Review Article Open Access
D-Neuron: Is it Ligand-Producing Neuron of Taar1? From Schizophrenia Research
Keiko Ikemoto*Department of Psychiatry, Iwaki Kyoritsu General Hospital, Iwaki 973-8555, Japan
- Corresponding Author:
- Keiko Ikemoto
Department of Psychiatry, Iwaki Kyoritsu General Hospital
Iwaki 973-8555, Japan
E-mail: ikemoto@iwaki-kyoritsu.iwaki.fukushima.jp
Received Date: May 20, 2013; Accepted Date: June 27, 2013; Published Date: July 01, 2013
Citation: Ikemoto K (2013) D-Neuron: Is it Ligand-Producing Neuron of Taar1? From Schizophrenia Research. J Community Med Health Educ 3:221. doi: 10.4172/2165-7904.1000221
Copyright: © 2013 Ikemoto K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Community Medicine & Health Education
Abstract
Recent pharmacological studies has been shown the importance of trace amine-associated receptor, type 1 (TAAR1), a subtype of trace amine receptors, as a prospective target receptor for novel neuroleptics. In the present article, the author shows D-neuron research in psychiatric field. Although dopamine (DA) dysfunction is a well-known hypothesis of etiology of schizophrenia, its molecular basis has not yet been clarified. To explain this, modulating function of trace amines on DA neurotransmission was considered. The TAAR1 has a large number of ligands, including tyramine, β-phenylethylamine and methamphetamine that influence on human mental state. Reduced stimulation of TAAR1 on DA neurons in the midbrain ventral tegmental area (VTA) has been revealed to increase firing frequency of VTA DA neurons. Previously, the author and her colleagues reported D-neuron decrease in the striatum including nucleus accumbens of postmortem brains of patients with schizophrenia. This implies the decrease of trace amine synthesis and consequent reduction of TAAR1 stimulation on terminals of midbrain VTA DA neurons, and may lead to mesolimbic DA hyperactivity in schizophrenia. Striatal D-neuron decrease of postmortem brains, due to neural stem cell dysfunction in the subventricular zone of lateral ventricle, might be pivotal in etiology of schizophrenia. The new “D-cell hypothesis”, in which D-neurons and TAAR1 are involved, is concordant with recent reports showing effectiveness of TAAR1 ligands for schizophrenia model animals.
Keywords
Dopamine; D-neuron; Trace amine; Schizophrenia; TAAR1; Aromatic L-amino acid decarboxylase
Introduction
Dopamine (DA) dysfunction [1,2], glutamate dysfunction [3,4], neurodevelopmental deficits [5,6], or neural stem cell dysfunction [7,8] are well-known hypotheses for etiology of schizophrenia. DA dysfunction hypothesis suggested that mesolimbic DA hyperactivity caused positive symptoms such as paranoid-hallucinatory state of schizophrenia [1,2]. It is also explained by the efficacy of DA D2 blockers for paranoid-hallucinatory state and also by hallucinogenic acts of DA stimulants including methamphetamine or amphetamine [1,2]. Glutamate dysfunction theory was induced by the fact that intake of phencyclidine (PCP), an antagonist of NMDA receptor, produces equivalent to negative symptoms of schizophrenia, such as withdrawal or flattened affect, as well as positive symptoms [3,4]. The neurodevelopmental deficits hypothesis implicates that schizophrenia is the consequence of prenatal abnormalities resulting from the interaction of genetic and environmental factors [5,6]. Neural stem cell dysfunction has also been shown to be a cause of schizophrenia [7,8]. Although mesolimbic DA hyperactivity [1,2] has been well documented in pathogenesis of schizophrenia, the molecular basis of this mechanism has not yet been detailed. In the present article, the author hypothesized the involvement of striatal D-neurons and trace amine-associated receptor, type 1 (TAAR1) in the pathogenesis of mesolimbic DA hyperactivity of schizophrenia [9].
D-neuron
The “D-cell” was described in 1983 in the rat central nervous system and was defined “the non-monoaminergic aromatic L-amino acid decarboxylase (AADC)-containing cell” [10]. The D-cell contains AADC but not dopaminergic nor serotonergic [10]. D-cells produce trace amines [11,12], and may also act as an APUD (amine precursor uptake and decarboxylation) system that takes up amine precursors and transforms them to amines by decarboxylation [13]. The localizations of D-cells were specified into 14 groups, from D1 (the spinal cord) to D14 (the bed nucleus of stria terminalis) in caudo-rostral orders of the rat central nervous system using AADC immunohistochemistry [14,15]. In this usage, the classification term “D” means decarboxylation. In rodents [13,16,17], a small number of D-cells in the striatum were rostrally described and confirmed to be neurons by electron-microscopic observation [13]. I reported in 1997, “dopa-decarboxylating neurons specific to the human striatum [18-21]”, that is, “D-neurons” in the human striatum [20,22] (classified to be D15) [20], and later, the reduction of the number of D-neurons in the striatum, including nucleus accumbens of patients with schizophrenia [9,22].
Trace Amine-Associated Receptor, Type 1 (TAAR1)
Cloning of trace amine receptors in 2001 [23,24], elicited enormous efforts for exploring signal transduction of these G-protein coupled receptors whose genes are located on chromosome focus 6q23.1 [25]. The receptors have been shown to co-localize with dopamine or adrenaline transporters in monoamine neurons and to modulate the functions of monoamines [26-28]. The trace amine-associated receptor, type 1 (TAAR1) having a large number of ligands, including tyramine, β-phenylethylamine (PEA) and psychostimulants, for example methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA) and lysergic acid diethylamide (LSD) [23,25,29], has become a target receptor for exploring novel neuroleptics [30,31]. TAAR1 knockout mice showed schizophrenia-like behaviors with a deficit in prepulse inhibition [32]. TAAR1 knockout mice showed greater locomotor response to amphetamine and released more DA (and noradrenaline) in response to amphetamine than wild type mice [32]. It has been shown that TAAR1 has a thermoregulatory function [33]. It was clarified that increased stimulation of TAAR1 receptors on cell membranes of DA neurons in the midbrain ventral tegmental area (VTA) reduced firing frequency of VTA DA neurons [30-32].
A new “D-Cell Hypothesis” of schizophrenia
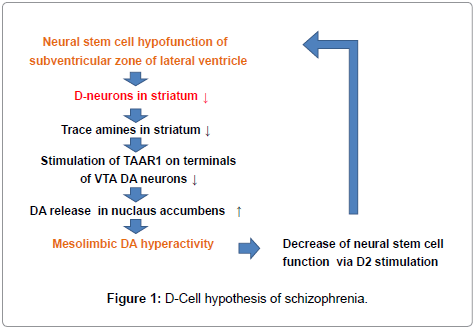
A new theory, “D-cell hypothesis”, for explaining mesolimbic DA hyperactivity in pathogenesis of schizophrenia is shown in Figure 1. In brains of patients with schizophrenia, dysfunction of neural stem cells in the subventricular zone of lateral ventricle causes the decrease of D-neurons in the striatum and nucleus accumbens [8,34]. This leads to the decrease of the amounts of trace amines in the nuclei, though direct evidences have not yet been demonstrated. Enlargement of the lateral ventricle [35,36], a usual finding documented in brain imaging studies of schizophrenia, is possibly due to dysfunction of neural stem cells of the subventricular zone [7,8].
The reduction of TAAR1 stimulation on DA terminals of VTA DA neurons, caused by trace amine decrease, would increase the firing frequency of VTA DA neurons [30,32]. This leads to the increase of DA release in the nucleus accumbens, resulted in mesolimbic DA hyperactivity. It has been shown that D2 stimulation of neural stem cells in the striatum inhibited forebrain neural stem cell proliferation [37]. Then, striatal DA hyperactivity may accelerate D-neuron decrease, which accelerates hyperactivity of mesolimbic DA system. Actions of D2 blocking agents in pharmacotherapy of schizophrenia might partially be explained by the decrease of inhibition to forebrain neural stem cell proliferations. It is consistent with clinical evidences that initial pharmacotherapy using D2 blockers is proved to be critical for preventing progressive pathognomonic procedures of schizophrenia.
Conclusion
The D-neuron, i.e., the possible trace amine-producing neuron, is a clue for schizophrenia research. Further exploration of signal transduction of the D-neuron is essential.
Acknowledgements
The present study was supported by Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (C-22591265).
References
- Hökfelt T, Ljungdahl A, Fuxe K, Johansson O (1974) Dopamine nerve terminals in the rat limbic cortex: aspects of the dopamine hypothesis of schizophrenia. Science 184: 177-179.
- Toru M, Nishikawa T, Mataga N, Takashima M (1982) Dopamine metabolism increases in post-mortem schizophrenic basal ganglia. J Neural Transm 54: 181-191.
- Watis L, Chen SH, Chua HC, Chong SA, Sim K (2008) Glutamatergic abnormalities of the thalamus in schizophrenia: a systematic review. J Neural Transm 115: 493-511.
- Olbrich HM, Valerius G, Rüsch N, Buchert M, Thiel T, et al. (2008) Frontolimbic glutamate alterations in first episode schizophrenia: evidence from a magnetic resonance spectroscopy study. World J Biol Psychiatry 9: 59-63.
- Christison GW, Casanova MF, Weinberger DR, Rawlings R, Kleinman JE (1989) A quantitative investigation of hippocampal pyramidal cell size, shape, and variability of orientation in schizophrenia. Arch Gen Psychiatry 46: 1027-1032.
- McGlashan TH, Hoffman RE (2000) Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry 57: 637-648.
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, et al. (2007) Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130: 1146-1158.
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, et al. (2006) Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry 11: 514-522.
- Ikemoto K, Nishimura A, Oda T, Nagatsu I, Nishi K (2003) Number of striatal D-neurons is reduced in autopsy brains of schizophrenics. Leg Med (Tokyo) 5: S221-224.
- Jaeger CB, Teitelman G, Joh TH, Albert VR, Park DH, et al. (1983) Some neurons of the rat central nervous system contain aromatic-L-amino-acid decarboxylase but not monoamines. Science 219: 1233-1235.
- Boulton AA (1974) Letter: Amines and theories in psychiatry. Lancet 2: 52-53.
- Boulton AA, Juorio AV (1979) The tyramines: are they involved in the psychoses? Biol Psychiatry 14: 413-419.
- Komori K, Fujii T, Karasawa N, Yamada K, Ikuko N (1991) Some neurons of the mouse cortex and caudo-putamen contain aromatic L-amino acid decarboxylase but not monoamines. Acta Histochem Cytochem 24: 571-577.
- Jaeger CB, Ruggiero DA, Albert V R, Joh TH, Reis DJ (1984) Immunocytochemical localization of aromatic-L-amino acid decarboxylase, in Handbook of Chemical Neuroanatomy. Classical Transmitters in the CNS, Part I. (Vol 2), Elsevier, Amsterdam 387-408.
- Jaeger CB, Ruggiero DA, Albert VR, Park DH, Joh TH, et al. (1984) Aromatic L-amino acid decarboxylase in the rat brain: immunocytochemical localization in neurons of the brain stem. Neuroscience 11: 691-713.
- Tashiro Y, Kaneko T, Sugimoto T, Nagatsu I, Kikuchi H, et al. (1989) Striatal neurons with aromatic L-amino acid decarboxylase-like immunoreactivity in the rat. Neurosci Lett 100: 29-34.
- Mura A, Linder JC, Young SJ, Groves PM (2000) Striatal cells containing aromatic L-amino acid decarboxylase: an immunohistochemical comparison with other classes of striatal neurons. Neuroscience 98: 501-511.
- Ikemoto K, Kitahama K, Jouvet A, Arai R, Nishimura A, et al. (1997) Demonstration of L-dopa decarboxylating neurons specific to human striatum. Neurosci Lett 232: 111-114.
- Ikemoto K, Nagatsu I, Kitahama K, Jouvet A, Nishimura A, et al. (1998) A dopamine-synthesizing cell group demonstrated in the human basal forebrain by dual labeling immunohistochemical technique of tyrosine hydroxylase and aromatic L-amino acid decarboxylase. Neurosci Lett 243: 129-132.
- Kitahama K, Ikemoto K, Jouvet A, Nagatsu I, Sakamoto N, et al. (1998) Aromatic L-amino acid decarboxylase- and tyrosine hydroxylase-immunohistochemistry in the adult human hypothalamus. J Chem Neuroanat 16: 43-55.
- Kitahama K, Ikemoto K, Jouvet A, Araneda S, Nagatsu I, et al. (2009) Aromatic L-amino acid decarboxylase-immunoreactive structures in human midbrain, pons, and medulla. J Chem Neuroanat 38: 130-140.
- Ikemoto K (2004) Significance of human striatal D-neurons: implications in neuropsychiatric functions. Prog Neuropsychopharmacol Biol Psychiatry 28: 429-434.
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, et al. (2001) Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol 60: 1181-1188.
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, et al. (2001) Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A 98: 8966-8971.
- Miller GM (2011) The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem 116: 164-176.
- Xie Z, Miller GM (2007) Trace amine-associated receptor 1 is a modulator of the dopamine transporter. J Pharmacol Exp Ther 321: 128-136.
- Xie Z, Miller GM (2009) Trace amine-associated receptor 1 as a monoaminergic modulator in brain. Biochem Pharmacol 78: 1095-1104.
- Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, et al. (2008) Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther 324: 948-956.
- Zucchi R, Chiellini G, Scanlan TS, Grandy DK (2006) Trace amine-associated receptors and their ligands. Br J Pharmacol 149: 967-978.
- Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, et al. (2009) The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci U S A 106: 20081-20086.
- Revel FG, Moreau JL, Pouzet B, Mory R, Bradaia A, et al. (2013) A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry 18: 543-556.
- Panas HN, Lynch LJ, Vallender EJ, Xie Z, Chen GL, et al. (2010) Normal thermoregulatory responses to 3-iodothyronamine, trace amines and amphetamine-like psychostimulants in trace amine associated receptor 1 knockout mice. J Neurosci Res 88: 1962-1969.
- Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, et al. (2007) The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav 6: 628-639.
- Ikemoto K (2008) Striatal D-neurons: in new viewpoints for neuropsychiatric research using post-mortem brains. Fukushima J Med Sci 54: 1-3.
- Degreef G, Ashtari M, Bogerts B, Bilder RM, Jody DN, et al. (1992) Volumes of ventricular system subdivisions measured from magnetic resonance images in first-episode schizophrenic patients. Arch Gen Psychiatry 49: 531-537.
- Horga G, Bernacer J, Dusi N, Entis J, Chu K, et al. (2011) Correlations between ventricular enlargement and gray and white matter volumes of cortex, thalamus, striatum, and internal capsule in schizophrenia. Eur Arch Psychiatry Clin Neurosci 261: 467-476.
- Kippin TE, Kapur S, van der Kooy D (2005) Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci 25: 5815-5823.
Relevant Topics
- Addiction
- Adolescence
- Children Care
- Communicable Diseases
- Community Occupational Medicine
- Disorders and Treatments
- Education
- Infections
- Mental Health Education
- Mortality Rate
- Nutrition Education
- Occupational Therapy Education
- Population Health
- Prevalence
- Sexual Violence
- Social & Preventive Medicine
- Women's Healthcare
Recommended Journals
Article Tools
Article Usage
- Total views: 14966
- [From(publication date):
July-2013 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 10395
- PDF downloads : 4571