Research Article Open Access
Development and Validation of a High Performance Liquid Chromatography Method for Determination of Telmisartan in Rabbit Plasma and its Application to a Pharmacokinetic Study
Prasad S. Virkar*, Satish G. Pingale and Kiran V. Mangaonkar
Department of Chemistry, Mithibai College of Arts, Chauhan Institute of Science and Amrutben Jivanlal College of Commerce and Economics, Vile Parle (W), Mumbai 400056, India
- *Corresponding Author:
- Prasad S. Virkar
Department of Chemistry
Mithibai College of Arts
Chauhan Institute of Science and Amrutben
Jivanlal College of Commerce and Economics
Vile Parle (W), Mumbai 400056, India
Tel: +91-9867605952
E-mail: prasadvirkar@rediffmail.com
Received date: March 03, 2012; Accepted date: May 26, 2012; Published date: May 30, 2012
Citation: Virkar PS, Pingale SG, Mangaonkar KV (2012) Development and Validation of a High Performance Liquid Chromatography Method for Determination of Telmisartan in Rabbit Plasma and its Application to a Pharmacokinetic Study. J Anal Bioanal Tech 3:133. doi: 10.4172/2155-9872.1000133
Copyright: © 2012 Virkar PS et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A simple and sensitive High-Performance Liquid Chromatography (HPLC) method with fluorescence detection for quantitation of telmisartan in rabbit plasma was developed and validated. Separation of telmisartan from plasma components was achieved on a Chromolith RP18e column (100 x 4.6 mm 5 μ). Losartan was used as an internal standard. A mobile phase consisting of 50 mM sodium phosphate buffer and acetonitrile in the volume ratio of 65:35 v/v was delivered at a flow rate of 3.5 mL/min. A simple and rapid sample preparation involved solid phase extraction using Varian Bond Elute C-18 cartridge. The linearity range of the proposed method was 4 to 375 ng/mL. The intra-day and inter-day coefficients of variation obtained for telmisartan were less than 4.90% and relative error was less than 9.08%. The overall recoveries for telmisartan and losartan were 101.7% and 100.0%, respectively. This validated method was used successfully for analysis of plasma samples from a pharmacokinetic study.
Keywords
Column liquid chromatography; Fluorescence; Telmisartan in rabbit plasma; Pharmacokinetic study
Introduction
Telmisartan is an angiotensin II receptor antagonist used in the management of hypertension. High blood pressure is quantitatively the largest single risk factor for premature death and disability due to changed life style. Despite considerable success in treatments, hypertension still remains one of the greatest public health problems.
Telmisartan,2-(4-{[4-methyl-6-(1-methyl-1H-1,3-benzodiazol- 2-yl)-2-propyl-1H-1,3-enzodiazol-1-yl]methyl}phenyl) benzoic acid (Figure 1) shows high affinity for the angiotensin II type 1, with a binding affinity 3000 times greater for AT1 [1,2]. It has a longer half life than any Angiotensin Receptor blocker (ARB - 24 Hrs) and has the largest volume of distribution [3].
Several HPLC-UV [4-7], LC-MS/MS [8-17] and HPLC-Fluorescence [18-31] methods have been reported for quantification of telmisartan in biological fluids.
Most of the bio-analytical methods involve liquid-liquid extraction [18-20,22,26,28,32,33], while few methods involve protein precipitation [25,34] for sample preparation.
Few bio-analytical methods are reported for the quantification of telmisartan in plasma using solid phase extraction [17,35].
The method reported by Kuang R et al. [35], is an HPLC method with fluorescent detection, for the determination of telmisartan concentration in human plasma. Telmisartan from plasma was extracted using solid phase extraction method. A Diamonsil C18 (100 x 4.6 mm, 5 μ) column was used. The mobile phase consisted of acetonitrile: potassium dihydrogen phosphate buffer solution, and its pH was adjusted to 3.74 using orthophosphoric acid, (61:39 v/v), at a flow rate of 1 mL/ min. Candesartan was used as the internal standard (IS). The fluorescence detector was operated at excitation wavelength of 305 nm and emission wavelength of 365 nm. The calibration curve of telmisartan was linear in the range of 0.5-144 ng/mL (r2=0.9998).The absolute recovery was 80%-86%, and the relative recovery was 102%-109%.The intra- day and inter-day precision were found to be 4.23-9.80% and 4.03- 9.95%, respectively. This method possesses merits of good sensitivity, accuracy and simplicity and is suitable for pharmacokinetics studies of telmisartan in humans.
The method reported by Torrealday N et al. [17], is a HPLC-fluorescence method of telmisartan in urine, using a Novapak C18 column (100 x 3.9 mm, 4 μ).The mobile phase consisting of a mixture of ace-tonitrile: 5 mM phosphate buffer-pH 6.0 (45: 55, v/v) was pumped at a flow rate of 0.5 mL/min. The effluent was monitored at excitation and emission wavelengths of 305 and 365 nm, respectively. The method utilizes solid-phase extraction procedure with C8 cartridges and methanol as an eluent. The method has LOQ of 1 ng/mL with a run time of 5 minutes but a poor peak shape was observed in the urine matrix.
A high throughput protein precipitation method followed by HPLC- Fluorometry detection has been reported by H. Zhang et al. 36]. This method has a linearity range of 1 -200 ng/mL with a run time of 2 minutes. The linearity domain not being high enough is the disadvantage of the method reported by H. Zhang et al. [36]. The dilution integrity and analysis of the samples were performed outside the validated assay range. A poor peak shape was also observed for the LOQ level using this method.
The present method reports analysis of telmisartan in plasma using Chromolith column. Chromolith column, which was introduced recently, offers a number of advantages such as higher flow rate , therefore quick separations and better resolution resulting in higher sample throughput.
The present paper describes a simple and reproducible HPLC-fluorescence method using solid phase extraction to detect plasma concentration in rabbits. The method described here is found to be rapid, sensitive, precise and accurate. It was successfully applied for pharmacokinetics study of telmisartan in rabbit.
Experimental
Materials
Telmisartan (99.0%) was provided by Glenmark pharmaceuticals (Navi Mumbai, India) while losartan was provided by Dr. Reddy’s laboratory (Hyderabad, India). HPLC grade acetonitrile, methanol and GR grade sodium phosphate were purchased from Merck (Mumbai, India). Varian Bond Elute C -18 cartridge (1 cc/30 mg) was purchased from Varian (Mumbai, India). High purity water was prepared in-house, using a Milli-Q water purification system obtained from Millipore (Bangalore, India).
Instrumentation and chromatographic conditions
HPLC system consisted of HPLC Agilent 1200 along with same series column thermostat, pump, auto sampler and (Fluoresence Detector) FLD Detector. The fluorescence detector was set at an excitation wavelength of 259 nm and emission wavelength of 399 nm. The other instrumental parameters, gain and attenuation, were set at normal values.
A Chromolith RP 18e, 100 x 4.6 mm, 5 μ (Merck, Mumbai, India) column was used to perform separation. The column oven temperature was maintained at 45°C during analysis. 50 mM sodium phosphate buffer, with pH adjusted to 3.8 using ortho-phosphoric acid was used as mobile phase A, while Acetonitrile was used as mobile phase B. The mobile phase was run under isocratic condition in the ratio of 65:35 v/v (mobile phase A: mobile phase B) at the flow rate of 3.5 mL/min. The diluent used was a mixture of methanol and water in the ratio of 50:50 v/v. The Injection volume was 5 μL and the auto sampler temperature was maintained at 10°C.
Calibration curve and quality control samples
A stock solution of telmisartan was prepared at the concentration of 100 μg/mL in diluent and further dilutions were made at the concentration of 10 μg/mL and 1 μg/mL for working solutions. A stock solution of losartan (Internal standard) was prepared at the concentration of 300 μg/mL. An eight point calibration curve was prepared, by spiking the blank plasma with an appropriate amount of telmisartan, to obtain concentrations of 4, 12, 50, 100, 125, 200, 300 and 375 ng/mL of telmisartan. Quality control (QC) samples 4, 12, 125 and 300 ng/mL were independently prepared in the same manner.
Extraction procedure
250 μL of rabbit plasma was taken in a 5 mL vial. 25 μL of stock solution of 300 μg/mL losartan (Internal standard) was added to it and vortexed for 2 minutes. 750 μL of 0.5% acetic acid (v/v) was added to it and the mixture was vortexed for 2 minutes. It was then centrifuged at 4600 rpm for 15 minutes at 15°C. The Sample mixture was loaded into a Varian Bond Elute C18 extraction cartridge, pre-conditioned with 1 mL methanol followed by 1 mL water. The extraction cartridge was washed with 1 mL of water, followed by 1 mL of 5% methanol. It was then eluted with 1 mL of water:methanol (1:1 v/v) into a test tube.
The solution was evaporated at 40°C under nitrogen stream. The residue was then reconstituted with 250 μL of mobile phase. This solution was transferred into an auto sampler vial and injected into the HPLC.
Validation
The validation was performed as per USFDA guidelines [37]. Calibration data was generated by spiking blank plasma samples with appropriate volume of stock solutions of telmisartan yielding 4, 12, 50, 100, 125, 200, 300 and 375 ng/mL. The calibration curves were represented by plots of the peak area versus concentrations and were fitted to the linear regression equation Y= mX + C.
The limit of quantification is defined as the lowest concentration that can be measured with acceptable precision and accuracy. A maximum intra-day coefficient of variation of 20% and a maximum deviation from nominal value of 20% were allowed. It was determined by using seven samples spiked with appropriate volume of telmisartan working solution.
The Intra-day and Inter-day variability of Telmisartan were assayed (n=7) at 4, 12, 125 and 300 ng/mL on the same day and on different days respectively. Precision was characterized by the percent coefficient of variation (%CV) whereas accuracy was expressed as a percentage relative error (%RE) of nominal versus measured concentration. The limits of variability were set at 15% except for the limit of quantitation level, which should be less than 20%.
The autosampler sample stability was evaluated by comparing stored samples (at 10°C for 18 hours.) in an autosampler, with freshly prepared samples at LQC and HQC level. The freeze–thaw stability was conducted by comparing the stability samples that had been frozen at -20°C and thawed three times, with freshly spiked quality control samples. Seven aliquots of each, low and high concentration were used for the freeze–thaw stability evaluation.
The extraction efficiencies of telmisartan and losartan were determined, by analysis of seven replicates at low and high quality control concentrations for telmisartan and losartan. The percent recovery was evaluated, by comparing the peak area of extracted analytes to the peak area of non extracted standards [38,39].
Pharmacokinetics study
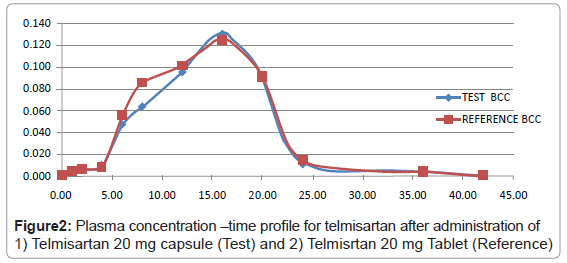
The plasma concentration-time profile, after a single dose of delayed release 20 mg telmisartan capsule, along with a 20 mg telmisartan tablet Telsar 20 (Make: Unichem) was determined. The study was conducted after approval from an independent ethics committee. The results of the pharmacokinetic study for the capsule and tablet formulation are provided in Table 4. The Cmax for the prepared capsule formulation was found to be 131 ng/mL while the Cmax for the marketed formulation was found to be 125 ng/mL. Similarly the Tmax for the formulated capsule formulation as well as the marketed formulation was found to be 16 Hrs.
Results and Discussions
Method development
During method development different options were evaluated to optimize detection parameters, chromatography and sample extraction.
Optimization of detector condition: The existence of several fluorescent functional groups in the molecular structure of telmisartan makes fluorescence as the obvious choice for detection. It has an advantage of minimizing the interfering responses from other substances present in the matrix. The wavelengths were standardized based on the optimum response obtained at those conditions.
Chromatography: Initially, mobile phases containing acetic acid solution and acetonitrile in varying combinations were tried. Poor peak shape was observed in these experiments. The mobile phase containing 0.2% formic acid in water: acetonitrile (20:80 v/v) and 0.2% formic acid in water: methanol (20:80 v/v) exhibited better separation, but the response was very low, and was insufficient to quantify LOQ for telmisartan. The best signal for telmisartan and losartan (IS) was obtained using an optimized mobile phase. The optimized mobile phase consisted of a phosphate buffer (50 mM sodium phosphate, adjusted to pH 3.8with orthophosphoric acid) and acetonitrile in the ratio of 65:35. A two-fold increase in its area count was observed, when compared with the mobile phase containing 0.2% formic acid in water : acetonitrile (20:80 v/v) and 0.2% formic acid in water : methanol. Moreover, a marked improvement in the peak shape of telmisartan and losartan was also observed using this mobile phase. Columns such as, Inertsil C18 (50 x 4.6 mm, 5 μ), Symmetry Shield RP18 (50 x 2.1 mm, 3.5 μ), HyPURITY C18 (50 x 4.6 mm, 5 μ), Nova-pak C18 column (100 x 3.9 mm, 4 μ) and Chromolith RP 18e (100 x 4.6 mm, 5 μ) were tried during the method development. Poor chromatography was observed in matrix sample, using columns like Inertsil C18, Nova-pak C18 and Symmetry Shield RP18. HyPURITY C18 gave a relatively good peak shape but the response was low. The best signal was obtained using the Chromolith RP 18e, 100 x 4.6 mm, 5 μ column, which gave satisfactory peak shapes, for all the analytes and a flow rate of 3.5 mL/min reducing the run time to as low as 4.5 min. The fluorescence detector was able to detect analytes with high selectivity. The column oven temperature was kept at a constant temperature of about 45°C.
Extraction: Prior to LC injection, the co-extracted proteins should be removed from the sample. Several organic solvents were employed to extract analytes from the plasma sample. Out of the tested solvents (methyl tertiary butyl ether, ethyl acetate, dichloromethane, chloroform, hexane and acetonitrile) acetonitrile, precipitated proteins more effectively than other solvents, but the extraction efficiency was low in acetonitrile precipitation. In the state of nonionic forms, the strong binding of analytes to the copolymer of the SPE cartridge, enabled sufficient clean-up and suitable eluting solution helped to elute analytes with more efficiency. So the method was optimized to achieve maximum extraction efficiency. The high recovery and selectivity was observed in the solid phase extraction method, which was used in the present work. These optimized detection parameters, chromatographic conditions and extraction procedure resulted in reduced analysis time with accurate and precise detection of telmisartan in matrix.
Method Validations
Selectivity
Selectivity was performed, by analyzing the rabbit blank plasma samples from seven different sources to test for interference at the retention time of telmisartan and internal standard losartan. No interference was observed, at the retention times of analyte as well as the internal standard. Seven replicates of extracted samples at the LOQ level in the plasma sample were prepared and analyzed. The % RSD of the area ratios of these seven replicates of samples was 5.68% for telmisartan, confirming that interference does not affect the quantification at LOQ level. Utilization of selected wavelengths for both compounds enhanced fluorometric selectivity. Hence it is concluded to be specific for telmisartan and losartan.
Linearity
The peak area ratios of calibration standards were proportional to the concentration of analytes in each assay, over the nominal concentration range of 4-375 ng/mL for telmisartan. The calibration curve appeared linear and was well described by least-squares linear regression lines. The correlation coefficient was 0.999 for telmisartan. The deviation of the back calculated values from the nominal standard concentrations were less than 15% except LOQ, where it should be less than 20%.
Sensitivity
The LOQ for telmisartan was 4 ng/mL. The intra-run precision of the LOQ plasma samples containing telmisartan was ≤ 3.17. The intrarun accuracy (%RE) of the LOQ plasma samples containing telmisartan was -9.08. These details are illustrated in Table 1 along with the data for other quality control samples tested during the precision and accuracy exercise. Cmaxof 131 ng/mL was obtained for telmisartan. As per guidelines, LOQ should be below 5% of Cmax. So for telmisartan, the LOQ was satisfactory. All the values obtained below 4 ng/mL for telmisartan were excluded from statistical analysis as they were below the LOQ values.
| Spiked concentration ng/mL | Mean calculated concentration ng/mL | %RSD | %RE |
|---|---|---|---|
| 4.004 | 3.64 | 3.17 | -9.08 |
| 12.01 | 11.38 | 4.90 | -5.18 |
| 125.12 | 123.4 | 2.67 | -1.28 |
| 300.30 | 298.23 | 2.66 | -0.59 |
Table 1: Intra-run precision and accuracy (n=7 for telmisartan)
Precision and accuracy
The intra-run precision and accuracy were determined, by pooling all individual assay results of replicate (n = 7) quality control of two separate batch runs analyzed on the same day. The intra-run precision was ≤ 3.17% at (Lower Limit of Quantification) LLOQ level. The intrarun accuracy (%RE) at LLOQ level (Table 1) was -9.08. The inter-run precision and accuracy were determined, by pooling all individual assay results of replicate (n = 7) quality control over three separate batch runs, analyzed on 3 different days. The inter-run precision was 3.81% at LLOQ level. The inter-run accuracy (%RE) at LLOQ level was -8.18, (Table 2). All these data indicate that the method is precise and accurate.
| Spiked concentration ng/mL | Mean calculated concentration ng/mL | %RSD | %RE |
|---|---|---|---|
| 4.004 | 3.67 | 3.81 | -8.18 |
| 12.01 | 11.44 | 3.25 | -4.65 |
| 125.12 | 121.73 | 2.09 | -1.02 |
| 300.30 | 289.90 | 1.92 | -0.37 |
Table 2: Inter-run precision and accuracy (n=7 for telmisartan)
Recovery
Seven aqueous replicates, at low and high quality control concentration levels for telmisartan were prepared for recovery determination, and the area ratios obtained were compared versus the area ratios obtained for extracted samples of the same concentration levels, from a precision and accuracy batch run on the same day. The mean recovery for telmisartan was 101.74 with a precision of 4.11%. The mean recovery for losartan was 100.0% with a precision of 2.62%. The recovery obtained for telmisartan was higher than that of other reported methods.
Matrix effect
The assessment of matrix effect constitutes an important and integral part of validation for quantitative methods for supporting pharmacokinetics studies. It was performed by processing plasma samples in quadruplet (n = 4). LQC and HQC working solutions were spiked post extraction in duplicate. The results obtained were well within the acceptable limits, as the %RSD of the area ratios of post spiked recovery samples at LQC were 1.85 and at HQC were 0.38 which were within 10%. Hence minor suppression or enhancement of analyte signal due to endogenous matrix interferences did not affect the quantification of analytes and IS peak.
Stability
Bench top, post preparative, Long term stability and Freeze thaw cycle for telmisartan were investigated at LQC (12 ng/mL) and HQC (300 ng/mL). The results revealed that telmisartan was stable in plasma for at least 6 hrs at room temperature, 48 hrs post preparation. It was confirmed that repeated freezing and thawing (three cycles) of plasma samples spiked with telmisartan at LQC and HQC levels did not affect their stability. The long term stability results also indicated that telmisartan was stable in matrix upto 15 days at a storage temperature of -20°C. The results obtained from all these stability studies are tabulated in Table 3 for telmisartan.
| Stability | Spiked final Conc ng mL-1 | Mean | SD | %RSD | %RE |
|---|---|---|---|---|---|
| ST | 11.6 | 11.3 | 0.27 | 2.38 | -2.82 |
| 300 | 300.1 | 1.85 | 0.62 | -0.2 | |
| PP | 11.6 | 11.5 | 0.21 | 1.83 | -0.92 |
| 300 | 299.5 | 1.04 | 0.35 | -0.19 | |
| FT | 11.6 | 11.6 | 0.24 | 2.06 | 0.02 |
| 300 | 298 | 1.3 | 0.44 | -0.52 | |
| LT | 11.7 | 12.4 | 0.42 | 3.35 | 6.61 |
| 300 | 305.8 | 1.6 | 0.53 | 0.53 |
ST: Short term stability – 6Hrs; PP: Post preparative stability (48Hrs)
FT: freeze thaw – 3 cycle LT: Long term (15 Days)
Table 3: Stability results for Cyc (n=6)
| Parameter | Value for test formulation | Value for the reference formulation |
|---|---|---|
| Cmax | 0.131 | 0.125 |
| Tmax | 16 | 16 |
| T1/2 | 4.0027392 | 4.7905686 |
| AUC(0-last) | 1.735 | 1.8375 |
| AUC(0-infinity) | 1.7407747 | 1.8444113 |
Table 4: Results of pharmacokinetic analysis
Application
The validated method has been successfully used to quantitate telmisartan concentrations in rabbits, after administration of a single combination dosage form (Tablet and Capsule containing 20 mg telmisartan as an oral dose). The statistical data evaluated were Cmax (maximum observed drug concentration during the study), AUC0- t (area under the plasma concentration–time curve measured to the last quantifiable concentration, using the trapezoidal rule), AUC0–inf (AUC0-t plus additional area extrapolated to infinity, calculated using the formula AUC0-t + Ct/Kel, where Ct is the last measurable drug concentration), Tmax (time to observe maximum drug concentration). The mean Cmax data obtained, justified the LOQ levels selected, as they are at least less than five half-lives of the obtained Cmax values. The mean Cmax observed for telmisartan, for test and reference formulations were 131 and 125 ng/mL, respectively (Figure 2). The corresponding mean Tmax for telmisartan, for test and reference formulations were 16.0 h. The mean AUC0-t for telmisartan, for test and reference formulations were 1735 and 1837 ng x h/mL, respectively while AUC0–inf were 1740 and 1844 ng x h/mL, respectively. The 90% confidence intervals of the ratios of means Cmax, AUC0-t and AUC0–inf all fell within the acceptance range of 0.8–1.25, demonstrating the bioequivalence of the two formulations of telmisartan.
Conclusions
Although, many other researchers have provided breakthrough methods for quantification of telmisartan, as can be seen from the references listed, we provide another alternative, simple, specific, sensitive and rapid method for estimation of telmisartan from rabbit plasma. The method provided excellent Selectivity and linearity with a limit of quantification of 4 ng/mL, and has been successfully applied to pharmacokinetic study.
References
- United States Pharmacopoeia Convention Inc (2006) The United States Pharmacopoeia, Asian edition. (29thedn), Rockville, Maryland.
- Hermida RC, Ayala DE, Fernandez JR, Calove C (2007) Comparison of the Efficacy of Morning Versus Evening Administration of Telmisartan in Essential Hypertension. Hypertension 50: 715-722.
- Benson SC, Pershadsingh H, Ho C, Chittiboyina A, Desai P, et al. (2004) Hypertension 43: 993.
- Shao Z, Wang T, Shang Z, Lu D (2007) Determination of telmisartan in human plasma concentration by HPLC. Xuzhou Yixueyuan Xuebao 27: 669-671.
- Shen J, Jiao Z, Li ZD, Shi XJ, Zhong MK (2005) HPLC determination of telmisartan in human plasma and its application to a pharmacokinetic study. Pharmazie 60: 418-420.
- Wen Y, Wu Y, Liu Y, Ma C (2006) Determination of telmisartan content in blood plasma by HPLC. Zhongguo Linchuang Yaoxue Zazhi 15: 245-247.
- Zhao X, Xu J; Liu W (2003) RP-HPLC determination of telmisartan in rat plasma. Zhongguo Yaoke Daxue Xuebao 34: 247-249.
- Yan T, Li H, Deng L, Guo Y, Yu W, et al. (2008) Liquid chromatographic-tandem mass spectrometric method for the simultaneous quantitation of telmisartan and hydrochlorothiazide in human plasma. J Pharm Biomed Anal 48: 1225-1229.
- Hempen C, Glaesle L, Kunz U, Karst U (2006) Determination of telmisartan in human blood plasma. Anal Chim Acta 560: 41-49.
- Li P, Wang Y, Wang Y, Tang Y, Fawcett JP, et al. (2005) Determination of telmisartan in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatograph B: Analyt Technol Biomed Life Sci 828: 126-129.
- Peng X, Zou J, Liu S, Li B, Wang X, et al. (2006) Determination of telmisartan in human plasma by HPLC mass spectrometry and studies on its pharmacokinetics and relative bioavailability. Zhongguo Linchuang Yaoxue Zazhi 15: 200-203.
- Tan W, Liu C, Huang D, Ding L, Xiao H, et al. (2005) Rapid determination of telmisartan in human plasma and study of bioequivalence of its tablets in hea. Yaoxue Jinzhan 29: 78-82.
- Liu S, Liu Y, Peng X, Zou J, Xiao D (2005) Study on pharmacokinetics and bioequivalence of telmisartan in human plasma by HPLC-MS. Yaowu Fenxi Zazhi 25: 519-522.
- Chen B, Liang YZ, Wang Y, Deng F, Zhou P, et al. (2005) Development and validation of liquid chromatography-mass spectrometry method for the determination of telmisartan in human plasma Anal Chim Acta 540: 367-373.
- Wu J, Feng F, Jiang J, Tian Y (2004) Determination of telmisartan in human plasma by HPLC-MS and study of its pharmacokinetics. Zhongguo Yaoke Daxue Xuebao 35: 545-548.
- Zhang H, Fang Y (2004) HPLC/APCI-MS Determination of telmisartan in Human Plasma. Yaowu Fenxi Zazhi 24: 497-499.
- Torrealday N, Gonzalez L, Alonso RM, Jimenez RM, Ortiz-Lastra E (2003) Experimental design approach for the optimisation of a HPLC-fluorimetric method for the quantitation of the angiotensin II receptor antagonist telmisartan in urine. J Pharm Biomed Anal 32: 847-857.
- Chen H, Lin Yang, W Shaojun, H Min, Zhou Z (2007) Determination of telmisartan concentration in human plasma by means of RP-HPLC with fluorescence detector. Shoudu Yike Daxue Xuebao 28: 646-648.
- Shen J, Jiao Z, Li Z, Shi X, Zhong M (2005) Determination of telmisartan in human plasma by HPLC with fluorimetric detection. Yaowu Fenxi Zazhi 25: 47-49.
- Wang M, Hou J, Wu J, Zhang J, Du W, et al. (2007) Improvement of HPLC-fluorometric method in determination of telmisartan in human plasma. Zhongguo Yaofang 18: 1556-1558.
- Chen X, Long E (2007) Determination of telmisartan concentration in human plasma by FLD-HPLC. Zhongguo Yaoye 16: 48-49.
- Zhao R, Yu J, Ma K, Li G, Jiang Z (2006) Determination of telmisartan concentration in human blood plasma by HPLC with fluorescence detection. Yiyao Daobao 25: 763-764.
- Guo Z, Ma J, Jia Z, Wang R, Fei G, et al. (2006) Column-switching HPLC determination of telmisartan in human plasma. Yaowu Fenxi Zazhi 26: 1071-1073.
- Qi Y, Zhang P, Lian J, Li X, Ding Y, et al. (2007) Determination of telmisartan in human plasma by HPLC with fluorimetric detection and study on its pharmacokinetics. Xibei Yaoxue Zazhi 22: 163-165.
- Dai Q, Dai Yu M, Jiang M (2006) Determination of telmisartan in human plasma by HPLC. Jiangxi Yixueyuan Xuebao 46: 33-34.
- Liu J, Liu H, Xue H, Wu X, Cao L (2005) HPLC determination of telmisartan in human plasma with fluorescence detection. Yaowu Fenxi Zazhi 25: 1318-1321.
- Tao D, Sun L, Shen J (2006) Determination of the content of telmisartan in human plasma by HPLC. Zhongguo Yaoshi (Wuhan, China) 9: 221-222.
- Wen Y, Wu Y, Liu Y, Ma C (2006) Determination of telmisartan content in blood plasma by HPLC. Zhongguo Linchuang Yaoxue Zazhi 15: 245-247.
- Ye Z, Liu D, Zhou J, Yang J (2005) Determination of telmisartan in plasma by HPLC with fluorescent. Zhongguo Yaoshi (Wuhan, China) 8: 405-407.
- Liu J, Wang J (2006) Fluorimetric Optimization of determination of telmisartan concentration by HPLC and fluorimetric detection. Zhongguo Yaoye 15: 48-49.
- Sun L, Shen J, Tao D, Zhu D (2006) Determination of telmisartan by HPLC. Zhongguo Xinyao Zazhi 15: 1210-1212.
- Miao H, She J, Yun Y, Liu G (2005) Ion-pair HPLC determination and pharmacokinetic study of Telmisartan in human plasma. Zhongguo Xiandai Yingyong Yaoxue 22: 479-481.
- Li X, Zhou Y, Shi Q, Lan C (2005) Determination of telmisartan concentration in human plasma by high performance liquid chromatography-fluorometry (HPLC-FLU) method. Zhongguo Yiyuan Yaoxue Zazhi 25: 715-717.
- Feng Y, Yan Y, Yin Q, Liu L, Shi A, et al. (2005) Studies on plasma level determination of telmisartan by HPLC. Yaowu Fenxi Zazhi 25: 618-620.
- Kuang R, Zhang H, Xiong Y (2005) HPLC determination with fluorescent detection of telmisartan concentration in plasma and its application. Yaowu Fenxi Zazhi 25: 629-632.
- Zhang H, Jiang Y, Wen J, Zhou T, Fan G, et al. (2009) Rapid determination of telmisartan in human plasma by HPLC using a monolithic column with fluorescence detection and its application to a bioequivalence study. J chromatograph B Analyt Technol Biomed Life Sci 877: 3729-3733.
- Bioanalytical Method Validation (2001) Guidance for industry: bioanalytical method validation. U.S. Department of health and human services, food and drug administration centre for drug evaluation and research (CDER), centre for veterinary medicine (CVM).
- del Rosario Brunetto M, Contreras Y, Clavijo S, Torres D, Delgado Y, et al. (2009) Determination of losartan, telmisartan, and valsartan by direct injection of human urine into a column-switching liquid chromatographic system with fluorescence detection. J Pharm Biomed Anal 50: 194-199.
- Nie J, Zhang M, Fan Y, Wen Y, Xiang B, et al. (2005) Biocompatible in-tube solid-phase microextraction coupled to HPLC for the determination of angiotensin II receptor antagonists in human plasma and urine. J Chromatogr B Analyt Technol Biomed Life Sci 828: 62-69.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15096
- [From(publication date):
July-2012 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10411
- PDF downloads : 4685