Research Article Open Access
Determination of Cremophor EL in Rat Plasma by LC-MS/MS: Application to a Pharmacokinetic Study
Vijaya Bhaskar V1*, Anil Middha2, Sudhir Tiwari1 and Savithiri Shivakumar1
1DMPK Laboratory (Biology Division), GVK BIO, Nacharam, Hyderabad, India
2Department of Pharmacy, Jagadish Prasad Jhabermal Tibrewala University, Vidyanagari, Rajasthan, India
- *Corresponding Author:
- Vijaya Bhaskar V
DMPK Laboratory (Biology Division), GVK BIO, Nacharam
Hyderabad, Andhra Pradesh, India-500076
Tel: +918143853440
E-mail: veeravalli.bhaskar@gmail.com
Received date: February 25, 2013; Accepted date: March 25, 2013; Published date: March 27, 2013
Citation: Vijaya Bhaskar V, Middha A, Tiwari S, Shivakumar S (2013) Determination of Cremophor EL in Rat Plasma by LC-MS/MS: Application to a Pharmacokinetic Study. J Anal Bioanal Tech 4:163. doi: 10.4172/2155-9872.1000163
Copyright: © 2013 Vijaya Bhaskar V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A rapid sensitive, and selective pseudo MRM based method for the determination of Cremophor EL (CrEL) in rat plasma was developed using Liquid chromatography/Tandem mass spectrometry (LC-MS/MS). The analytes were detected using atmospheric pressure chemical ionization (APCI) tandem mass spectrometry. Plasma concentrations of CrEL were quantified after administration through oral and intravenous routes in male sprague dawley rats at a dose of 0.26 g/kg. The standard curve was linear (0.9982) over the concentration range of 2.10 to 2100.00 μg/mL. The lower limit of quantitation for CrEL was 2.10 μg/mL using 50 μL plasma. The coefficient of variation and relative error for inter and intra assay at three QC levels were 1.23 to 8.87 and -12.08 to 4.10 respectively. CrEL has poor oral bioavailability with mean absolute bioavailability of 2.32%. CrEL plasma concentration profiles after oral and intravenous dosing were without spiky concentration levels. Spiky plasma concentration profiles for new chemical entities (NCEs) are a common phenomenon in drug discovery, which could be due to entrohepatic circulation, sample handling errors. A novel proposal was conveyed to the scientific community, where formulation vehicle can be analysed as qualifier in the analysis of NCEs to address the spiky profiles.
Keywords
CrEL; LC-MS/MS; NCE; Method validation; Bioavailability
Introduction
Polyoxyethyleneglycerol triricinoleate (Cremophor EL; CrEL) is a liquid product formed by the reaction of ethylene oxide with castor oil at a molar ratio of 35:1 [1]. CrEL is used as a excipient in formulations for the solubilization of various hydrophobic drugs including anesthetics [2], antineoplastic agents [2,3] immunosuppressive agents [4], analgesics [5,6] vitamins [7] and new synthetic water insoluble compounds. Although CrEL is considered relatively nontoxic [2,8], several reports suggest that drugs administered in CrEL induce serious complications like anaphylactoid hypersensitivity [7], axonal swelling, degeneration and demyelination [3,4]. Moreover, it has been proposed that CrEL plays a role in the etiology of peripheral neuropathy observed after intravenous paclitaxel [3] or cyclosporin A treatment and recent evidence supports the notion that CrEL causes nerve conduction [3,9]. Pharmacokinetic disposition of drugs depends on CrEL pharmacokinetics [10].
In early stages of drug discovery, rat is the most commonly studied animal in pharmacokineitc and drug metabolism and disposition studies as it is relatively inexpensive and can be easily acquired and handled [11]. In a typical pharmacokientic (PK) study new chemical entities (NCEs) are administered to rats via intravenous and oral routes. Serial blood samples are collected and assayed by Liquid chromatography/Tandem mass spectrometry (LC-MS/MS) and final pharmacokinetic parameters are calculated. Few NCEs had spiky plasma concentration profiles and various reasons for such profiles could be due to enterohepatic circulation, or discrepancies in sample collection/sample processing. Spiky profiles in elimination phase will lead to inaccurate quantification of PK parameters. Extensive studies needed to be carried to characterize enterohepatic circulation behavior of test compounds. Drugs that undergo enterohepatic cycling to a significant extent include colchicine, phenytoin, leflunomide and tetracycline antibiotics. As formulation excipients has fixed plasma concentration profiles irrespective of NCEs dosed, monitoring the plasma concentration levels of excipient along with NCEs will help to take a decision on the spiky plasma concentration profiles of NCEs. A thoroughly developed and validated bioanalytical method is required to fix the plasma concentration profile and understand the pharmacokinetic disposition of formulation excipient studied. Integrity of results from pharmacokinetic studies can be cross verified if formulation excipients that had fixed Plasma concentration profile/ PK parameters are monitored along with the test compound studied.
There exists different analytical methods for the quantitative measurement of CrEL such as colorimetric [3,12] chromatographic [13-15], electrophoresis [16], potentiometric [17-20] and pyrolysis mass spectrometry [21]. Cleavage and derivatisation of CrEL with UV absorbing group outweighs the usage of colorimetric methods for the analysis of CrEL as it is time taking and involve multiple steps in the process. Also both the chromatographic and electrophoresis methods are time consuming due to tedious derivatisation procedures involved in the process. The potentiometric methods can’t be used for measuring lower levels of the CrEL due to limited sensitivity. Pyrolysis mass spectrometry needs different mass spectrometry design and throughput of analysis was a consideration. In the present work an attempt was made to develop and validate bioanalytical method for the quantitative estimation of CrEL using LC-MS/MS and presented the plasma concentration profiles/PK parameters in male sprague dawley rats. This was the first approach of its kind to quantify the concentration levels of CrEL with pseudo MRM based approach.
Experimental Section
Materials
Cremophor EL, dimethyl sulfoxide (DMSO) and telmisartan (internal standard) were procured from sigma Aldrich Co. (St. Louis, MO, USA). Acetonitrile, water and acetone (HPLC grade) were obtained from Merck specialities Pvt ltd (Mumbai, India). Ammonium acetate was procured from Merck specialities Pvt ltd (Mumbai, India). Sprague dawley rats were procured from Bioneeds ltd (Bangalore, India). Blood collection vacutainers (Lithium Heparin as anticoagulant) were sourced from BD (Franklin lakes, USA).
All mass spectrometric estimations were performed on a sciex 3200 QTrap triple quadrupole instrument with turboionspray (AB Sciex, Toronto, Canada). The HPLC system consisted two of LC20AD UFLC pumps and a SIL HTC autosampler (Shimadzu, Kyoto, Japan). The stationary phase was XBridge C18 with 3.5 μm particle diameter (Waters, Ireland). The column dimensions were 50×4.6 mm.
Preparation of calibration standards and quality control samples
Master stock solution of telmisartan (Purity: 98%) (1 mg/ml) was prepared in DMSO. Working standard solutions of CrEL (Purity: 100%) were prepared by serial diluting from master stock (CrEL provided by supplier with density of 1.05 g/mL was used as master stock) with Acetonitrile: DMSO: water (2:2:1). Working standard solutions were prepared at 25 fold higher concentration than plasma calibration standards and quality control samples. A total of nine calibration standards and three quality control samples were prepared. Plasma calibration standards (2.10, 4.20, 21.00, 105.01, 420.05, 1050.13, 1680.21, 1890.00, 2100.00 μg/mL) and quality control samples (6.64, 995.40, 1659.00 μg/mL) of CrEL was prepared by spiking 2 μL of the working standard solutions into 48 μL of blank rat plasma. The working solution for internal standard (100 ng/mL) was prepared by diluting an aliquot of master stock solution with acetonitrile. Master stock solutions were stored at 40C when not in use.
Sample preparation
A 50 μL aliquot of plasma (blank control plasma, plasma samples from rats dosed with CrEL, blank plasma spiked with calibration standards and QC samples) was pipetted in to a 96 well polypropylene plate and extracted with 200 μL of acetonitrile containing internal standard. Samples were vortex mixed for 10 min at 1200 rpm and centrifuged at 4000 rpm for 10 min at 4°C. 50 μL of supernatant was pipette transferred in to a fresh analysis plate and diluted with 450 μL of acetonitrile: water (1:1, v/v) and 5 μL aliquots were injected for LC-MS/ MS analysis.
LC-MS/MS Analysis
A generic gradient LC method with a short run time of 3.5 min was developed for the analysis of CrEL in plasma samples. The mobile phase flow rate was 1.0 mL/min with a split ratio of 1:1 to the ionization source. The mobile phase consisted of 10 mM ammonium acetate in water as aqueous component and 100% acetone as organic modifier. The column and autosampler were maintained at 40°C and 4°C respectively. The turboionspray source was operated with typical settings as follows: ionization mode, positive; curtain gas, 20 psi; nebulizer gas (GS1), 50 psi; heater gas (GS2), 50 psi; nebulizer current (NC), 5A; temperature, 500°C. The molecular ions of CrEL and telmisartan were formed using the declustering potentials of 110 V, 65 V respectively. Molecular ion of telmisartan was fragmented at collision energy of 65 V by collisionactivated dissociation with nitrogen as the collision gas at a pressure setting of ‘medium’ on the instrument. Molecular ion of CrEL didn’t generate distinct fragment ions in product ion scan. So pseudo MRM mode was used, where molecular ion is monitored as a fragment ion with minimal collision energy of 5 V. Multiple reactions monitoring (MRM) mode was employed for the quantification: m/z 133.10→133.10 for CrEL (Pseudo MRM) and m/z 515.3→276.1 for telmisartan. Peak areas for all components were automatically integrated using Analyst software version 1.5.
Method validation
Three precision and accuracy batches, consisting of calibration standards (2.10, 4.20, 21.00, 105.01, 420.05, 1050.13, 1680.21, 1890.00, 2100.00 μg/mL) were analyzed on three different days to check the repeatability of the developed method. In each precision and accuracy batch, six replicates of each QC sample at 6.64, 995.40 and 1659.00 μg/ mL were assayed to evaluate the intra, inter-day precision and accuracy. Relative error (RE) indicates accuracy and coefficient of variation (CV) serves as the measure of precision of the developed method. The selectivity was evaluated by analyzing blank plasma samples obtained from different animals. Extraction efficiency of CrEL was determined by comparing peak areas of analyte spiked before extraction into the six different lots of plasma with those of the analyte post spiked into plasma extracts. Matrix effect was evaluated from matrix factor values. Matrix factor was calculated by dividing mean peak areas of analyte post spiked in to plasma extracts with those of analyte spiked in to neat solutions at three QC levels. To assess post-preparative stability, six replicates of QC samples at each of the low, mid and high concentrations were processed and stored under autosampler conditions for 24 h before analysis. To assess bench top stability, six replicates of QC samples at each of the low, mid and high concentrations were kept at room temperature for 8 h before analysis. Freeze thaw stability was assessed at three QC levels for three freeze thaw cycles. To assess long term stability, six replicates of QC samples at each of the low, mid and high concentrations were kept at -80°C for 60 days before analysis.
Application
Individual rats (male Sprague-Dawley) were dosed at 0.26 g/kg intravenously (Bolus) through tail vein and 0.26 g/kg orally through oral gavage needle. Dosing volume administered was 5 mL/kg. The composition of dosing vehicle used for the study was Ethanol/CrEL/ water (10:5:85, % v/v) [22,23]. Serial blood samples were collected into vacutainers containing lithium heparin (anticoagulant) at 0.08, 0.25, 0.50, 1, 2, 4, 8 and 24 h post dose [24] after intravenous administration and 0.25, 0.50, 1, 2, 4, 8 and 24 h post dose [24] after oral administration. At each time point 200 μL of blood was collected in to vacutainers. Blood Samples were collected using retro orbital puncture method. Plasma was isolated by centrifugation at 10000 rpm for 10 min and stored frozen at -80°C until assay. Pharmacokinetic parameters such as Elimination rate constant (Kel), Half life (T1/2), Extrapolated drug concentration (C0), AUC0-last, AUC0-inf, AUC%Extrapolated, Volume of distribution (Vd), Clearance (Cl), Tmax, Cmax, MRTlast and absolute bioavailability were calculated using phoenix winnonlin software (v6.3). Absolute bioavailability was calculated using AUC0-inf values as AUC%Extrapolated was less than 20%.
Results and Discussion
LC-MS/MS Analysis
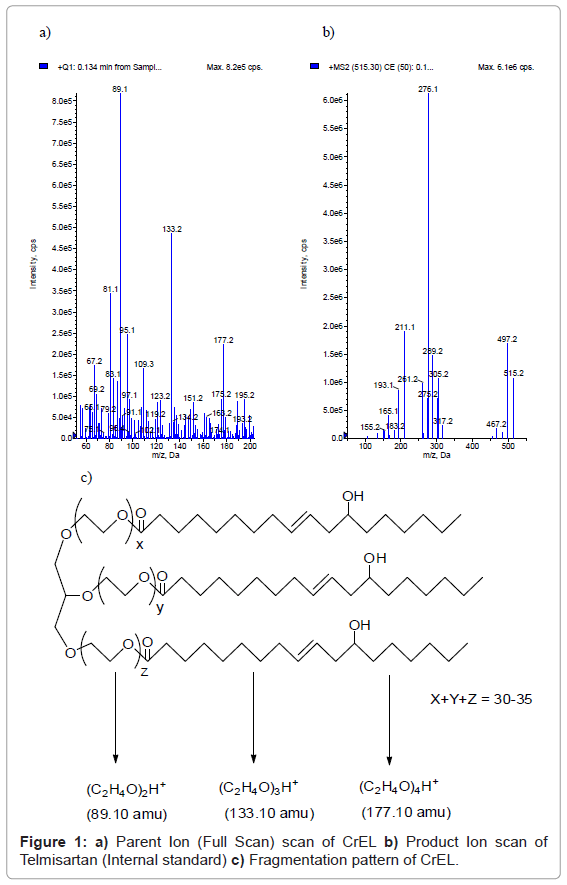
The atmospheric chemical ionization of CrEL produced the abundant protonated molecules ([MH]+) at m/z 89.10, 133.20, 177.20 (Figure 1a) under positive ionization conditions. These masses correspond to insource fragmentation of CrEL and didn’t generate further distinct fragment ions. Molecular ion with 133.10 amu mass (three ethylene oxide units) [25] was selected as both precursor, fragment ion and analyzed in pseudo MRM mode. The atmospheric pressure chemical ionization of telmisartan produced abundant protonated molecules ([MH]+) at 515.20 amu and generated an intense fragment at 276.10 amu (Figure 1b). Figure 1c shows the insource fragmentation pattern of CrEL. Of the three molecular ions, molecular ion with 133.10 amu mass was selected as it had better selectivity without any interference from endogenous components. So, pseudo MRM transition of m/z; 133.10/133.10 was used to monitor the PK plasma concentration profiles of CrEL in both oral and intravenous routes of administration. The selected transition wasn’t selective in ESI mode of analysis with interfering peak eluting at the same RT of CrEL from blank plasma. Due to selectivity issues in ESI mode of ionization, CrEL was analyzed in APCI mode of ionization. Pseudo MRM 133/10/133/10 was free from blank interference in APCI mode of ionization. However, interference from blank was observed for molecular ion at m/z, 89.10 even in APCI mode of ionization and molecular ion at m/z, 177.20 isn’t sensitive enough to be selected as molecular ion for pseudo MRM generation. Calibration standards were prepared by serial dilution from the master stock. Various diluents combinations such as methanol: water (1:1, v/v), water, acetonitrile: water (1:1, v/v), methanol: acetonitrile: water (2: 2: 1, v/v) were tested for serial dilution of calibration standards. Calibration linearity wasn’t achieved with any of the solvent combinations tested. Acetonitrile: DMSO: water (2: 2: 1, v/v) combination as a diluent helped in achieving better linearity than the other solvent combinations tested.
LC-MS/MS methods operated with the C18 column and a 3.5 min generic gradient LC method (Time (min)/%B=0.01/5, 1.50/95, 2.50/95, 2.60/5, 3.50/5) was developed for the analysis of CrEL in plasma. Use of acidic aqueous modifier (0.1% formic acid) didn’t result in good separation of CrEL from blank interfering peaks.
Use of 10 mM ammonium acetate as aqueous modifier had better baseline separation from interfering peaks than acidic aqueous modifiers. Various organic modifiers such as acetonitrile, methanol and acetone were tested for achieving better peak shape and address the response saturation observed at higher calibration standards for CrEL. Both acetonitrile and methanol didn’t produce sharp peak and response saturation was observed at higher calibration standards. However, acetone as organic modifier helped to achieve sharper peak shapes and response saturation at higher calibration standards wasn’t an issue here. Final mobile phase composition used for the analysis of CrEL was 10 mM ammonium acetate in water as aqueous modifier and 100% acetone as organic modifier.
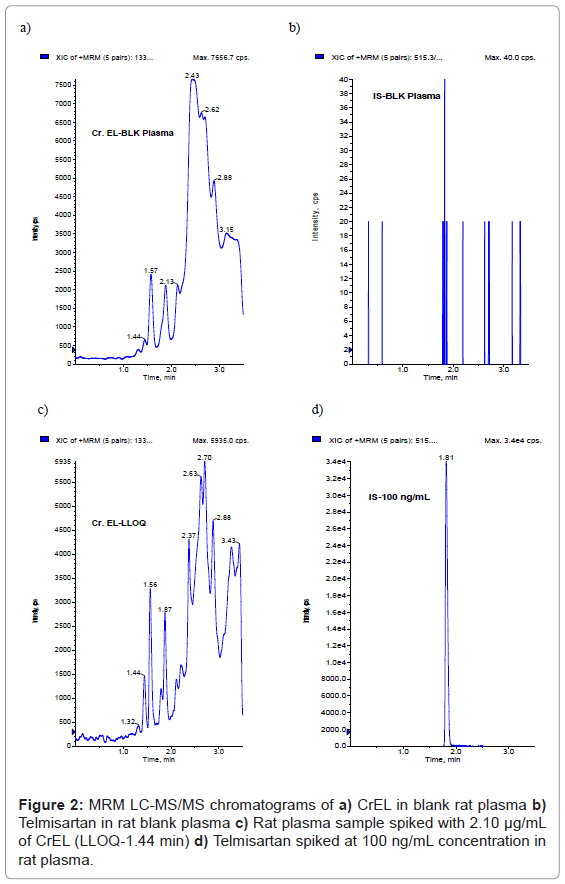
Because of the higher sensitivity of LC-MS/MS method compared to that of HPLC or colorimetric methods, lesser plasma sample volume (50 μL) is sufficient to obtain an LLOQ of 2.10 μg/mL. No interference at the retention times of CrEL (1.45 min) (Figure 2a) and telmisartan (1.81 min) (Figure 2b) was observed in any of the lots screened as shown in representative chromatogram of the extracted blank plasma sample, confirming the selectivity of the present method. Representative chromatogram of CrEL at LLOQ was shown in Figure 2c. Representative chromatogram of Telmisartan at 100 ng/mL spiked concentration was shown in Figure 2d. The LLOQ was set at 2.10 μg/mL for CrEL using 50 μL of rat plasma. The signal-to-noise ratio for CrEL is greater than 5 (S/N; 6.4) at 2.10 μg/mL. The retention times of CrEL and telmisartan were reproducible throughout the experiment and no column deterioration was observed after analysis of plasma samples.
Method validation
The developed method was validated as per the FDAs guidance for bioanalytical method validation [26]. Calibration curves were obtained over the concentration range of 2.10-2100.00 μg/mL of CrEL in plasma. Quadratic regression with a weighting of 1/(x×x) generated optimum accuracy for the calculated concentrations at each level (Table 1). The low CV value at each concentration level indicates the precision of the present method (Table 1). Summary of intra, inter-day precision and accuracy data for QC samples was shown in table 2. Intra and interassay CV values for CrEL ranged from 1.23 to 8.87% across three QC levels. Intra and inter-assay RE values for CrEL ranged from -12.08 to 4.10% across three QC levels. These results of acceptable RE and %CV indicate the acceptable accuracy and precision of developed method. As shown in table 3, the overall extraction efficiency of CrEL was 89.99%, which was consistent with a total % CV less than 9% at three QC concentration levels. Mean matrix factor values of 0.95 (Table 3) at three QC levels shows that the developed method is totally free of matrix effects. Acceptable matrix factor range for qualifying the method to be free from matrix effects is 0.85-1.15. Protein precipitation as extraction procedure has been used successfully used to extract CrEL from rat plasma. Autosampler stability for 24 h at 4°C with <9% (Table 3) difference from theoretical concentration, benchtop stability for 8 h at room temperature with <4% (Table 3) difference from theoretical concentration was proved for extracted QC samples at three concentration levels. Spiked QC samples were stable for three freeze thaw cycles (freeze thaw stability) with <6% (Table 3) difference from theoretical concentration. Long term stability at -80°C was proved for a period of 60 days with <5% (Table 3) difference from theoretical concentration.
| Concentration (μg/mL) | Statistical parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Actual conc. | Calculated Conc. | Mean | SD | % CV | Relative Error (%) | % Accuracy | ||
| Set-1 | Set-2 | Set-3 | ||||||
| 2.10 | 2.22 | 2.18 | 2.16 | 2.19 | 0.031 | 1.40 | 4.13 | 104.13 |
| 4.20 | 3.72 | 3.86 | 4.00 | 3.86 | 0.140 | 3.63 | -8.10 | 91.90 |
| 21.00 | 20.32 | 20.82 | 19.59 | 20.24 | 0.619 | 3.06 | -3.60 | 96.40 |
| 105.01 | 110.63 | 108.64 | 107.23 | 108.83 | 1.708 | 1.57 | 3.64 | 103.64 |
| 420.05 | 441.42 | 425.99 | 440.79 | 436.07 | 8.732 | 2.00 | 3.81 | 103.81 |
| 1050.13 | 1090.86 | 1083.66 | 1166.40 | 1113.64 | 45.833 | 4.12 | 6.05 | 106.05 |
| 1680.21 | 1525.08 | 1602.62 | 1653.67 | 1593.79 | 64.748 | 4.06 | -5.14 | 94.86 |
| 1890.00 | 1898.61 | 2027.52 | 1838.80 | 1921.64 | 96.445 | 5.02 | 1.67 | 101.67 |
| 2100.00 | 2147.43 | 1937.97 | 1894.39 | 1993.26 | 135.279 | 6.79 | -5.08 | 94.92 |
Table 1: Calculated concentrations and statistical parameters of crel calibration standards prepared in rat plasma (N=3).
| Type | Statistical parameter | Concentration (μg/mL) | ||
|---|---|---|---|---|
| LQC (6.64) | MQC (995.40) | HQC (1659.00) | ||
| Intra Day-Set-1 (N=6) | Mean | 5.96 | 1036.20 | 1717.39 |
| SD | 0.22 | 12.76 | 88.53 | |
| % CV | 3.61 | 1.23 | 5.15 | |
| % Accuracy | 89.71 | 104.10 | 103.52 | |
| Relative Error (%) | -10.29 | 4.10 | 3.52 | |
| Intra Day-Set-2 (N=6) | Mean | 6.32 | 997.50 | 1458.58 |
| SD | 0.35 | 54.43 | 51.99 | |
| % CV | 5.60 | 5.46 | 3.56 | |
| % Accuracy | 95.21 | 100.21 | 87.92 | |
| Relative Error (%) | -4.79 | 0.21 | -12.08 | |
| Intra Day-Set-3 (N=6) | Mean | 6.04 | 1007.73 | 1503.41 |
| SD | 0.50 | 38.43 | 45.10 | |
| % CV | 8.21 | 3.81 | 3.00 | |
| % Accuracy | 90.99 | 101.24 | 90.62 | |
| Relative Error (%) | -9.01 | 1.24 | -9.38 | |
| Inter Day (N=18) | Mean | 6.11 | 1013.81 | 1559.79 |
| SD | 0.19 | 20.06 | 138.31 | |
| % CV | 3.13 | 1.98 | 8.87 | |
| % Accuracy | 91.97 | 101.85 | 94.02 | |
| Relative Error (%) | -8.03 | 1.85 | -5.98 | |
Table 2: Precision and accuracy of CrEL in quality control samples.
| Validation parameter | Statistical parameter | Result |
|---|---|---|
| Extraction Recovery | Mean | 89.99 |
| SD | 7.77 | |
| % CV | 8.64 | |
| Matrix factor (Matrix effect) | Mean | 0.95 |
| SD | 0.06 | |
| % CV | 6.20 | |
| Autosampler stability | Mean | 91.21 |
| SD | 8.38 | |
| % CV | 9.19 | |
| Bench Top stability | Mean | 96.03 |
| SD | 4.38 | |
| % CV | 4.56 | |
| Freeze thaw stability | Mean | 94.53 |
| SD | 6.83 | |
| % CV | 7.22 | |
| Long term stability | Mean | 95.29 |
| SD | 5.66 | |
| % CV | 5.94 |
Table 3: Summary of validation parameters for CrEL in rat plasma.
Application study
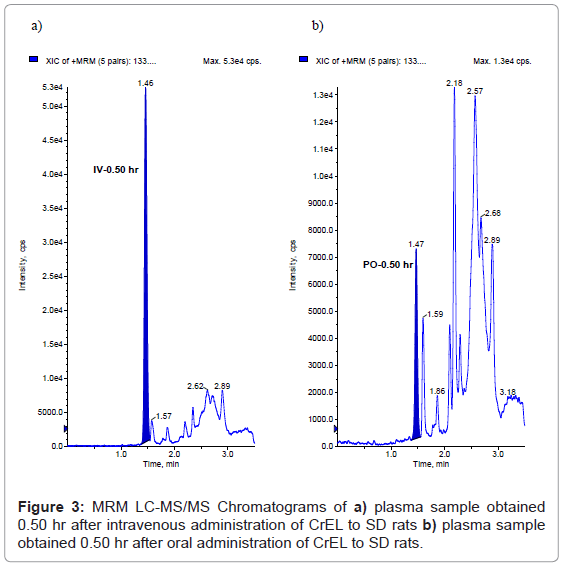
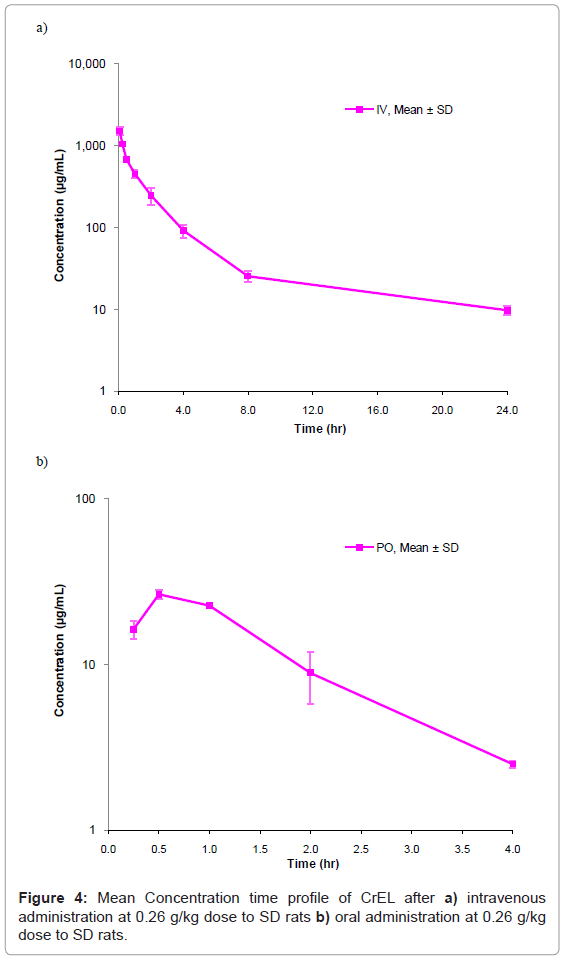
This method has been successfully applied to the bioanalysis of rat plasma samples in absolute bioavailability study of CrEL. Representative chromatograms of CrEL from intravenous (0.50 hr), oral (0.50 hr) study samples were shown in figures 3a and 3b respectively. The Intravenous and oral concentration/time profiles of CrEL is represented in figures 4a and 4b respectively. As CrEL had a clear absorption and elimination phase in oral route of administration and clear elimination phase in intravenous route of administration, monitoring CrEL along with NCEs helps to take a decision on the spiky profile of NCEs. Monitoring formulation excipient concentrations in PK study samples act as quality control check for in vivo and bioanalytical processes. Intravenous and oral pharmacokinetic parameters of CrEL were listed in tables 4 and 5 respectively. The oral bioavailability of CrEL was measured as 2.32% which shows the excipient had very poor absorption. Mean terminal half life of CrEL after oral and intravenous administration was 0.97 hr and 7.16 hr respectively. Mean Tmax and Cmax after oral administration of CrEL to sprague dawley rats was 0.50 hr and 26.35 μg/mL respectively. Mean residence time of CrEL after intravenous and oral administration of to sprague dawley rats was 3.42 and 1.36 respectively. Mean volume of distribution after intravenous administration (1.34 L/kg)) was greater than total normalized body water (0.7 L/kg) in rats. Mean clearance of CrEL after intravenous administration was much less (2.15 mL/min/kg body wt) than hepatic blood flow in rats (70 mL/min/kg body wt).
| Subject | Kel (1/hr) | T1/2 (hr) | C0 (μg/mL) | AUClast (hr×μg/mL) | AUCINF_obs (hr×μg/mL) | AUC_ %Extrap_obs (%) | Vz_obs (L/kg) | Cl_obs (mL/min/kg) | MRTlast (hr) |
|---|---|---|---|---|---|---|---|---|---|
| RAT-1 | 0.10 | 6.98 | 1770.47 | 1855.24 | 1946.55 | 4.69 | 1.36 | 2.25 | 3.39 |
| RAT-2 | 0.09 | 7.47 | 1606.31 | 1762.12 | 1858.79 | 5.20 | 1.52 | 2.35 | 3.39 |
| RAT-3 | 0.10 | 7.03 | 2065.39 | 2248.00 | 2361.61 | 4.81 | 1.13 | 1.85 | 3.50 |
| Mean | 0.10 | 7.16 | 1814.06 | 1955.12 | 2055.65 | 4.90 | 1.34 | 2.15 | 3.42 |
| SD | 0.00 | 0.27 | 232.62 | 257.88 | 268.58 | 0.27 | 0.20 | 0.26 | 0.06 |
| CV% | 3.69 | 3.77 | 12.82 | 13.19 | 13.07 | 5.44 | 14.83 | 12.28 | 1.82 |
Table 4: Pharmacokinetic parameters of CrEL after intravenous administration at 0.26 g/kg dose in male Sprague dawley rats.
| Subject | Kel(1/hr) | T1/2(hr) | Tma×(hr) | Cma× (μg/mL) | AUClast (hr×μg/mL) | AUCINF_obs (hr×ug/mL) | AUC_%Extrap_obs (%) | Vz_F_obs (L/kg) | Cl_F_obs (mL/min/kg) | MRTlast (hr) |
|---|---|---|---|---|---|---|---|---|---|---|
| RAT-1 | 0.69 | 1.01 | 0.50 | 28.04 | 40.77 | 44.42 | 8.22 | 8.60 | 98.49 | 1.33 |
| RAT-2 | 0.69 | 1.01 | 0.50 | 26.01 | 42.40 | 46.20 | 8.22 | 8.24 | 94.69 | 1.34 |
| RAT-3 | 0.77 | 0.90 | 0.50 | 24.99 | 49.12 | 52.18 | 5.88 | 6.51 | 83.84 | 1.42 |
| Mean | 0.72 | 0.97 | 0.50 | 26.35 | 44.10 | 47.60 | 7.44 | 7.78 | 92.34 | 1.36 |
| SD | 0.05 | 0.06 | 0.00 | 1.55 | 4.42 | 4.07 | 1.35 | 1.12 | 7.60 | 0.05 |
| CV% | 6.79 | 6.53 | 0.00 | 5.89 | 10.03 | 8.54 | 18.19 | 14.34 | 8.23 | 3.73 |
Table 5: Pharmacokinetic parameters of CrEL after oral administration at 0.26 g/kg dose in male Sprague dawley rats.
Conclusion
A rapid, sensitive and reliable pseudoMRM based LC-MS/MS method with protein precipitation as extraction technique has been successfully developed and validated for the analysis of CrEL in Sprague dawley rat plasma. The developed assay method has acceptable sensitivity (LLOQ: 2.10 μg/mL), precision, accuracy, selectivity, recovery, stability and can be used to address the spiky concentration profiles observed with NCEs. The validated method was successfully applied to assay rat plasma samples. The plasma concentration profiles/ PK parameters of CrEL after intravenous and oral administration in sprague dawley were demonstrated.
References
- Webster LK, Woodcock DM, Rischin D, Milliward MJ (1997) Review : Cremophor: Pharmacological activity of an "inert" solubiliser. J Oncol Pharm Pract 3: 186-192.
- Sparreboom A, Loos WJ, Verweij J, de Vos AI, van der Burg ME, et al. (1998) Quantification of Cremophor EL in Human Plasma Samples Using a Colorimetric Binding Microassay. Anal Biochem 255: 171-175.
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F (2000) Description of a short-term Taxol-induced nociceptive neuropathy in rats. Brain Res 887: 239-249.
- Windebank AJ, Blexrud MD, de Groen PC (1994) Potential neurotoxicity of the solvent vehicle for cyclosporine. J Pharmacol Exp Ther 268: 1051-1056.
- Burstein SH, Friderichs E, Kögel B, Schneider J, Selve N (1998) Analgesic effects of 1',1'-dimethylheptyl-delta8-THC-11-oic acid (CT3) in mice. Life Sci 63: 161-168.
- Janssen PA, Niemeggers CJ, Dony JG (1963) The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawal reflex in rats. Arzneimittelforschung 13: 502-507.
- Mounier P, Laroche D, Divanon F, Mosquet B, Vergnaud MC, et al. (1995) [Anaphylactoid reactions to an injectable solution of a cremophor-containing solution of multivitamins]. Therapie 55: 571-573.
- Zhao FK, Chuang LF, Israel M, Chuang RY (1989) Cremophor EL, a widely used parenteral vehicle, is a potent inhibitor of protein kinase C. Biochem Biophys Res Commun 159: 1359-1367.
- Jack AM, Cameron NE, Cotter MA (1999) Effects of the diacylglycerol complexing agent, Cremophor, on nerve conduction velocity and perfusion in diabetic rats. J Diabetes Complications 13: 2-9.
- Gelderblom H, Verweij J, van Zomeren DM, Buijs D, Ouwens L, et al. (2002) Influence of Cremophor EL on the bioavailability of intraperitoneal paclitaxel. Clin Cancer Res 8: 1237-1241.
- Tse FLS, Jaffe JM (1991) Preclinical drug disposition-A Laboratory Handbook. Marcel Dekker Inc, New York.
- Brouwer E, Verweij J, Hauns B, Loos WJ, Nooter K, et al. (1998) Linearized colorimetric assay for Cremophor EL: Application to pharmacokinetics after 1 hour paclitaxel infusions. Anal Biochem 261: 198-202.
- Sparreboom A, van Tellingen O, Huizing MT, Nooijen WJ, Beijnen JH (1996) Determination of polyoxyethyleneglycerol triricinoleate 35 (Cremophor EL) in plasma by pre-column derivatization and reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Appl 681: 355-362.
- Faouzi M el-A, Dine T, Luyckx M, Brunet C, Mallevais ML, et al. (1995) Stability, compatibility and plasticizer extraction of miconazole injection added to infusion solutions and stored in PVC containers. J Pharm Biomed Anal 13: 1363-1372.
- Huizing MT, Rosing H, Koopmans FP, Beijnen JH (1998) Influence of Cremophor EL on the quantification of paclitaxel in plasma using high performance liquid chromatography with solid phase extraction as sample pretreatment. J Chromatogr B Biomed Sci Appl 709: 161-165.
- Kunkel M, Bohler J, Keller E, Frahm AW (1997) Separation of Cremophor EL by capillary electrophoresis. Pharmazie 52: 109-121.
- Kunkel M, Bohler J, Keller E, Frahm AW (1998) Titrimetric determinations of cremophor EL in aqueous-solutions and biofluids part-1-validation of the potentiometric titration method. Pharmazie 53: 314-321.
- Kunkel M, Meyer T, Bohler J, Keller E, Frahm AW (1999) Titrimetric determination of Cremophor (R) EL in aqueous solutions and biofluids Part 2: Ruggedness of the method with respect to biofluids. J Pharm Biomed Anal 21: 911-922.
- Meyer T, Bohler J, Frahm AW (2001) Determination of Cremophor EL in plasma after sample preparation with solid phase extraction and plasma protein precipitation. J Pharm Biomed Anal 24: 495-506.
- Carbini M, Stevanato R, Rovea M, Traldi P, Favretto D (1996) Curie-point Pyrolysis-Gas Chromatography/Mass Spectrometry in the Art Field. 2--The Characterization of Proteinaceous Binders. Rapid Commun Mass Spectrom 10: 1240-1242.
- Boon JJ (1992) Analytical Pyrolysis mass spectrometry: new vistas opened by temperature-resolved in-source PYMS. Int J Mass Spectrom Ion Proc 118-119: 755-787.
- Neeravannan S (2006) Preclinical formulations for drug discovery toxicology: physicochemical challenges. Expert Opin Drug Metab Toxicol 2: 715-731.
- Li P, Zhao L (2007) Developing early formulations: Practice and Perspective. Int J Pharm 341: 1-19.
- Kwon Y (2002) Pharmacokinetic study design and data interpretation. Kluwer Academic Publishers, New York, 21-46.
- James L (2011) Identification of surfactants by Liquid Chromatography-Mass spectrometry. 5-10.
- US Department of Health and Human Services (2001) Guidance for Industry-Bioanalytical Method Validation. Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15982
- [From(publication date):
April-2013 - Nov 28, 2025] - Breakdown by view type
- HTML page views : 11165
- PDF downloads : 4817