Research Article Open Access
Cryopreservation of Human Adipose Tissues using Human Plasma
Jong Bin Kim1, Hee Mi Lee1, Seo Yoon Kim1, Kyoung Sik Park2, Jaeman Bae3, Ju Yun Jang1 and Soo-Shin Kim1*1Real Cosmetic Surgery Clinic , Asia Building 580 Sinsa-dong, Gangnam-gu, Seoul 152-892, Korea
2Department of Surgery, Konkuk University School of Medicine, Hwayang-dong, Gwangjin-gu, Seoul, 143-729, Korea
3Department of Obstetrics and Gynecology, Konkuk University School of Medicine, Hwayang-dong, Gwangjin-gu, Seoul, 143-729, Korea
- Corresponding Author:
- Soo-Shin Kim, M.D., Ph.D
Real Department of Plastic Surgery
Asea Building 580 Sinsa-dong
Gangnam-gu, Seoul 152-892, Korea
Tel: 82-2-760-2921
Fax: 82-2-512-1616
E-mail: kim1624@hotmail.com
Received date: April 19, 2011; Accepted date: June 22, 2011; Published date: June 24, 2011
Citation: Kim JB, Lee HM, Kim SY, Park KS, Bae J, et al. (2011) Cryopreservation of Human Adipose Tissues using Human Plasma. J Biotechnol Biomaterial 1:107. doi:10.4172/2155-952X.1000107
Copyright: © 2011 Kim JB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Human adipose tissue or derived cells have been used clinically in several fields. Animal serum is used in storing and culturing human adipose tissue or derived cells. Thus far, few problems have been associated with these sera in current research fields. However, animal sera may cause some problems in clinical applications because of the toxic viruses and proteins that can be present in animal sera. Human plasma can resolve these problems associated with animal serum. In this study, we investigated the effects of human plasma as a cryopreservation solution for human adipose tissues. We observed that adherent cells derived from adipose tissues were isolated effectively by collagenase treatment. We did not observe any adherent cells when human adipose tissues were preserved without cryopreservation solution at ?20°C. Adherent adipose-derived cells exhibited different growth rates based on the cryopreservation solution and storage temperature. We also did not observe adherent cells when human adipose tissues were preserved with cryopreservation solution at ?20°C and ?70°C. Adherent adipose tissue-derived cells exhibited a similar growth rate as cryopreserved tissues when cryopreserved in 10% dimethyl sulfoxide (DMSO) + 90% fetal bovine serum (FBS) or 10% DMSO + 90% human plasma cryopreservation solution but exhibited a low growth rate when cryopreserved in 10% DMSO + 10% FBS + 80% Dulbecco’s modi?ed Eagle’s medium (DMEM) cryopreservation solution. In conclusion, these results demonstrated that human plasma is useful as a cryopreservation solution for human adipose tissues.
Keywords
Adipose tissues; Adipose-derived cells; Adipose stem cells; Cryopreservation; Human plasma
Introduction
Human adipose tissue is composed of fat derived from lipoblasts [1]. The amount of fat is associated with obesity in humans. Human adipose tissues store energy and produce several hormones and cytokines such as insulin, adiponectin, leptin, resistin, interleukins, and tumor necrosis factor (TNF)-alpha [2]. There are also several types of cells in human adipose tissue, such as multipotent adipose stem cells, adipose stromal cells, and adipocytes [3]. Adipose stem cells can differentiate into adipogenic, chondrogenic, and osteogenic cells [4]. Because of these characteristics, adipose tissue has been used clinically not only as a source of adult stem cells but also as biomaterial for tissue engineering [5]. Adipose tissue derived-cells were isolated by two different methods, mechanical (chopping) or enzyme treatment (collagenase). Compared to these isolation methods, the mechanical isolation of cell from adipose tissue can minimize cellular damage, whereas the enzyme treatment can obtain a large numbers of cells. Human adipose is mainly obtained through aspiration during operations [6]. These tissues are injected to humans immediately or stored in vitro and then may be reused after being designed. These tissues must be stored and maintained without damage to be used in vitro.
It is very important to select a proper freezing temperature and cryopreservation solution to minimize tissue damage during the freezing process [7]. Animal serum, culture media, and dimethyl sulfoxide (DMSO) have been used as cryopreservation solutions [8], and methods have been develop to slowly decrease temperatures during the freezing process to minimize tissue damage [9]. There is a need for rapid thawing to isolate cells from cryopreserved tissue or to reuse cryopreserved tissue.
Sera from several animals have been used to culture cells isolated from tissues or to cryopreserve tissue [10]. Sera are derived from several animals such as bovine fetuses and goats [11]. Among them, fetal bovine serum is broadly used as a cell culture or cryopreservation solution. Animal serum has many advantages in cell culture and cryopreservation. These sera are easy to obtain from animals. Animal sera must undergo many steps of sterilization to be used in human tissues. However, animal serum may contain viruses or toxic proteins that can cause fatal disease in humans even when the serum is sterilized [12]. For this reason, human applications of animal serum are strictly regulated in many countries [13]. In this study, we investigated the effects of two different isolation methods, which are mechanical (chopping) and enzyme treatment (collagenase), on primary adipose derived-cells, and the effect of human plasma on efficiency of cryopreservation solution for human adipose-derived cells.
Materials and Methods
Culture of human adipose-derived cells
Each donor of adipose tissue was asked to provide written informed consent for participation in the study. Fresh (female, 10 cases) or stored (about 3 months (female, 19 cases), about 6 months (female, 18 cases), about 12 months (female, 31 cases) human adipose tissues (10 mL) were obtained from the Real Department of Plastic Surgery Hospital. Tissues were immediately transported to a clean bench and centrifuged for 5 min at 80 × g. The upper layer was moved to a 15- or 50-mL tube. The tissues were washed three times with phosphate buffered saline (PBS) and chopped for mechanical cell isolation or digested with collagenase for enzymatic cell isolation. Chopped tissues were then seeded in tissue culture dishes and cultured in media (Dulbecco’s modified Eagle’s medium [DMEM] supplemented with 10% fetal bovine serum [FBS]) at 37°C in a humidified atmosphere containing 5% CO2. Tissues digested with collagenase and inactivated with media (DMEM containing 10% FBS) were dissociated mechanically and filtered with a 40-μm cell strainer. They were centrifuged for 5 min at 80 × g. Pellets were washed with PBS, seeded in Petri dishes, and cultured in media (DMEM supplemented with 10% FBS) at 37°C in a humidified atmosphere containing 5% CO2. Photographs were taken with an inverted system microscope (CKX41 model; Olympus, Tokyo, Japan) equipped with a DP50 camera system (Olympus), stored on a personal computer, and then processed using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA). To assay the growth rate, cells were harvested by trypsin-ethylenediaminetetraacetic acid (EDTA) treatment after 7 days. Growth rates were determined by cell counting with 0.4% trypan blue dye in a Neubauer chamber. All experiments were performed in triplicate.
Apoptosis analysis with Annexin V staining
To analyze fraction of survival and apoptotic cells in isolated adipose- derived cells from 3-, 6-, or 12-month stored human adipose tissues, 10 mL of human adipose tissues stored for 3, 6, or 12 months were obtained from the Real Department of Plastic Surgery Hospital. Cells were isolated by collagenase treatment as described above. The analysis of cell death was performed using annexin V and propidium iodide (PI) staining according to the manufacturer’s protocol (BD Pharmingen, NJ). Cells isolated from adipose tissues were washed with PBS and resuspended in binding buffer supplied in the manufacturer’s kit (BD Pharmingen) at a concentration of 1 × 106 cells/mL. Annexin V-FITC and PI were added to tubes containing 1 × 105 cells and incubated for 15 min at room temperature (25°C) in the dark. To analyze cell death, 400 μL of 1× binding buffer were added to each tube. Flow cytometry was performed using a FACS Caliber system within 1 h.
Storage of human adipose tissues
Ten microliters of fresh human adipose tissues and blood were obtained from the Real Department of Plastic Surgery Hospital. Human blood was centrifuged for 15 min at 1610 × g. The upper layer was moved to a 15 mL tube and centrifuged. The upper layer obtained as human plasma. Tissues were immediately transported to a clean bench and centrifuged for 5 min at 80 × g. The upper layer was moved to a 15- or 50-mL tube. Tissues were immediately frozen using one of the following three cryopreservation conditions: Condition 1: 10% DMSO + 90% FBS; Condition 2: 10% DMSO + 90% human plasma; Condition 3: 10% DMSO + 10% FBS + 80% DMEM. Tissues were also immediately frozen using each cryopreservation condition. To determine the effect of cryopreservation temperature on cell survival, tissues were also immediately frozen at different temperatures (−20°C, −70°C, −180°C) in 10% DMSO + 90% FBS and 10% DMSO + 90% human plasma. After cryopreservation for 7 days, cells were isolated and cultured as described above. Growth rates were determined by cells counting with 0.4% trypan blue dye as described above.
Statistical analyses
All experiments were repeated a minimum of three times. SAS version 8.2 (SAS Inc, Cary, NC, USA) was used for all data analyses. Data is presented as the mean ± standard error (SE) of the mean. Statistical significance was determined using a one-way analysis of variance (ANOVA). When ANOVAs indicated a significant difference, Tukey and Dunnette’s tests were used performed to compare the means for three different storage and culture media conditions. Paired Student’s t-tests were used to compare efficiency of two different isolation methods. P-values less than 0.05 were determined to be significant.
Results
Human adipose derived cells were efficiently isolated by collagenase treatment
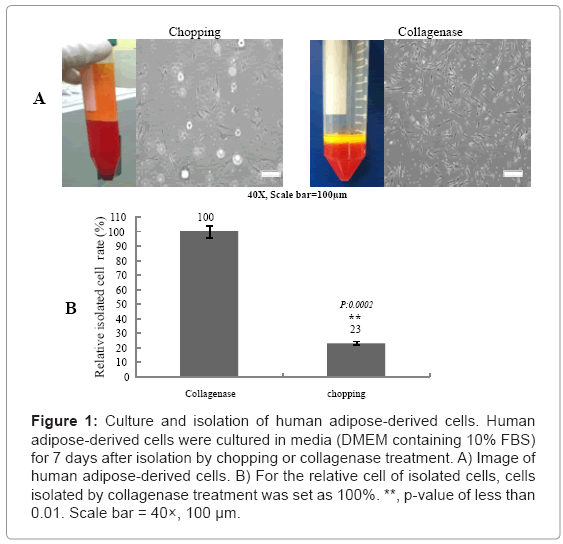
Equal volumes (10 mL) of human adipose tissues were used to isolate adipose-derived cells. As shown in Figure 1, most human adipose tissue floated in the culture dish after chopping treatment, and a low rate of adherent adipose-derived cells was observed. In contrast, a significantly higher rate of adherent adipose-derived cells was observed after collagenase treatment. These results demonstrated that adipose cells derived from human adipose tissues could be isolate efficiently by collagenase treatment.
Figure 1: Culture and isolation of human adipose-derived cells. Human adipose-derived cells were cultured in media (DMEM containing 10% FBS) for 7 days after isolation by chopping or collagenase treatment. A) Image of human adipose-derived cells. B) For the relative cell of isolated cells, cells isolated by collagenase treatment was set as 100%. **, p-value of less than 0.01. Scale bar = 40×, 100 μm.
Cells derived from human adipose tissues cryopreserved at −20°C
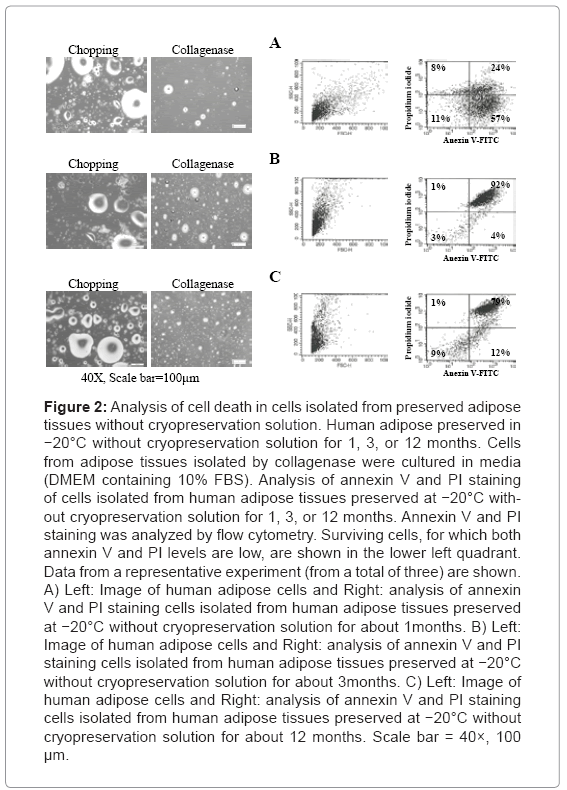
To examine viability of adipose cells in adipose tissues cryopreserved at −20°C without cryopreservation solution, adipose-derived cells were isolated and cultured, and cell death was analyzed in these tissues. Adipose-derived cells were isolated by chopping or collagenase treatment, and adherent cells were counted. As shown in Figure 2, adherent adipose-derived cells were not observed when cultured in DMEM containing 10% FBS regardless of the storage period. To confirm these results, the surviving cell fraction was analyzed for cell death using annexin V and propidium iodide (PI) staining. A little survival cell the fraction observed in analysis of cell death. The fraction may be blood cells including adipose tissues.
Figure 2: Analysis of cell death in cells isolated from preserved adipose tissues without cryopreservation solution. Human adipose preserved in −20°C without cryopreservation solution for 1, 3, or 12 months. Cells from adipose tissues isolated by collagenase were cultured in media (DMEM containing 10% FBS). Analysis of annexin V and PI staining of cells isolated from human adipose tissues preserved at −20°C without cryopreservation solution for 1, 3, or 12 months. Annexin V and PI staining was analyzed by flow cytometry. Surviving cells, for which both annexin V and PI levels are low, are shown in the lower left quadrant. Data from a representative experiment (from a total of three) are shown. A) Left: Image of human adipose cells and Right: analysis of annexin V and PI staining cells isolated from human adipose tissues preserved at −20°C without cryopreservation solution for about 1months. B) Left: Image of human adipose cells and Right: analysis of annexin V and PI staining cells isolated from human adipose tissues preserved at −20°C without cryopreservation solution for about 3months. C) Left: Image of human adipose cells and Right: analysis of annexin V and PI staining cells isolated from human adipose tissues preserved at −20°C without cryopreservation solution for about 12 months. Scale bar = 40×, 100 μm.
Cells derived from human adipose tissues cryopreserved in cryopreservation solution containing human plasma
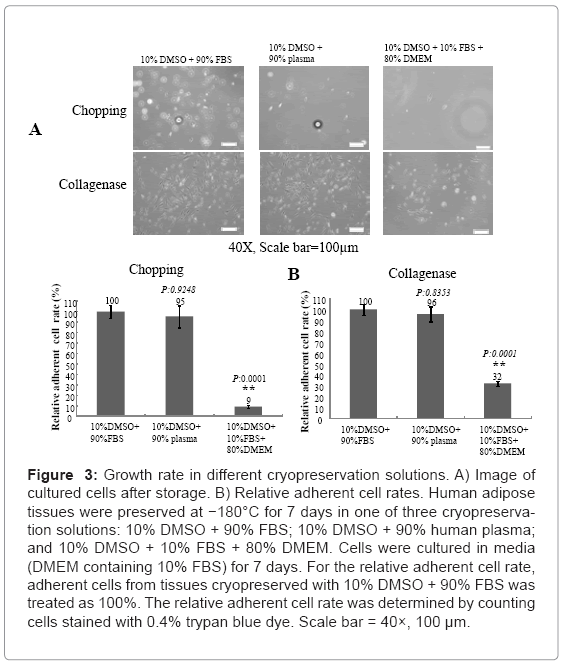
Human adipose tissues were cryopreserved using a cryopreserva- tion solution containing DMSO, cell culture media, and FBS. To observe the effect of human plasma as a cryopreservation solution, fresh plasma and human adipose tissues from the same donor were cryopreserved in one of three cryopreservation solutions (Condition 1: 10% DMSO + 90% FBS; Condition 2: 10% DMSO + 90% human plasma; Condition 3: 10% DMSO + 10% FBS + 80% DMEM) at −180°C for 7 days and cultured in DMEM medium containing 10% FBS for 7 days. As shown in Figure 3, the number of adherent cells was similar in conditions 1 and 2, but a lower number of adherent cells was observed with condition 3 (cryopreservation solution containing media; Figure 3A). Cells preserved with conditions 1 and 2 had similar growth rates, but cells preserved using condition 3 exhibited a slower growth rate (Figure 3B).
Figure 3: Growth rate in different cryopreservation solutions. A) Image of cultured cells after storage. B) Relative adherent cell rates. Human adipose tissues were preserved at −180°C for 7 days in one of three cryopreservation solutions: 10% DMSO + 90% FBS; 10% DMSO + 90% human plasma; and 10% DMSO + 10% FBS + 80% DMEM. Cells were cultured in media (DMEM containing 10% FBS) for 7 days. For the relative adherent cell rate, adherent cells from tissues cryopreserved with 10% DMSO + 90% FBS was treated as 100%. The relative adherent cell rate was determined by counting cells stained with 0.4% trypan blue dye. Scale bar = 40×, 100 μm.
Cells derived from human adipose tissues cryopreserved in cryopreservation solution containing human plasma at −20°C and −70°C
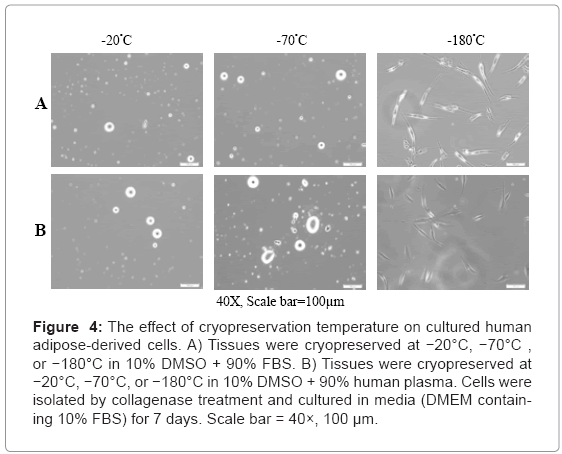
We observed that cells could not attach to tissue culture dish when preserved without cryopreservation solution at −20°C as demonstrated above, but adherent cells were observed when cells were preserved in cryopreservation solution containing FBS and human plasma at −180°C. To investigate the effect of temperature on cryopreservation, fresh human adipose tissues were cryopreserved in cryopreservation solution containing human plasma and FBS at −20°C, −70°C, and −180°C. As shown in Figure 4, adherent adipose-derived cells were observed only after cryopreservation at −180°C. The cryopreservation solution did not affect the isolation of adipose-derived cells from human adipose tissues.
Figure 4: The effect of cryopreservation temperature on cultured human adipose-derived cells. A) Tissues were cryopreserved at −20°C, −70°C , or −180°C in 10% DMSO + 90% FBS. B) Tissues were cryopreserved at −20°C, −70°C, or −180°C in 10% DMSO + 90% human plasma. Cells were isolated by collagenase treatment and cultured in media (DMEM containing 10% FBS) for 7 days. Scale bar = 40×, 100 μm.
| Tissues | Age (average) | Gender | Height (average) | Weight (average) |
|---|---|---|---|---|
| Freash | 23-70 (44) | F | 150-165 (157) | 42-64 (53) |
| 3 months | 27-70 (41) | F | 150-168 (161) | 41-62 (54) |
| 6 months | 25-58 (41) | F | 153-183 (162) | 46-89 (56) |
| 12 months | 22-81 (41) | F | 153-174 (162) | 42-62 (55) |
Table 1: Clinical information of tissues used in this study.
Discussion
It is easy to obtain human adipose tissues. Human adipose tissues have been used in a variety of fields clinically [14]. Recently, multipotent stem cells have been identified in adipose tissue through the in vitro culture of adipose-derived cells [4]. Most cells of adipose tissues except some blood cells grow on adherent condition in vitro. Animal serum has been used to culture and cryopreserve adipose-derived cells in vitro [15]. In this study, we investigated the in vitro effects of human plasma in cryopreservation solution that did not contain animal serum. We examined the rate of cell isolation from human adipose tissues, the rate of cell survival for cells derived from cryopreserved human adipose tissues, the effect of human plasma as a cryopreservation solution, and the effect of temperature on cryopreservation.
Since there has been a few studies reported isolation protocol An efficient isolation protocol to facilitate the clinical use of human adipose tissue needs to be established. To optimize protocol for isolating human adipose-derived cells from the tissues, mechanical treatment (chopping) and collagenase treatment were investigated. Collagenase was efficient in isolating adipose cells from human adipose tissues. Collagenases are enzymes that metabolize collagen, and have been used to isolate cells from tissues. Our results are consistent with previous results [16], and Yu et al also reported that adipose stem cells can be isolated efficiently by collagenase treatment [17].
Human adipose tissues are used mainly for fat grafts in plastic surgery [18]. Most plastic surgery hospitals in Korea preserve human adipose tissues at −20°C without cryopreservation solution. Additionally, extracted human adipose tissues from a patient can be clinically used multiple times to the same patient. Clinically, these extracted human adipose tissues improve aging process such as wrinkles and effectively increase wound healing. However, the mechanism behind these beneficial effects has not been demonstrated. Adherent adipose-derived cells were investigated in adipose tissue cryopreserved without cryopreservation solution, as this cryopreservation protocol is used in many plastic surgery hospitals in Korea. We could not observe adherent adiposederived cells after the in vitro culturing of cryopreserved tissues. These results were confirmed by annexin V staining. These results demonstrated that adherent adipose-derived cells could not be culture when tissues were preserved without cryopreservation solution at −20°C. In addition, adherent adipose-derived cells were not observed after cryopreservation at −20°C or −70°C, even though adipose tissues were preserved in cryopreservation solution. These results revealed that to derive adipose cells, adipose tissues should be preserve at −180°C with cryopreservation solution. These findings are in contrast to reports that indicated that cryopreservation solution was not necessary to cryopreserve tissues. Liu et al reported that cryopreservation solution did not affect the growth and osteogenesis of adipose stem cells compared with the control group without cryopreservation solution [6]. Pu suggested that cryopreservation solution was needed for the long-term storage of adipose tissues [19]. Our results were consistent with previous results indicating an effect of temperature of storage [9]. Son et al reported that adipose tissue should be cryopreserved at −70°C to maintain surviving adipose cells [9]. Moscatello et al demonstrated that fresh tissue should be cryopreserved at −180°C for in vitro applications [20].
Human plasma is useful for cryopreserving and culturing cells from human tissues from the viewpoints of immune response and infection. We observed that human plasma was similar to animal serum as a cryoprotective agent regarding the isolation of adipose-derived cells. These results demonstrated that human plasma could be use as an agent to cryopreserve adipose tissues. Similar to our results, Thirumala et al reported that a DMSO concentration of 2% is needed for the cryopreservation of tissue and survival of adult stem cells, which was similar to the concentration of animal or human serum in cryopreservation solution [15]. Our results suggested that human plasma could be an alternative replacement of animal serum for cryopreservation of adipose tissues.
In conclusion, we found that cryopreservation solutions are needed to culture adherent adipose derived-cells from preserved adipose tissue and that adipose tissues must be cryopreserved at −180°C. Human plasma has similar effects as animal serum regarding cryopreservation. Moreover, the potential application of human plasma as alternative replacement of animal serum should be investigated for many different types of tissues, beside of adipose tissue. Further investigation is needed to efficiently isolate adherent adipose derived-cells when adipose tissues are cryopreserved with cryopreservation solution containing human plasma.
References
- Engstrom K, Willen H, Kabjorn-Gustafsson C, Andersson C, Olsson M, et al. (2006) The myxoid/round cell liposarcoma fusion oncogene FUS-DDIT3 and the normal DDIT3 induce a liposarcoma phenotype in transfected human fibrosarcoma cells. Am J Pathol 168: 1642-1653.
- Guerre-Millo M (2004) Adipose tissue and adipokines: for better or worse. Diabetes Metab 30: 13-19.
- Festy F, Hoareau L, Bes-Houtmann S, Pequin AM, Gonthier MP, et al. (2005) Surface protein expression between human adipose tissue-derived stromal cells and mature adipocytes. Histochem Cell Biol 124: 113-121.
- Yamamoto N, Akamatsu H, Hasegawa S, Yamada T, Nakata S, et al. (2007) Isolation of multipotent stem cells from mouse adipose tissue. J Dermatol Sci 48: 43-52.
- Labbe B, Marceau-Fortier G, Fradette J (2011) Cell sheet technology for tissue engineering: the self-assembly approach using adipose-derived stromal cells. Methods Mol Biol 702: 429-441.
- Liu G, Li Y, Sun J, Zhou H, Cui L (2010) Effect of cryopreservation on growth and osteogenesis of human adipose-derived stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 24: 1224-1227.
- Grewal N, Yacomotti L, Melkonyan V, Massey M, Bradley JP, et al. (2009) Freezing adipose tissue grafts may damage their ability to integrate into the host. Connect Tissue Res 50: 14-28.
- De Rosa A, De Francesco F, Tirino V, Ferraro GA, Desiderio V, et al. (2009) A new method for cryopreserving adipose-derived stem cells: an attractive and suitable large-scale and long-term cell banking technology. Tissue Eng Part C Methods 15: 659-667.
- Son D, Oh J, Choi T, Kim J, Han K, et al. (2010) Viability of fat cells over time after syringe suction lipectomy: the effects of cryopreservation. Ann Plast Surg 65: 354-360.
- van der Sanden B, Dhobb M, Berger F, Wion D (2010) Optimizing stem cell culture. J Cell Biochem 111: 801-807.
- Baker H, DeAngelis B, Frank O (1988) Vitamins and other metabolites in various sera commonly used for cell culturing. Experientia 44: 1007-1010.
- Kurita M, Aiba-Kojima E, Shigeura T, Matsumoto D, Suga H, et al. (2008) Differential effects of three preparations of human serum on expansion of various types of human cells. Plast Reconstr Surg 122: 438-448.
- Felka T, Schafer R, De Zwart P, Aicher WK (2010) Animal serum-free expansion and differentiation of human mesenchymal stromal cells. Cytotherapy 12: 143-153.
- Gonda K, Shigeura T, Sato T, Matsumoto D, Suga H, et al. (2008) Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast Reconstr Surg 121: 401-410.
- Thirumala S, Gimble JM, Devireddy RV (2010) Evaluation of methylcellulose and dimethyl sulfoxide as the cryoprotectants in a serum-free freezing media for cryopreservation of adipose-derived adult stem cells. Stem Cells Dev 19: 513-522.
- Gimble J, Guilak F (2003) Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 5: 362-369.
- Yu G, Floyd ZE, Wu X, Halvorsen YD, Gimble JM (2011) Isolation of human adipose-derived stem cells from lipoaspirates. Methods Mol Biol 702: 17-27.
- Minn KW, Min KH, Chang H, Kim S, Heo EJ (2010) Effects of fat preparation methods on the viabilities of autologous fat grafts. Aesthetic Plast Surg 34: 626-631.
- Pu LL (2007) Comparison of techniques for long-term storage of fat grafts. Plast Reconstr Surg 120: 813.
- Moscatello DK, Dougherty M, Narins RS, Lawrence N (2005) Cryopreservation of human fat for soft tissue augmentation: viability requires use of cryoprotectant and controlled freezing and storage. Dermatol Surg 31: 1506-1510.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16136
- [From(publication date):
July-2011 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 11396
- PDF downloads : 4740