Review Article Open Access
Currently Used Markers for CTC Isolation - Advantages, Limitations and Impact on Cancer Prognosis
Yicun Man, Qing Wang and Wolfgang Kemmner*Research Group, Surgical Oncology, Experimental Clinical Research Center, Max-Delbrueck-Center for Molecular Medicine, Berlin, Germany
- *Corresponding Author:
- Wolfgang Kemmner
ECRC at the Max-Delbrueck-Center for Molecular Medicine
Research Group Surgical Oncology
Robert Roessle Str. 10, 13125 Berlin, GERMANY
Tel: 49 030 9406 2506
Fax: 49 030 9406 2846; E-mail: wkemmner@mdc-berlin.de
Received Date: September 08, 2011; Accepted Date: November 18, 2011; Published Date: November 19, 2011
Citation: Man Y, Wang Q, Kemmner W (2011) Currently Used Markers for CTC Isolation - Advantages, Limitations and Impact on Cancer Prognosis. J Clinic Experiment Pathol 1:102. doi: 10.4172/2161-0681.1000102
Copyright: © 2011 Man Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Introduction
Metastasis caused by the entry of circulating tumor cells (CTC) into the bloodstream or lymphatic vessels is the major factor contributing to death in cancer patients [1]. Meanwhile, it is generally accepted that the detection of CTC is beneficial for early metastasis detection and provides independent prognostic information [2]. There is clear evidence that the presence of CTC in the blood of tumor patients correlates with poor overall survival of patients suffering from metastatic prostate, breast, and colon cancers [3]. For instance, a large study including 467 cases with metastatic colorectal cancer [4] showed that patients with a high number of CTC in the blood had a significantly decreased progression-free survival and overall survival. This study confirmed previous findings [5] demonstrating that a high CTC count was a strong indicator for poor survival. Accordingly, Cristofanilli [6] found that breast cancer patients with more than 5 CTC per 7.5 ml of whole blood had a shorter median progression-free survival in comparison to patients with less than 5 CTC per 7.5 ml. Thus, counting CTC yields prognostic information in almost every type of solid malignancy and is related to metastatic-tumor spread [7]. In the future, monitoring of CTC may become a routine assay for prediction of therapeutic efficacy in cancer patients, as well as for improving the stratification of patients in need of adjuvant therapies. Follow this assumption, separation, detection and characterization of CTC is relevant not only to understand what drives cancer diseases and metastasis, but also for improving prognosis and novel therapeutic strategies of metastatic cancer [8,9].
Isolation and detection CTC is hampered by the fact that their number is low and that few of them are mixed with approximately 10 million leukocytes and 5 billion erythrocytes per 1ml blood [10]. Several properties distinguish CTC (antigen independent) from most normal blood cells, including the larger size of most epithelial tumor cells compared to peripheral blood leukocytes [11,12] and differences in density [13], charge, migratory properties, and properties of specific cell types (e.g. melanocytic granules in melanoma cells). Approaches based on these properties to filter out CTC from blood have shown to be suitable for capturing cancer cells spiked into blood. However, further validation for the use of clinical specimens is needed, in which both heterogeneous CTC size [14], the high number of contaminating blood cells, and the resistance to filtering shear stress [15] is likely to be significant variables.
The most widely used CTC isolation technique relies on antibodybased capture of CTC, which target epithelial-specific markers or tumor-specific antigens present on the CTC surface. Each of the current CTC-isolating markers (Table 1) reveals virtually advances and might provide a basis from which to anticipate ongoing technological developments. Nonetheless, false positive selection may occur due to expression of the epithelial markers in non-epithelial cells [13]. In addition, false negative selection may be due to the heterogeneity of tumor cells which may variably or not at all express a given marker [16]. Moreover, the specific method to capture CTC with markers varies widely between studies, leading to a broad range of reported CTC frequencies even in patients with the same type of tumor [17]. In the absence of any gold standard used to measure the efficiency of the various markers, defining the sensitivity (true-positive, without a wrong identification of “tumor cells” as “non-tumor cells”) and specificity (true-negative, without a wrong identification of “nontumor cells” like epithelial non tumor cells as “tumor cells”) of these markers for selection of CTC remains a challenge.
| Specificity and Sensitivity of different membrane molecules used to detect CTC | ||||
|---|---|---|---|---|
| Marker | Tumor tissue | % cases in which CTC have been reported | false positive in healthy donors | References |
| EpCAM | Lung, Prostate, Colon, Breast, Bladder | Breast cancer: 70% (n=92) | 0 (n=5) | [19-22] |
| EphB4 | Breast, Colorectal, Head & Neck | Breast cancer: 77.5% (n=40) | 0 (n=47) | [41-43] |
| EGFR | Breast, Colon, Gastric | Colon cancer: 18% (n=19) | 0.5% (n=38) | [54-60] |
| CEA | Breast, Gastric, Pancreatic | Colon cancer: 66% (n=25) | 20% (n=10) | [47, 54, 55, 65-68] |
| HER2 | Breast, Gastric | Advanced breast cancer: 37% (n=15) Early stage breast cancer: 48.6% (n=35) |
0 (n=42) | [48, 49, 51, 52] |
| MUC-1 | Colorectal, Ovarian, Breast, Prostate | Breast cancer: 88.2% (n=34) | 24% (n=29) | [67, 71-75] |
| N - Patient number involved in study | ||||
Table 1: Specificity and Sensitivity of different membrane molecules used to detect CTC.
Yet, apart from the lack of common specific markers applicable for CTC screening, which are related to the requirement of high sensitivity combined with high specificity, two more major challenges for CTC still exist: the extraordinarily low frequency of CTC, comprising as few as one CTC per one billion normal blood cells in the circulation of patients with advanced cancer, and secondly the heterogeneity and plasticity of CTC, including subpopulations which have lost characteristic epithelial features [18]. Our understanding of CTC’ biological properties is thus been limited by the availability of technologies capable of isolating them in sufficient numbers. Subsequent improvements to efficiently yield purer CTC populations amenable to better cellular and molecular characterization in a viable and intact state are thought to be of vital significance.
Markers
EpCAM
Background: The Epithelial Cell Adhesion Molecule (EpCAM) is ubiquitously expressed on the surface of epithelial cells and carcinoma cells.
Current state: Using EpCAM, CTC were detected in peripheral blood in approximately 70% of 92 metastatic breast cancer patients [19]. Similarly, EpCAM-positive CTC were detected in the blood of 61.3% colon (n=31) and 70.8% prostate (n=24) metastatic cancer patients [20], who have had ≥2 CTC/7.5 ml (a CTC count ≥2 was defined as clinically significant), as well as 33% of 6 metastatic stomach cancer patients [21] and 30% of 50 patients with bladder cancer [22]. A current study indicated that EpCAM-positive CTC were also detected in the blood of 43% (n=26) patients with neuroendocrine tumors (NETs), who showed midgut metastasis. Similarly, 21% of 6 patients with pancreatic metastasis and 31% (n=13) with bronchopulmonary NETs had detectable EpCAM-positive CTC [23].
Regarding the specificity of EpCAM [24], no positive sample was observed among five healthy donors confirming previous results from Allard [25] who found 1 CTC per 7.5 ml blood only in 8 of 145 samples from healthy women and no sample with ≥2 CTC. This study demonstrated that EpCAM is indeed an exclusive selection factor for the isolation of CTC. Moreover, Antolovic [24] reported recently that the EpCAM-specific monoclonal antibody KS1/4 retrieved 10 fold more CTC than the commonly used BerEP4 antibody. Thus, the use of different EpCAM antibodies might enhance its CTC capture rate. After receiving chemotherapy treatment for metastatic breast cancer, 41.4% (n=58) patients had decreased CTC levels according to EpCAM expression and enumeration, and these patients survived significantly longer than patients with increased CTC levels (17.67±5.90 months versus 4.53±0.54 months) [26]. Similarly, in a study of 430 patients with metastatic colorectal cancer, Cohen [27] found that the overall survival of patients with more than 3 CTC per 7.5 ml was shorter (9.4 months versus 20.6 months) than that of patients with less than 3 CTC per 7.5ml.
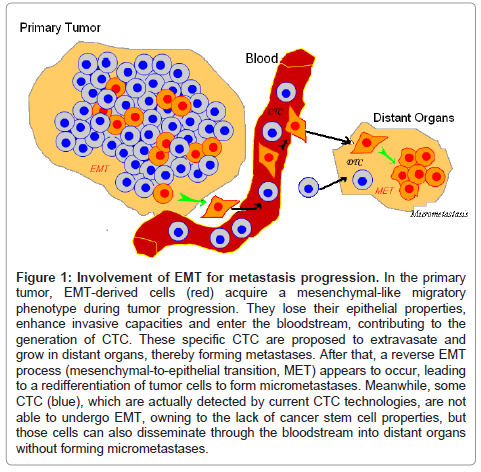
Limitations: Zhong XY has illustrated that EpCAM is not a perfect marker for CTC selection due to the high variation in its gene expression between tumor subtypes and its illegitimate transcription in leukocytes [28]. Another major drawback is that EpCAM can be downregulated during epithelial-to-mesenchymal transition (EMT), a process attributed to disseminating cancer cells (Figure 1) [29]. This transition affects tumor cells with stem cell-like properties in particular [30], which means that assays targeting epithelial cells in blood are susceptible to missing the detection of the most invasive tumor cells. This might be the reason why EpCAM has been found to be expressed in only 70% of 134 tumors with different histologic types [31]. Of note, 62% of CTC isolated from the blood of 69 patients with metastatic breast cancer were positive for at least one EMT marker [32], such as Twist1, Akt2 or PI3Kalpha.
Figure 1: Involvement of EMT for metastasis progression. In the primary tumor, EMT-derived cells (red) acquire a mesenchymal-like migratory phenotype during tumor progression. They lose their epithelial properties, enhance invasive capacities and enter the bloodstream, contributing to the generation of CTC. These specific CTC are proposed to extravasate and grow in distant organs, thereby forming metastases. After that, a reverse EMT process (mesenchymal-to-epithelial transition, MET) appears to occur, leading to a redifferentiation of tumor cells to form micrometastases. Meanwhile, some CTC (blue), which are actually detected by current CTC technologies, are not able to undergo EMT, owning to the lack of cancer stem cell properties, but those cells can also disseminate through the bloodstream into distant organs without forming micrometastases.
Techniques used: Among the EpCAM-based technologies (Table 2), the CellSearch CTC test has gained considerable attention and is the only diagnostic test that is currently approved by the US Food and Drug Administration for the automated detection and enumeration of circulating tumor cells [33]. In parallel, Nagrath et al. [34] proposed that the CTC-chip, a microfluidic platform which consists of an array of anti-EpCAM antibody-coated microposts for single-step isolation of CTC from unprocessed blood specimens, enabled a high yield of capture (median, 50 CTC/ml) and purity (average, 49% in metastatic prostate, 60% in metastatic breast and 67% in metastatic colon carcinomas). Another automated immunomagnetic separator, the slowly rotating MagSweeper, coated with antibody to EpCAM has also been tested to capture CTC from blood samples [35]. Recently, a new CTC-capture platform, named Nanostructured Substrates that integrates two functional components, a patterned SiNP substrate with anti-EpCAM and an overlaid polydimethylsiloxane (PDMS) chip with a serpentine chaotic mixing channel was employed for recognizing/ capturing CTC [36].
| Summary of different EpCAM-based CTC isolation approaches | |||
|---|---|---|---|
| Isolation process | Advantages | Disadvantages | References |
| CellSearch | High sensitivity CTC quantification |
Subjective images interpretation no further analysis possible |
[33] |
| CTC Chip | 98% cell viability high identification rate further analysis possible |
lack of validation studies in clinical settings not commercially available |
[34] |
| MagSweeper | high degree of purity further analysis possible |
not commercially available | [35] |
| Nanostructured Substrates |
increasing cell-substrate contact frequency & high affinity |
not commercially available | [36] |
Table 2: Summary of different EpCAM-based CTC isolation approaches.
Summary: EpCAM remains the most frequently used marker for isolating CTC. Its usefulness has been confirmed in many studies of different kinds of solid tumors. However, the loss of EpCAM during EMT reduces its value for CTC isolation. Furthermore, EpCAM-based CTC isolation strongly depends on the CTC’s EpCAM expression. Of note, EpCAM shows a good correlation with the prognosis for cancer survival. Thus, EpCAM might be useful for monitoring treatment benefit and its detection might allow finding a significantly earlier time point for optimized therapy.
EphB4
Background: The ephrin receptor (EphB4) is a member of the Eph-family of receptor protein tyrosine kinases and has recently been reported to be up-regulated in several cancers [37-39]. These receptor tyrosine kinases and their membrane-anchored ephrin ligands mediate cell-cell contact signalling and are versatile regulators of cell migration and tissue patterning, which are often exploited by cancer cells during tumor progression [40]. Because of their high expression, these surface markers have also been used for CTC-selection.
Current state: EphB4 is strongly over-expressed in the blood of patients with advanced head and neck cancers: eleven were positive for CTC in a study encompassing 16 patients [41]. EphB4 has also been used successfully for CTC isolation in breast cancer [42], 36% of patients (n=56) had moderate to strong expression on CTC. With regard to the specificity, blood samples from 10 normal donors analysed in a similar study regarding breast cancer were all found to be negative for EphB4 [42]. In agreement another study showed that expression of EphB4 marker in peripheral blood samples (n=47) was indeed 100% negative [43].
Limitations: As an epithelial-specific marker, EphB4 might label also non-tumor epithelial cells, thus giving false positive results [44]. Furthermore, the non-specific binding involves also Fc-receptorbearing leukocytes and monocytes or illegitimate expression of epithelial antigens in normal hematopoietic cells [25]. Additionally, some of these positive cells are morphologically difficult to distinguish from CTC, therefore leading to an increased amount of false positives. Variable numbers of EphB4-positive epithelial cells [45] have been found in peripheral blood of subjects without malignancy, being related to benign epithelial proliferative diseases, inflammation and surgical interventions [46].
Summary: EphB4 has a high sensitivity, especially for CTC from patients with head and neck cancer, but is seldomly used for other solid tumors. Expression of EphB4 was not found to be significantly associated with disease-free survival, although a trend towards poorer disease-free survival of patients with high EphB4 expression was observed [42].
HER2
Background: The epidermal growth factor receptor (EGFR) or HER/ erbB family of tyrosine kinase receptors is widely recognized as a component of signal transduction network that is deregulated in several major cancers. EGFR/ HER1, ErbB2/HER2, ErbB3/HER3 and ErbB4/HER4 are members of this family and play a major role in the pathogenesis of many solid tumors including breast, prostate, and gastric cancer. At present, the HER2/ERBB2 immunohistochemical score of breast carcinomas is used to guide therapy decisions for the application of humanized anti-HER2 monoclonal antibodies, leading to a significantly improved disease free and overall survival [47].
Current state: Several studies have reported that isolation of CTC from the peripheral blood of breast cancer patients is possible by using HER2/ERBB2 as target gene. As for sensitivity, HER2 was shown to be worse than EpCAM. Out of 66 advanced breast cancer patients, forty (61%) had EpCAM-positive CTC samples, and of those, 15 (37%) had HER2-positive CTC [48]. Of note, results from HER2-positive CTC have shown that metastasis of breast carcinoma might be an unexpectedly early event. Wulfing [49] showed that already 17 from 35 patients (48.6%) in the early stages Ι or II showed positive CTC. These cells might represent an undetected spread of the tumor and thus might serve as surrogate markers for prognosis estimates. Besides breast cancer, HER2 was also used for CTC selection in gastric cancer [50]. Considering the specificity, no HER2-positive CTC (n=42) were detected in healthy women [51]. Nevertheless, recently an unexpectedly high per cell level of HER2 was discovered in peripheral blood natural killer cell and granulocyte population [10], which might increase the background noise and compromise its specificity of CTC isolation.
Limitations: High concentrations of HER2 were found in the serum of patients with HER2-negative primary tumors. Only a part of the HER2-overexpressing cancer cells may have the potential to disseminate [49]. Discordant HER2 expression was also found in HER2-positive primary breast carcinomas, where 5 of 12 (42%) patients had exclusively HER2-negative CTC [48,52].
Summary: HER2 is mostly used in breast cancer with high specificity, and may be employed for early recognition of tumor sensitivity to a given therapeutic regimen. Its value for CTC selection is similar to that of other markers. Patients with HER2-positive CTC show a reduced disease-free and overall survival [53].
EGFR
Background: EGFR (also known as Her/ErbB1) is a member of the ErbB family of receptor tyrosine kinases responsible for regulating an intricate network of signalling pathways during cell growth, survival, adhesion and motility and is overexpressed in a variety of epithelial carcinomas, including neuronal, lung and breast.
Current state: Recently, it was found that the CTC of 18% (n=19) patients with metastatic colorectal carcinomas were positive for EGFR [54]. Moreover, there were similar findings from other groups demonstrating low EGFR expression on CTC in about 15% of the metastases of 25 colorectal cancer patients [55], and in 10% of 20 cases with metastatic colon carcinoma [56]. EGFR was also reported to be used for CTC selection in breast [57] and gastric [58] cancers. However, it has been showed that EGFR is also expressed in 10.5% (n=38) of peripheral blood of healthy donors [59,60].
Limitations: Interestingly, there is now evidence that EGFR can induce metastasis and promote EMT [61], However, inhibition of EGFR does not block the epithelial–mesenchymal transition (EMT) induced by TGFbeta1 in hepatocytes, which indicates that activation of EGFR plays an essential role in impairing apoptosis, but it is dispensable for the EMT process [62]. This probably explains why nearly no EGFR expression was detected in most CTC-positive patients with metastases, whereas approximately half of the patients express EGFR as demonstrated by immunohistochemistry.
Summary: Sensitivity of EGFR is not high, although it has been used for CTC-isolation in a variety of carcinomas, especially breast and colon cancer. Of note, EGFR-expression has been found more frequently on CTC in early stages of carcinomas than on CTC of patients with metastasis [55]. However, there was no direct correlation between the EGFR expression in CTC and that in the metastasis.
CEA
Background: Another marker often used for CTC selection is the carcinoembryonic antigen (CEA/CEACAM5) which is known to have a role for several biological functions, including cell–cell adhesion [63,64]. CEA is described as an epithelial marker as well as an oncofetal marker.
Current state: In comparison to EGFR, CEA has a higher sensitivity for metastatic colorectal cancer [55]. CEA expression was found in 66% of the CTC of already metastasised patients (n=25), but EGFR expression was only detected on 15% of the CTC. In another study, CEA expression was found in 91% (n=19) of CTC positive blood samples in metastatic colorectal patients [54]. In contrast to EGFR, the CTC expression of CEA was reduced in early stages of colorectal cancer patients [55]. In metastatic breast cancers (n=50), five (10%) had CEApositive CTC in the peripheral blood [65]. High expression of CEA on CTC was also reported for gastric [47] and pancreatic carcinomas [66] With regard to specificity, however, CEA has been detected in the blood of healthy donors and in patients with inflammatory bowel diseases [67]. In comparison only 4 of 38 (10.5%) healthy donors showed EGFR, nearly half-fold than CEA with 20% (n=10) during the same study [68].
Limitations: False negative results occur generally, since CEA is not present on all tumor cells.
Summary: CEA has a higher sensitivity for metastatic tumors than for early staged carcinomas, but its specificity is poor. Remarkably, detection of CEA in metastatic breast cancer patients has been shown to be an independent prognostic factor regardless whether determined before or after surgery. Patients with CEA-positive CTC either preor post-operative had a significant shorter disease-free survival than patients without CEA-positive CTC [69].
MUC-1
Background: Most glandular epithelial cells express the membranebound mucin encoded by the mucin-1 (MUC-1) gene at their apical cell surface. Expression of MUC-1 is increased considerably in the majority of carcinomas. Recent studies have shown that a high amount of MUC- 1 on the cell surface decreases cell adhesion, favors dissemination and is associated with poor prognosis [70].
Current state: In epithelial ovarian tumors (n=86), 19% showed high MUC-1 reactivity [71]. In metastatic breast cancer (n= 35), CTC were selected by MUC-1 specific expression, with a rate of 88.2% before chemotherapy [72]. In advanced pancreatic adenocarcinoma (n=39), 49.3% peripheral blood samples expressed CTC [73]. Moreover, MUC- 1-positive CTC have been detected in the peripheral blood of patients with colorectal [74] and prostate [75] cancers. However, MUC-1- positive CTC have been repeatedly shown to be expressed on leukocytes in blood samples from healthy donors and controls. In one study, it was reported to be expressed in 70% blood samples from 40 normal subjects and 73% of 15 patients with haematological malignancies [67].
Summary: MUC-1 has a relatively highly sensitivity for ovarian tumors, but its specificity is poor. CTC’s MUC-1 expression in metastatic colorectal cancer [74] showed a prognostic value for the estimation of the survival time of CTC-positive Dukes A/B patients. In pancreatic cancer, a high amount of MUC-1-positive CTC has been associated with a higher aggressiveness and a shorter median overall survival [76].
Conclusion
Detection and characterization of circulating tumor cells (CTC) in the blood of tumor patients undoubtedly is a valuable and revolutionary tool for detecting metastasis and has the potential to become a standard tool for tumor diagnosis and prognosis. This notion is underlined by the fact that CTC are also able of self-seeding back to their original organs [77] and thus lead to the formation of local recurrences. Furthermore, characterization of CTC will improve our understanding of the resistance to cytotoxic therapies.
Several selection methods, especially those which target specific tumor markers expressed on CTC, have been utilized in various clinical settings. Effort is being made to validate these tumor markers, but of course there is no unique one identified which will be specific enough to capture all rare CTC. Actually, there is no antibody available which is 100% tumor- or tissue-specific [16]. Another limitation, which confounds the interpretation of the results, is that tumorassociated markers are not always cancer-specific but also expressed in healthy samples or by non-epithelial leukocytes due to illegitimate transcription. Besides, due to genomic instability of malignant cells, the intermittent shedding of CTC surface markers can lead to false negative results [78,79]. For instance, EpCAM is not a perfect marker for CTC selection due to the high variation in its gene expression between tumor subtypes and its illegitimate transcription in leukocytes [28]. Furthermore, the phenomenon of metastatic inefficiency (i.e. not all CTC which are detected with current methods are viable and able to settle in secondary organs) can obscure the prognostic value of a strategy that is based simply on the presence or absence of CTC. Therefore, only markers that are stably expressed because of an essential role for cancer-cell survival or aggressiveness should be taken into consideration.
A logical way to deal with these issues may be to obtain multiple samples from each patient and to analyze the expression of multiple markers, thereby increasing the probability of detecting CTC. In general, the healthy controls of all studies reported so far were never positive for two markers [80]. Thus, the more molecular markers used for CTC detection, the higher the specificity of the method becomes. Using a combination of two or three markers could be considered. For instance negative selection in order to get rid of leukocytes, performed by using magnetic beads coated with anti-CD45 antibody specific for a surface marker expressed only on leukocytes, is able to complement positive selection by EpCAM excellently [81,82]. Accordingly, an anti-CD61 antibody can be used for the surface marker expressed only on megakaryocytes and platelets [81,82]. In this way, the cellular population is depleted of unwanted cells, while the epithelial cells, not expressing CD45 or CD61, are left in solution. Furthermore, the combination of E-selectin and EpCAM has been proved to result in a more than 3-fold enhancement of CTC separation capacity and capture efficiency [83]. Similar results were found by Mostert et al. [84], who introduced CD146 for the detection of EpCAM-negative CTC in breast cancer patients. In addition, Bischoff et al. demonstrated that by using antibody mixtures, additional EpCAM-negative and CK-negative CTC could be collected [85]. This in agreement with other studies [1,86] confirming that the rate of positive findings of marker combinations was significantly higher than that of an individual maker. The use of a combination of several molecular markers is hence important for obtaining a specific and reliable positive result.
Stem cell and EMT markers could complement the ones discussed above. CTC might have tumor-initiating properties of cancer stem cells (CSC) but also own additional intravasation and extravasation properties. Therefore, preliminary experiments could focus on the overlap with markers of CSC and their levels in CTC (ie.CD133+). Encouragingly, a recent finding indicates that a CD26+ subpopulation of CD133+ colorectal cancer cells has a unique metastatic potential [87]. These cells exclusively form liver metastasis when injected into the cecal wall of mice. Future efforts to improve CTC selection could also explore the possibility of low EpCAM expression by CTC, as this has been noted in epithelial–mesenchymal transitions, a process that CTC may undergo during early stages of metastasis. It is possible, although not proven that Neural Cadherin (N-cadherin), a mesenchymal adhesion molecule, which is associated with a heightened invasive potential in cancer [88] and upregulated in several aggressive cancers [89], can be used as an innovative marker for CTC selection. Interestingly, this marker molecule increases in EMT [90-92]. Furthermore, according to studies of Armstrong et al, who were collecting patients with metastatic prostate cancer and metastatic breast cancer, a high frequency of CTC co-expressed epithelial, mesenchymal and stem-cell markers CTC [93].
Together, as new markers evolve further, a significant and maximum optimization is necessary to enable highly sensitive and reliable CTC isolation before the characterization of CTC by the full array of currently available molecular tools is no longer a fairy tale.
References
- Wharton RQ, Jonas SK, Glover C, Khan ZA, Klokouzas A, et al. (1999) Increased detection of circulating tumor cells in the blood of colorectal carcinoma patients using two reverse transcription-PCR assays and multiple blood samples. Clin Cancer Res 5: 4158-4163.
- Lianidou ES, Mavroudis D, Sotiropoulou G, Agelaki S, Pantel K (2010) What's new on circulating tumor cells? A meeting report. Breast Cancer Res 12: 307.
- Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, et al. (2010) Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A 107: 18392-18397.
- Tol J, Koopman M, Miller MC, Tibbe A, Cats A, et al. (2010) Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol 21: 1006-1012.
- Scher HI, Jia X, de Bono JS, Fleisher M, Pienta KJ, et al. (2009) Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol 10: 233-239.
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, et al. (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351: 781-791.
- Muller V, Stahmann N, Riethdorf S, Rau T, Zabel T, et al. (2005) Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res 11: 3678-3685.
- Pantel K, Muller V , Auer M, Nusser N, Harbeck N, et al. (2003) Detection and clinical implications of early systemic tumor cell dissemination in breast cancer. Clin Cancer Res 9: 6326-6334.
- Maheswaran S, Haber DA (2010) Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev 20: 96-99.
- You F, Roberts LA, Kang SP, Nunes RA, Dias C, et al. (2008) Low-level expression of HER2 and CK19 in normal peripheral blood mononuclear cells: relevance for detection of circulating tumor cells. J Hematol Oncol 1: 2.
- Vona G, Sabile A, Louha M, Sitruk V, Romana S, et al. (2000) Isolation by size of epithelial tumor cells : a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol 156: 57-63.
- Desitter I, Guerrouahen BS, Benali-Furet N, Wechsler J, Janne PA, et al. (2011) A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res 31: 427-441.
- Gertler R, Rosenberg R, Fuehrer K, Dahm M, Nekarda H, et al. (2003) Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. Recent Results Cancer Res 162: 149-155.
- Marrinucci D, Bethel K, Bruce RH, Curry DN, Hsieh B, et al. (2007) Case study of the morphologic variation of circulating tumor cells. Hum Pathol 38: 514-519.
- Mohamed H, Murray M, Turner JN, Caggana M (2009) Isolation of tumor cells using size and deformation. J Chromatogr A 1216: 8289-8295.
- Goeminne JC, Guillaume T, Symann M (2000) Pitfalls in the detection of disseminated non-hematological tumor cells. Ann Oncol 11: 785-792.
- Miller MC, Doyle GV, Terstappen LW (2010) Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J Oncol 2010: 617421.
- Riethdorf S, Pantel K (2010) Advancing personalized cancer therapy by detection and characterization of circulating carcinoma cells. Ann N Y Acad Sci 1210: 66-77.
- Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, et al. (2007) Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 13: 920- 928.
- Maestro LM, Sastre J, Rafael SB, Veganzones SB, Vidaurreta M, et al. (2009) Circulating tumor cells in solid tumor in metastatic and localized stages. Anticancer Res 29: 4839-4843.
- Balic M, Dandachi N, Hofmann G, Samonigg H, Loibner H, et al. (2005) Comparison of two methods for enumerating circulating tumor cells in carcinoma patients. Cytometry B Clin Cytom 68: 25-30.
- Rink M, Chun FK, Minner S, Friedrich M, Mauermann O, et al. (2010) Detection of circulating tumour cells in peripheral blood of patients with advanced nonmetastatic bladder cancer. BJU Int 107: 1668-1675.
- Khan MS, Tsigani T, Rashid M, Rabouhans JS, Yu D, et al. (2011) Circulating tumor cells and EpCAM expression in neuroendocrine tumors. Clin Cancer Res 17: 337-345.
- Antolovic D, Galindo L, Carstens A, Rahbari N, Buchler MW, et al. (2010) Heterogeneous detection of circulating tumor cells in patients with colorectal cancer by immunomagnetic enrichment using different EpCAM-specific antibodies. BMC Biotechnol 10: 35.
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, et al. (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10: 6897- 6904.
- Hartkopf AD, Waqner P, Wallwiener D, Fehm T, Rothmund R (2011) Changing levels of circulating tumor cells in monitoring chemotherapz response in patients with metastatic breast cancer. Anticancer Res 31: 979-984.
- Cohen SJ, Punt CJ, Lannotti N, Saidman BH, Sabbath KD, et al. (2009) Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol.20: 1223-1229.
- Zhong XY, Kaul S, Eichler A, Bastert G (1999) Evaluating GA733-2 mRNA as a marker for the detection of micrometastatic breast cancer in peripheral blood and bone marrow. Arch Gynecol Obstet 263: 2-6.
- Pantel K, Alix-Panabieres C (2010) Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med 16: 398-406.
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, et al. (2008) The epithelialmesenchymal transition generates cells with properties of stem cells. Cell 133: 704-715.
- Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, et al. (2004) Frequent EpCam protein expression in human carcinomas. Hum Pathol 35: 122-128.
- Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, et al. (2009) Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 11: R46.
- Food and Drug Administration (2004) Medical devices; immunology and microbiology devices; classification of the immunomagnetic circulating cancer cell selection and enumeration system. Final rule. Fed Regist 69: 26036-26038.
- Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, et al. (2007) Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450: 1235-1239.
- Talasaz AH, Powell AA, Huber DE, Berbee JG, Roh KH, et al. (2009) Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proc Natl Acad Sci U S A 106: 3970-3975.
- Wang S, Liu K, Liu J, Yu ZT, Xu X, et al. (2011) Highly Efficient Capture of Circulating Tumor Cells by Using Nanostructured Silicon Substrates with Integrated Chaotic Micromixers. Angew Chem Int Ed Engl 50: 3084-3088.
- Stephenson SA, Slomka S, Douglas EL, Hewett PJ, Hardingham JE (2001) Receptor protein tyrosine kinase EphB4 is up-regulated in colon cancer. BMC Mol Biol 2: 15.
- Nikolova Z, Djonov V, Zuercher G, Andres AC, Ziemiecki A (1998) Cell-type specific and estrogen dependent expression of the receptor tyrosine kinase EphB4 and its ligand ephrin-B2 during mammary gland morphogenesis. J Cell Sci 111: 2741-2751.
- Tang XX, Brodeur GM, Campling BG, Ikegaki N (1999) Coexpression of transcripts encoding EPHB receptor protein tyrosine kinases and their ephrin-B ligands in human small cell lung carcinoma. Clin Cancer Res 5: 455-460.
- Wang B (2011) Cancer cells exploit the eph-ephrin system to promote invasion and metastasis: tales of unwitting partners. Sci Signal 4: e28.
- Winter SC, Stephenson SA, Subramaniam SK, Paleri V, Ha K, et al. (2009) Long term survival following the detection of circulating tumour cells in head and neck squamous cell carcinoma. BMC Cancer 9: 424.
- Raynor MP, Stephenson SA, Pittman KB, Walsh DC, Henderson MA, et al. (2009) Identification of circulating tumour cells in early stage breast cancer patients using multi marker immunobead RT-PCR. J Hematol Oncol 2: 24.
- Lloyd JM, McIver CM, Stephenson SA, Hewett PJ, Rieger N, et al. (2006) Identification of early-stage colorectal cancer patients at risk of relapse postresection by immunobead reverse transcription-PCR analysis of peritoneal lavage fluid for malignant cells. Clin Cancer Res 12: 417-423.
- Paterlini-Brechot P, Benali NL (2007) Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett 253: 180-204.
- Fehm T, Solomayer EF, Meng S, Tucker T, Lane N, et al. (2005) Methods for isolating circulating epithelial cells and criteria for their classification as carcinoma cells. Cytotherapy 7: 171-185.
- Crisan D, Ruark DS, Decker DA, Drevon AM, Dicarlo RG (2000) Detection of circulating epithelial cells after surgery for benign breast disease. Mol Diagn 5: 33-38.
- Pantel K, Brakenhoff RH (2004) Dissecting the metastatic cascade. Nat Rev Cancer 4: 448-456.
- Pestrin M, Bessi S, Galardi F, Truglia M, Biggeri A, et al. (2009) Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat 118: 523-530.
- Wulfing P, Borchard J, Buerger H, Heidl S, Zanker KS, et al. (2006) HER2- positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res 12: 1715-1720.
- Ross JS (2011) Update on HER2 testing for breast and upper gastrointestinal tract cancers. Biomark Med 5: 307-318.
- Ignatiadis M, Rothe F, Chaboteaux C, Durbecq V, Rouas G, et al. (2011) HER2-positive circulating tumor cells in breast cancer. PLoS One 6: e15624.
- Riethdorf S, Muller V, Zhang L, Rau T, Loibl S, et al. (2010) Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res 16: 2634-2645.
- Apostolaki S, Perraki M, Kallergi G, Kafousi M, Papadopoulos S, et al. (2009) Detection of occult HER2 mRNA-positive tumor cells in the peripheral blood of patients with poerable breast cancer: evaluation of their prognostic relevance. Breast Cancer Res Treat 117: 525-534.
- Lankiewicz S, Rother E, Zimmermann S, Hollmann C, Korangy F, et al. (2008) Tumour-associated transcripts and EGFR deletion variants in colorectal cancer in primary tumour, metastases and circulating tumour cells. Cell Oncol 30: 463- 471.
- Zieglschmid V, Hollmann C, Mannel J, Albert W, Jaeschke-Melli S, et al. (2007) Tumor-associated gene expression in disseminated tumor cells correlates with disease progression and tumor stage in colorectal cancer. Anticancer Res 27: 1823-1832.
- Lankiewicz S, Zimmermann S, Hollmann C, Hillemann T, Greten TF (2008) Circulating tumour cells as a predictive factor for response to systemic chemotherapy in patients with advanced colorectal cancer. Mol Oncol 2: 349- 355.
- Payne RE, Yague E, Slade MJ, Apostolopoulos C, Jiao LR, et al. (2009) Measurements of EGFR expression on circulating tumor cells are reproducible over time in metastatic breast cancer patients. Pharmacogenomics 10: 51-57.
- Dragovich T, Campen C (2009) Anti-EGFR-Targeted Therapy for Esophageal and Gastric Cancers: An Evolving Concept. J Oncol 2009: 804108.
- Grunewald K, Haun M, Urbanek M, Fiegl M, Muller-Holzner E, et al. (2000) Mammaglobin gene expression: a superior marker of breast cancer cells in peripheral blood in comparison to epidermal-growth-factor receptor and cytokeratin-19. Lab Invest 80: 1071-1077.
- Corradini P, Voena C, Astolfi M, Delloro S, Pilotti S, et al. (2001) Maspin and mammaglobin genes are specific markers for RT-PCR detection of minimal residual disease in patients with breast cancer. Ann Oncol 12: 1693-1698.
- Louderbough JM, Schroeder JA (2009) EGFR-induced EMT is modulated by the ECM: the role of hyaluronan in the inhibition of breast cancer metastasis. Clinical & Experimental Metastasis 26: 899.
- Murillo MM, del CG, Sanchez A, Fernandez M, Fabregat I (2005) Involvement of EGF receptor and c-Src in the survival signals induced by TGF-beta1 in hepatocytes. Oncogene 24: 4580-4587.
- Gangopadhyay A, Lazure DA, Thomas P (1998) Adhesion of colorectal carcinoma cells to the endothelium is mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp Metastasis 16: 703-712.
- Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, et al. (1989) Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell 57: 327-334.
- Stathopoulou A, Mavroudis D, Perraki M, Apostolaki S, Vlachonikolis I, et al. (2003) Molecular detection of cancer cells in the peripheral blood of patients with breast cancer: comparison of CK-19, CEA and maspin as detection markers. Anticancer Res 23: 1883-1890.
- Zach O, Lutz D (2006) Tumor cell detection in peripheral blood and bone marrow. Curr Opin Oncol 18: 48-56.
- Zieglschmid V, Hollmann C, Bocher O (2005) Detection of disseminated tumor cells in peripheral blood. Crit Rev Clin Lab Sci 42: 155-196.
- De LA, Pignata S, Casamassimi A, D'Antonio A, Gridelli C, et al. (2000) Detection of circulating tumor cells in carcinoma patients by a novel epidermal growth factor receptor reverse transcription-PCR assay. Clin Cancer Res 6: 1439-1444.
- Jotsuka T, Okumura Y, Nakano S, Nitta H, Sato T, et al. (2004) Persistent evidence of circulating tumor cells detected by means of RT-PCR for CEA mRNA predicts early relapse: a prospective study in node-negative breast cancer. Surgery 135: 419-426.
- Ligtenberg MJ, Buijs F, Vos HL, Hilkens J (2000) Suppression of cellular aggregation by high levels of episialin. Cancer Res 52: 2318-2324.
- Aktas B, Kasimir-Bauer S, Heubner M, Kimmig R, Wimberger P (2011) Molecular profiling and prognostic relevance of circulating tumor cells in the blood of ovarian cancer patients at primary diagnosis and after platinum-based chemotherapy. Int J Gynecol Cancer 21: 822-830.
- Cheng JP, Yan Y, Wang XY, Lu YL, Yuan YH, et al. (2011) MUC1-positive circulating tumor cells and MUC1 protein predict chemotherapeutic efficacy in the treatment of metastatic breast cancer. Chin J Cancer 30: 54-61.
- de Albuquerque A, Kubisch I, Ernst D, Breier G, Kaul S, et al. (2011) Prognostic significance of multimarker circulating tumor cells analzsis in patients with advanced pancreatic cancer. J Clin Oncol 29: e14657.
- Hardingham JE, Hewett PJ, Sage RE, Finch JL, Nuttall JD, et al. (2000) Molecular detection of blood-borne epithelial cells in colorectal cancer patients and in patients with benign bowel disease. Int J Cancer 89: 8-13.
- O'Hara SM, Moreno JG, Zweitzig DR, Gross S, Gomella LG, et al. (2004) Multigene reverse transcription-PCR profiling of circulating tumor cells in hormone-refractory prostate cancer. Clin Chem 50: 826-835.
- Benjamin P. Neign (2010) The 46thAnnual Meeting of the American Society of Clinical Oncology.
- Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, et al. (2009) Tumor self-seeding by circulating cancer cells. Cell 139: 1315-1326.
- Weigelt B, Bosma AJ, Hart AA, Rodenhuis S, van 't Veer LJ (2003) Marker genes for circulating tumour cells predict survival in metastasized breast cancer patients. Br J Cancer 88: 1091-1094.
- Witzig TE, Bossy B, Kimlinger T, Roche PC, Ingle JN, et al. (2002) Detection of circulating cytokeratin positive cells in the blood of breast cancer patients using immunomagnetic enrichment and digital microscopy. Clin Cancer Res 8: 1085- 1091.
- Gervasoni A, Monasterio Munoz RM, Wengler GS, Rizzi A, Zaniboni A, et al. (2008) Molecular signature detection of circulating tumor cells using a panel of selected genes. Cancer Lett 263: 267 -279.
- Panteleakou Z, Lembessis P, Sourla A, Pissimissis N, Polyzos A, et al. (2009) Detection of circulating tumor cells in prostate cancer patients: methodological pitfalls and clinical relevance. Mol Med 15: 101-114.
- Gerges N, Rak J, Jabado N (2010) New technologies for the detection of circulating tumour cells. Br Med Bull 94: 49-64.
- Myung JH, Launiere CA, Eddington DT, Hong S (2010) Enhanced tumor cell isolation by a biomimetic combination of E-selectin and anti-EpCAM: implications for the effective separation of circulating tumor cells (CTCs). Langmuir 26: 8589-8596.
- Mostert B, Kraan J, Bolt-de VJ, van der Spoel P, Sieuwerts AM, et al. (2010) Detection of circulating tumor cells in breast cancer may improve through enrichment with anti-CD146. Breast Cancer Res Treat 127: 33-41.
- Mikolajczyk SD, Millar LS, Tsinberg P, Coutts SM, Zomorrodi M, et al. (2011) Detection of EpCAM-Negative and Cytokeratin-Negative Circulating Tumor Cells in Peripheral Blood. J Oncol 2011: 252361.
- Conzelmann M, Linnemann U, Berger MR (2005) Molecular detection of clinical colorectal cancer metastasis: how should multiple markers be put to use? Int J Colorectal Dis 20: 137-146.
- Pang R, Law WL, Chu AC, Poon JT, Lam CS, et al. (2010) A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell 6: 603-615.
- Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, et al. (2004) N-cadherin expression and epithelial mesenchymal transition in pancreatic carcinoma. Clin Cancer Res 10: 4125-4133.
- Tanaka H, Kono E, Tran CP, Miyazaki H, Yamashiro J, et al. (2010) Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med 16: 1414-1420.
- Tran NL, Nagle RB, Cress AE, Heimark RL (1999) N-Cadherin expression in human prostate carcinoma cell lines. An epithelial-mesenchymal transformation mediating adhesion with Stromal cells. Am J Pathol 155: 787-798.
- Christiansen JJ, Rajasekaran AK (2006) Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 66: 8319-8326.
- Kang Y, Massague J (2004) Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 118: 277-279.
- Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting R, et al. (2011) Circulating Tumor Cells from Patients with Advanced Prostate and Breast Cancer Display Both Epithelial and Mesenchymal Markers. Mol Cancer Res 9: 997-1007.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 20433
- [From(publication date):
November-2011 - Dec 19, 2024] - Breakdown by view type
- HTML page views : 15468
- PDF downloads : 4965