Research Article Open Access
Determination of Tinidazole and Ciprofloxacin Hydrochloride in Single Formulation Tablet using Differential Pulse Polarography
Salvi V S*, Sathe P A and Rege P VDepartment of chemistry, Ramnarain Ruia College, Mumbai-400 019, India
- *Corresponding Author:
- V S Salvi
Department of chemistry
Ramnarain Ruia College
Mumbai– 400 019, India
E-mail: vaibhav.salvi27@gmail.com
Received date: October 19, 2010; Accepted date: November 24, 2010; Published date: November 26, 2010
Citation: Salvi VS, Sathe PA, Rege PV (2010) Determination of Tinidazole and Ciprofloxacin Hydrochloride in Single Formulation Tablet using Differential Pulse Polarography. J Anal Bioanal Tech 1:110. doi: 10.4172/2155-9872.1000110
Copyright: © 2010 Salvi VS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A simple and precise method for the determination of Tinidazole and Ciprofloxacin hydrochloride from its single formulation tablet has been developed using differential pulse polarography. Tinidazole and ciprofloxacin produces a cathodic wave at -0.38 V and -1.30 V respectively versus S.C.E in a solution of pH = 6.5 (Britton Robison buffer). The dynamic range for Tinidazole, is 0.50 to 279.31μgcm -3 and for Ciprofloxacin, it is 24.39 to 245.28 μgcm -3 . The method has been validated and applied successfully for the determination of the two drugs from their respective formulation.
Keywords
Tinidazole; Ciprofloxacin hydrochloride; Differential pulse paleography; Single formulation
Introduction
Tinidazole (TNZ) [1-(2-ethylsulfonylethyl)-2-methyl-5-nitroimidazole] is a 5-nitroimidazole derivative, an anti-parasitic drug used against protozoan infections. It is also used in the treatment of a variety of amoebic and parasitic infections. Ciprofloxacin Hydrochloride (CIPRO) [1-cyclopropyl- 6-fluoro- 4-oxo- 7-piperazin- 1-yl- quinoline- 3-carboxylic acid] is used in the treatment of bacterial infections. It is a second generation floroquinolone antibacterial. Several methods have been reported with the different voltammetry techniques such as adsorptive stripping voltammetry, polarography etc. [1-12].
Materials and Methods
Equipments
Differential pulse polarography was carried out using an Autolab PGSTAT 30 with 663 VA electrode stand of Metrohm. The three- electrode system consist of saturated calomel electrode as a reference electrode, HMDE as a working electrode and platinum electrode as a auxiliary electrode. The pH measurements were made with an Equip-Tronic pH meter model no. 610.
Chemicals
TNZ and CIPRO standards were obtained from Mayer Organics Pvt. Ltd. and formulations used for analysis were ‘Tiniba 300’ (Cadila Healthcare Ltd., India) and ‘Fasigyn 500’ (Pfizer, USA). 1M KCl was used as supporting electrolyte and Britton-Robinson buffer of pH 6.5 as a buffer solution in cell . All the solution were prepared in distilled water and analytical-reagent grade chemicals (Merck) were used.
Sample preparation
A stock solution of 1000µgcm-3 for both was prepared by dissolving 25mg of the standard to the respective volumetric flask and making up the volume to 25 cm3 with distilled water. Britton- Robinson buffer was prepared by mixing 0.04M boric acid, 0.04M phosphoric acid and 0.04M glacial acetic acid and adjusting the pH to required value with 1M NaOH.
Development of polarographic method and calibration curve
The optimization of experimental condition and parameters has been done to get uniform, less tailing, less broadening peak shape with normal baseline. The polarographic response for TNZ and CIPRO was examined in different buffer solutions such as Britton-Robinson, phosphate, carbonate and acetate buffer. The Britton-Robinson buffer of pH 6.5 was chosen as a best, as both the analytes give well defined peak in the same pH condition. As the concentration of TNZ increases the slight negative shift in potential was observed whereas the increase in the concentration of CIPRO tends a positive shift in the potential.
The polarogram was also observed with different supporting electrolyte like KNO3 , KCl, NaCl and peak height was found to be maximum in presence of KCl.
The peak current is linearly related to the pulse amplitude between 10 and 100mV. A pulse amplitude of 50mV was chosen as a optimum, as there is a distorted peak at high pulse amplitude value. The differential pulse polarogram of TNZ and CIPRO were recorded at various scan rates. At the scan rate higher than 15mVs-1 the width of the peak shape increases and gives distorted peak. At lower scan rate than 15mVs-1 the peak height is small as when compared with the 15mVs-1 scan rate.
No significance interference was observed from excipients which are commonly used in the formulation.
An aliquot of (18 cm3) of the Britton-Robinson buffer of pH 6.5 was placed in the voltammetric cell, 1M KCl (2 cm3) was added as a supporting electrolyte. The solution in cell was purged with nitrogen gas for 180 seconds. The recording of polarogram was carried out in voltage range of 0.0V to -2.0V vs SCE with scan rate of 15 mVs-1 and pulse amplitude of 50 mV. After recording blank polarogram, aliquot of definite volume was added to the system. It was observed that TNZ and CIPRO give a well defined cathodic peak at -0.38V and -1.30V respectively. The calibration curves were constructed for the concentration added against the current obtained for each addition.
Analysis of tablets
The two commercially available formulations ‘Tiniba 300’ and ‘Fasigyn 500’ were used for determination of TNZ whereas ‘Ciplox 250’ and ‘Cifran 250’ used for the determination of CIPRO. Ten tablets of each sample were weighed and powdered separately. The weight of the powder equivalent to 25 mg of analyte was weighed and transferred to a 25 cm3 volumetric flask and the volume was made up to the mark with distilled water. The solution was subjected to vigorous shaking for 5 minutes
Then, definite volume of the supernatant solution was transferred in to a volumetric cell containing de-aerated Britton- Robinson buffer of pH 6.5. The polarograms were recorded under the optimum experimental conditions. The amount of TNZ and CIPRO were calculated from resulting current values using already constructed calibrations graphs.
Results and Discussion
In determination of TNZ and Cipro , TNZ gives wide range of linearity from 0.50 to 279.31µgcm-3 where as linearity for Cipro is 24.39 to 245.28µgcm-3. The working range selected for TNZ was 14.28 to 100.0µgcm-3 and for CIPRO it was taken as 24.39 to 100µgcm-3. The quantitative determination of both the analytes has been done by both calibration and the standard addition method. The validation parameters and results for both analytes are shown tabulated in Table 1, Table 2, Table 3 and Table 4. The method is validated as per ICH guidelines [13].
| Brand Name | Tiniba 300 | Fasigyn 500 |
|---|---|---|
| Label claim (mg) | 300 | 500 |
| Amount found (mg) | 299.43 | 494.46 |
| % Assay ± RSD | 99.81 ± 0.53 | 98.89 ± 0.45 |
Table 1: Assay for TNZ.
| Brand Name | Ciplox 250 | Cifran 250 |
|---|---|---|
| Label claim (mg) | 250 | 250 |
| Amount found (mg) | 243.83 | 246.14 |
| % Assay ± RSD | 97.53 ± 2.28 | 98.46 ± 0.88 |
Table 2: Assay for CIPRO.
| Level | Amount of std. TNZ added in the cell in (µg/mL) | Amount of std. TNZ Recovered in (µg/mL) | % Recovery |
|---|---|---|---|
| 20 % | 4.85 | 4.81 | 99.18 |
| 120 % | 28.44 | 28.51 | 100.25 |
| 220 % | 50.92 | 50.41 | 99.00 |
| Mean | 99.48 | ||
| % RSD | 0.68 | ||
Table 3: Recovery for TNZ.
| Level | Amount of std. CIPRO added in the cell in (µg/mL) | Amount of std. CIPRO Recovered in (µg/mL) | % Recovery |
|---|---|---|---|
| 20 % | 4.85 | 4.68 | 96.89 |
| 120 % | 28.44 | 27.74 | 98.19 |
| 220 % | 50.92 | 50.07 | 98.02 |
| Mean | 97.70 | ||
| % RSD | 0.98 | ||
Table 4: Recovery for CIPRO.
Validation parameters
Specificity: The specificity of method was confirmed by observing the polarograms obtained from the drug sample with the respective standard solutions. The polarograms obtained from the drugs sample solution were found to be identical to those obtained for standard solution.
The addition of the standard solution to the drug sample solution did not change the characteristics of differential pulse polarogram. This gives the validity of method for the determination of analyte from its pharmaceutical formulation.
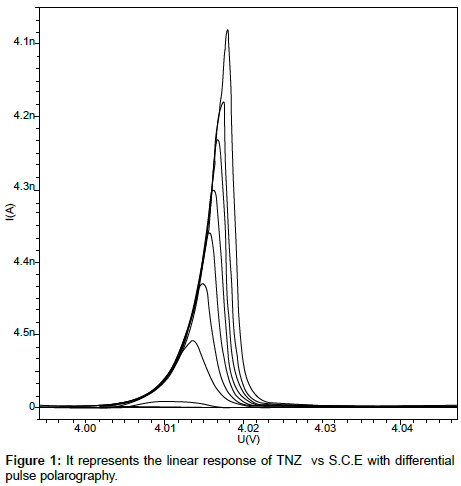
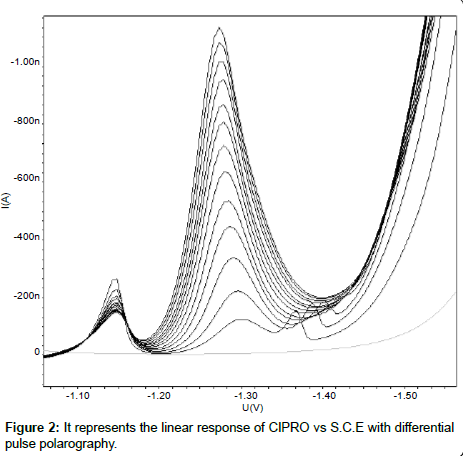
Linearity: The linearity range for the analyte was obtained by subsequent addition of analyte solution to the blank solution. TNZ gives wide linearity range from 0.50µgcm-3 to 279.31µgcm-3. CIPRO was found to be linear from 24.39µgcm-3 to 245.28µgcm-3. The calibration curves were constructed with concentration (C) against peak current (Ip) and calibration equations were found to be y = 37.4345x + 0.0482 and y = 4.6480x + 18.5140 for TNZ and CIPRO respectively. Coefficient of correlation for both the curves was found to be 0.999. The linear polarogram for TNZ and CIPRO is shown in Figure 1 and Figure 2 respectively.
Limit of detection and limit of quantification
Stock solutions containing analyte were diluted to series of appropriate concentration with distilled water and aliquots of diluted solutions were subjected to polarographic analysis. The limit of detection (LOD) and limit of quantification (LOQ) for the TNZ was calculated on the basis of signal-to- noise ratio (S/N) of 3 and 10 respectively and it was observed to be 0.24µgcm-3 and 0.50µgcm-3 respectively. For CIPRO limit of detection (LOD) and limit of quantification (LOQ) were obtained on the basis of visual evaluation, which were found to be 13.08µgcm-3 and 24.39µgcm-3 respectively.
Precision and repea tability
For TNZ, about ten concentrations in the working range of 14.28 – 100.00µgcm-3 were selected as a set and analysed thrice for the intra-day precision and for inter-day precision. The relative standard deviation was calculated for each concentration. Similarly for CIPRO, nine concentrations in the working range 24.39 to 100µgcm-3 were consider as a set and analysed same as TNZ.
It was observed that at each concentration the RSD value calculated was within the limit for intra – day as well as inter – day precision.
Robustness
The robustness of the method was tested by the measuring the peak height by bringing deliberately small changes in the experimental parameters. A slight changes in the pH value from the optimum, did not change the peak height for both analytes Also, a small change in scan rate and pulse amplitude was examined to be correct.
Accuracy
The accuracy of the method for the determination of both the analyte was tested by standard addition method. Recovery of both analytes has performed at three levels, 20 , 120 and 220% of amount of sample added, as shown in Table 3 and Table 4.
Conclusion
In this work, the optimum condition for the polarographic determination of TNZ and CIPRO in the tablet of single formulation is described. The presented method is simple and accurate, can be used for routine analysis for determination of both the analyte.
References
- Alvarez-Lueje A, Lopez C, Nunez-Vergara LJ, Squella JA (2001) Voltammetric behaviour and analytical application of lomefloxacin, an antibecterial fluroquinalone. J AOAC Inst 84: 649-658.
- Zhang Y Z, Yuan Z B (2001) Study of voltammetric behaviour of ampicillin with mercury film electrode. Fenxi Shiyan Study of voltammetric behaviour of ampicillin with mercury film electrode shi 20: 16-18.
- Vilchez JL, Araujo L, prieto A, Navolon A (2001) Differential pulse adsorptive stripping voltammetric determination of antibacterial lomefloxacin. J Pharm Biomed Anal 26: 23-29.
- Zhao JC, Shi RX, Kang XF, Song JF, Guo ZA (1999) Study on polarographic and catalytic behaviour of ciprofloxacin. Fenxi Shiyanshi 18: 1-4.
- Hu JB, Shang J, Li QL (2000) Votammetric behaviour of chloramphenicol at ion implementation modified electrode Pt/GC and its determination. Fenxi Shiyanshi 19: 27-29.
- Biryol I, Ulsu B, Kucukyavaz Z (1998) Voltammetric determination of amoxycillin using carbon paste electrode modified with poly-(4-vinyl pyridine). STP Pharma Sci 8: 383-386.
- Rizk M, Belal F, Aly FA, El- Enany NM (1998) Differential pulse polarographic determination of ofloxacin in pharmaceutical and biological fluids. Talanta 46: 83-89.
- Reddy GVS, Reddy SJ (1997) Estimation of cepholosporine antibiotics by d.p.p. Talanta 44: 627-631.
- Elsayed GO, Amin AS, Issa YM (1994) D.C polarographic determination of ampicillin inpharmaceutical dosage forms. Anal Lett 27: 2515-2521.
- Pan JH, Zhou GR, Kong XK, Wu J (1995) Polarographic and voltammetric behaviour of pefloxacin and its application. Fenxi Huaxue 23: 42-45.
- Jaber AMY, Lounici A (1994) Polarographic determination and behaviour of norfloxacin in tablet. Anal Chim Acta 291: 53-64.
- Bishop E, Hussein W (1984) Electroanalytical studies of antibacterial and diuretic drug at rotating disk electrode of gold and platinum. Analyst 109: 913- 921.
- ICH guidelines. Internet available- http://www.ich.org/LOB/media/MEDIA417. pdf.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17112
- [From(publication date):
December-2010 - Dec 04, 2024] - Breakdown by view type
- HTML page views : 12540
- PDF downloads : 4572