. We tested the influence of gas pressure on peak area at the optimal drift tube temperature. The optimal gas pressure was determined to be 30 psi (Figure 3).
Selection of solvent to dissolve sample
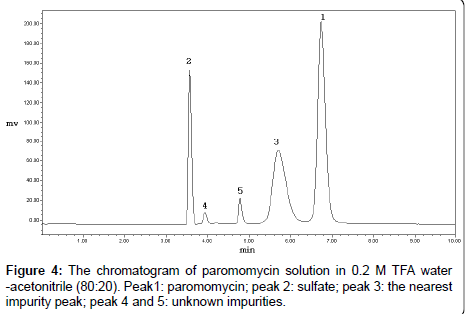
Paromomycin dissolved in the mobile phase was not stable and a new impurity appeared. The content of this impurity also changed with the ratios of 0.2 M TFA water to acetonitrile. When the ratio of 0.2 M TFA water and acetonitrile was 80:20, the content of the new impurity was the highest (Figure 4). Therefore, water was selected as the solvent to dissolve the sample. The RSD of paromomycin peak in the water solution was 1.1% (n =7) in 8 hours, indicating the water solution of paromomycin was stable.
Specificity
The impurities of paromomycin may have been carried over from its isolation and purification steps. Because paromomycin is produced by fermentation, the sample could contain other aminoglycosides such as neomycin and neamine, which have similar chemical structures. Therefore, the system suitability should be tested using the mixed solution of paromomycin, neomycin and neamine. However, our test showed that the major nearest impurity peak in paromomycin were not neomycin or neamine, and the resolution between neamine and paromomycin did not correlate with the results of the resolution between paromomycin and the nearest impurity. Therefore, it was not suitable to select the solution of neamine and paromomycin for system suitability test. We selected the 0.2 M TFA water-acetonitrile (80:20) solution of paromomycin as the system suitability solution for inspecting the specificity of the method (Figure 4). Furthermore, the specificity was also tested by the degradation solutions of paromomycin with acid, base, heat, illumination and hydrogen peroxide. The results showed that neomycin, neamine and other degraded impurities were well separated from the paromomycin peak.
System suitability
Paromomycin (0.5 mg/ml) degraded in 0.2 M TFA wateracetonitrile (80:20) was used as the system suitability solution (Figure 4). The resolution between paromomycin and the nearest impurity was defined.
Repeatability
The method repeatability was determined by carrying out six determinations at 100% of the test concentration (0.50 mg/ml). The relative standard deviation (RSD) was 0.8% for paromomycin, indicating high repeatability.
Intermediate precision
The intermediate precision was determined by six injections of the test solution (0.50 mg/ml) on 2 days. The inter-day precision was then calculated, and the relative standard deviation (RSD) was 1.5% for paromomycin.
Linearity
Light scattering is a complex process involving several mechanisms. It is usually described as a mixture of Rayleigh scattering, Mie scattering, diffraction and reflection. It is well known that ELSD gives no direct linear response [23]. The calibration curve was generated by preparing and analyzing nine calibration samples covering the concentration range of 23.6784.1 μg/ml. Each sample was injected in triplicate. The double logarithm linear equation for analysis of paromomycin was, y =1.3607 + χ7.3311, γ2 = 0.9997 indicating good linearity.
Limit of detection (LOD) and quantitation (LOQ)
A signal-to-noise ratio (S/N) of 3 is generally accepted for estimating the LOD, which is the lowest concentration that can be detected. The LOQ (S/N ≥10) is the lowest concentration of a substance that can be quantified with acceptable precision. The LOD and LOQ of paromomycin were 2.25 μg/ml and 25.5μg/ml respectively.
Robustness
Different columns such as GRACE Kromasil C18, Agilent Zorbax SBC 18, and Agilent Extend-C18 (250m×4.6mm. 5μm) were used, and the results were similar except that the peak response was a little larger with Agilent Zorbax SB-C18 and the peak response was a little smaller with GRACE Alltima C18.
Analysis of Commercial Samples
Determination of paromomycin
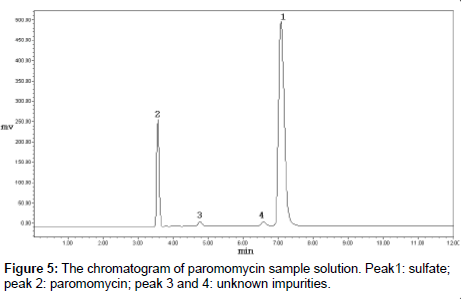
The commercial samples of paromomycin were analyzed by the developed HPLC-ELSD method (Figure 5). Samples from three different batches were dissolved and diluted with water to the appropriate concentrations. Paromomycin RS solutions of 0.2, 0.5 and 0.7 mg/ml were prepared, and a regression equation was made with logarithm of concentration to the logarithm of peak area correspondingly, and the correlation coefficient should not be less than 0.99. The concentrations of paromomycin in commercial samples were calculated with the regression equation. In the meantime, the potency was determined by the microbial assay with cylinder-plate method. Results are summarized in Table 1.
| Content of paromomycin (%) | Potency of paromomycin´╝?μ/mg´╝? | Content of sulfate (%) | |
| 20050409-004 | 64.8 | 654.1 | 26.4 |
| 20050412-005 | 66.0 | 668.1 | 25.2 |
| 20050420-007 | 68.8 | 686.9 | 26.0 |
Table 1: Analysis of paromomycin sulfate commercial samples.
Determination of sulfate
Sulfate was eluted before paromomycin and could be separated from the other peaks. Therefore, the content of sulfate could be determined by the standard curve method. Sulfate standard solutions at 0.075, 0.15, 0.20, and 0.50 mg/ml were prepared with sulfuric acid, and a regression equation was made with logarithm of concentration to the logarithm of peak area. The regression equation was y =1.7744 χ + 10.518, γ2 = 0.9992. The method was also validated. The liner range was 0.075–0.20mg/ml, and the accuracy was 1.35% (n =3). The contents of sulfate in commercial samples were calculated with the regression equation. Results are summarized in Table 1.
Discussion and Conclusion
The developed HPLC-ELSD method, using a reverse-phase C18 column, allowed the separation of paromomycin from its impurities. The nearest peak before paromomycin might be an enantiomer, and the other impurities were unknown. The experiments to identify these impurities by LC-MS are under way. The method was specific, robust, and fairly sensitive. Paromomycin sulfate was stable in water for 8 h, a time period that is sufficient for most assays. The method was used for the analysis of commercial samples, and it was also used to determine the inorganic counter ions (sulfates).
Paromomycin is a mixture of two isomers whose ratios vary from sample to sample [24]. The composition of paromomycin is not controlled in Chp2005 [25] and USP33 [26], but the isomers of aminoglycoside antibiotics have different antibacterial activity and toxicity. For example, the toxicity and the side effect of L and D neamine are different [27]. Paromomycin has paromomycin and isomers. Paromomycin is produced by fermentation, and the sample could contain other aminoglycosides such as neomycin and neamine. Moreover, impurities were carried over from its isolation and purification steps. Paromomycin II only possesses 32% of the antibacillus subtilis activity of paromomycin [28], and therefore the composition of paromomycin should be controlled. HPLC with evaporative light scattering detection appeared to be an efficient method for the determination of paromomycin and other minor components. The related substances also could be controlled by this method, and further study is needed.
We also used the Alltech 2000 ELSD, a high temperature evaporative light scattering detector. The influences of the gas pressure and the drift tube temperature on paromomycin response were also studied. The shift tube temperature was 100°C and the gas flow rate was 2.4 L/min. The Alltech 2000 ELSD has a better LOD than the Waters low temperature evaporative light scattering detector. Therefore, it is better to determine the related substances by using a high temperature evaporative light scattering detector such as Alltech 2000 ELSD.
Acknowledgements
Funded by the National Key New Drug R&D Program Foundation of China (No. 2011ZX0930).
References
- Lu J, Cwik M, Kanyok T (1997) Determination of paromomycin in human plasma and urine by reversed-phase high-performance liquid chromatography using 2,4-dinitrofluorobenzene derivatization. J Chromatogr B Biomed Sci Appl 695: 329-335.
- Davidson RN, den Boer M, Ritmeijer K (2009) Paromomycin. Trans R Soc Trop Med Hyg 103: 653-660.
- Kayok TP, Reddy MV, Chinnaswamy J, Danziger LH, Gangadharam PR (1994) Activity of aminosidine (paromomycin) for mycobacterium tuberculosis and mycobacterium avium. Antimicrob Chemother 33: 323-327.
- Li Lifen, Liu B, Li G (2005) Progress of antituberculous drug’s research. Journal of Datong Medical College 3: 36-39
- Danziger LH, Kanyok TP, Novak RM (1993) Treatment of cryptosporidial diarrhea in an AIDS patient with paromomycin. Ann Pharmacother 27:1460-1462.
- Fichtenbaum CJ, Ritchie DJ, Powderly WG (1993) Use of paromomycin for treatment of cryptosporidiosis in patients with AIDS. Clin Infect Dis 16: 298-300
- Stefani HN, Levi GC, Amato NV, Braz LM, Azevedo HD, et al. (1996) [The treatment of crytosporidiosis in AIDS patients using paromomycin]. Rev Soc Bras Med Trop 29: 355-357.
- Cirioni O, Giacometti A, Balducci M, Drenaggi D, Del Prete MS, et al. (1995) Anticryptosporidial activity of paromomycin. J Infect Dis 172:1169-1170.
- Ackermans MT Everaerts FM, Beckers JL (1992) Determination of aminoglycoside antibiotics in pharmaceuticals by capillary zone electrophoresis with indirect UV detection coupled with micellar electrokinetic capillary chromatography. J Chromatogr 606: 228-235
- Lin YF, Wang YC, Chang SY (2008) Capillary electrophoresis of aminoglycosides with argon-ion laser-induced fl uorescence detection. J Chromatogr A 1188: 331-333.
- Pastore P, Gallina A, Magno F (2000) Description and validation of an analytical method for the determination of paromomycin sulfate in medicated animal feeds. Analyst 125: 1955-1958.
- Oertel R, Neumeister V, Kirch W (2004) Hydrophilic interaction chromatography combined with tandem-mass spectrometry to determine six aminoglycosides in serum. J Chromatogr A 1058:197-201.
- Rona K, Klausz G, Keller E, Szakay M, Laczay P, et al. (2009) Determination of paromomycin residues in turkey tissues by liquid chromatography/mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 877: 3792-3798.
- Wang MT, Liu MH, Wang CR, Chang SY (2009) Silver-coated gold nanoparticles as concentrating probes and matrices for surface-assisted laser desorption/ ionization mass spectrometric analysis of aminoglycosides. J Am Soc Mass Spectrom 20:1925-1932.
- Szunyog J,Adams E, Roets E, Hoogmartens J (2000) Analysis of tobramycin by liquid chromatography with pulsed electrochemical detection. J Pharm Biomed Anal 23: 891-896.
- Lu J,Cwik M, Kanyok T (1997) Determination of paromomycin in human plasma and urine by reversed-phase high-performance liquid chromatography using 2,4-dinitrofl uoro benzene derivatization. J Chromatogr B Biomed Sci Appl 695: 329-335.
- Soltes L, Sebille B (1999) A simple chiral high-performance liquid chromatographic method to study the enantiomer-differentiating action of microorganisms. An assay with DL-tryptophan. Biomed Chromatogr13: 249- 251.
- Kennedy DG, McCracken RJ, Cannavan A, Hewitt SA (1998) Use of liquid chromatography–mass spectrometry in the analysis of residues of antibiotics in meat and milk. J Chromatogr A 812: 77-98.
- Roda A, Gatti R, Cavrini V, Cerre C, Simoni P (1993) HPLC study of the impurities present in different ursodeoxycholic acid preparations: comparative evaluation of four detectors. J Pharm Biomed Anal 11: 751-760.
- Sas B, Peys E, Helsen M (1999) Effi cient method for (lyso) phospholipid class separation by high-performance liquid chromatography using an evaporative light-scattering detector. J Chromatogr A 864: 179-182.
- Wang Mingjun,Hu Changqin. Jin Shao-hong (2002) Analysis of the response factors of different aminoglycoside antibiotics detected by evaporative lightscattering detector. Yao Xue Xue Bao 37: 204-206.
- Mengerink Y, de Man HCJ, Van der Wal SJ (1991) Use of an evaporative light scattering detector in reversed-phase high-performance liquid chromatography of oligomeric surfactants. J Chromatogr A 552: 593-604.
- Petritis K, Elfakir C, Dreux M (2002) A comparative study of commercial liquid chromatographic detectors for the analysis of underivatized amino acids. J chromatogr A 961: 9-21.
- Olson LL, Pick J, Ellis WY, Lim P (1997) A chemical assessment and HPLC assay validation of bulk paromomycin sulfate. J Pharm Biomed Anal 15: 783- 793.
- Chp, (2005) 2: 723.
- U.S. Pharmacopeia, (2009) 32: 3213.
- Ryu DH,Litovchick A, Rando RR (2002) Stereospecificity of aminoglycosideribosomal interactions. Biochemistry 41: 10499-10509.
- Reuter G, Liebermann B, Koester H (1975) Physiology and biochemistry of streptomycetes. 1. Isolation identification, and studies on the microbial synthesis of stereoisomers in the paromomycin complex. Pharmazie 30: 733- 736.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 19185
- [From(publication date):
October-2010 - Dec 06, 2025] - Breakdown by view type
- HTML page views : 13991
- PDF downloads : 5194