Research Article Open Access
Conversion of Palm Oil to Methyl and Ethyl Ester using Crude Enzymes
Liu Meng* and Jailani SalihonFaculty Science of Industrial and Technology, Lebuhraya Tun Razak 26300 Gambang Kuantan Pahang Malaysia
- Corresponding Author:
- MLiu Meng
Faculty Science of Industrial and Technology
Lebuhraya Tun Razak 26300 Gambang Kuantan Pahang Malaysia
Tel: +014- 9255090
E-mail: liuzhizi616@yahoo.com.cn
Received date: July 12, 2011; Accepted date: August 10, 2011; Published date: August 12, 2011
Citation: Meng L, Salihon J (2011) Conversion of Palm Oil to Methyl and Ethyl Ester using Crude Enzymes. J Biotechnol Biomaterial 1:110. doi:10.4172/2155-952X.1000110
Copyright: © 2011 Meng L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Biodiesel (methyl esters) is a clean alternative fuel which can be produced from many renewable resources. Palm oil like other vegetable oils can be used as feedstock for biodiesel production. It is processed through transesterification to produce palm oil methyl ester. Enzymatic reactions catalyzed by lipases are potentially excellent processes to produce biodiesel through the transesterification reaction. Enzymes have several advantages over chemical catalysts such as mild reaction conditions high specificity and renewability. Various microorganisms like bacteria and fungi produce different kinds of enzymes which could be used as catalysts in a series of degradation reactions. Enzymatic transesterification of crude palm oil with methanol was studied. The enzymes from the three bacterial strains with the most significant transesterification reactions were tested for yield of biodiesel by changing the molar ratio of alcohol to crude palm oil and by changing reaction temperature. The molar ratio of methanol to crude palm oil was varied in the range from 3:1 to 4:1. The reaction temperature was varied from 35°C to 60°C. It was found that the optimum ratio of methanol to crude palm oil is 3:1 and the optimum reaction temperature is 40°C.
Introduction
In recent years demand for fatty acid methyl esters (biodiesel) being used as fuel in diesel engines and heating systems increased due to rises in petroleum prices increase in earth population and their energy needs and development of government measures such as The EU Directive 2003/30/EC and The US Energy Policy Act [18].
Transesterification of triglycerides with alcohol or other acyl acceptors in the presence of a chemical catalyst or biocatalyst leads to the formation of alkyl ester commercially known as biodiesel. Biodiesel can be produced from vegetable oils like soybean oil jatropha oil rapeseed oil palm oil sunflower oil corn oil peanut oil canola oil and cottonseed oil [14]. Among the vegetable oils with a potential to obtain biodiesel palm oil stands out for being the second most abundant vegetable oil in the world next to soybean oil. Further palm oil is characterized as having superior productivity among all the crops [5].
Transesterification reaction can be catalyzed by both homogeneous (basic or acidic) and heterogeneous (basic acidic or enzymatic) catalysts [8]. Recent studies showed that biodiesel can be produced enzymatically by lipase-catalyzed transesterification which has become more attractive in biodiesel production since the glycerol can be recovered easily and the purification process for biodiesel is simple [17].
Microbial lipases are diversified in their properties and substrate specificities which improve their biotechnological importance and justify the search for novel lipases possessing entirely new properties and specific substrate specificities depending on their applications. Extracellular lipases have been proven to be efficient and selective biocatalysts in many industrial applications such as biosensors pharmaceuticals foods cosmetics detergents [12].
Most of the lipases are produced commercially from fungi [4] yeast [1] and bacteria by using a variety of methods involving ammonium sulphate precipitation and ion exchange chromatography followed by gel filtration [3]. Although a number of lipase producing bacterial sources are available only a few are commercially exploited. Of these the important genera are Achromobacter Alcaligenes Arthrobacter Aeromonas Hydrophila Bacillus Burkholderia Chromobacterium and Pseudomonas [11].
The Pseudomonas enzymes have found application in a number of processes namely the production of detergents glycerolysis of fats and oils direct transesterification chiral resolution and acrylate synthesis [7]. To date the three dimensional structures of the lipase of only three species of bacteria are known namely Pseudomonas cepacia (Burkholderia cepacia) Chromobacterium viscosum and Pseudomonas glumae [9].
In this research paper an evaluation of the suitability of palm oil as a feedstock in transesterificaion is reported as Malaysia in bound of the resource. Three crude lipases from Pseudomonas qeniculata Bacillus pseudomycoides and Stenotorophomonas maltophiliawhch were isolated by our labrotary were used in the transesterification as catalyst. The free enzyme on the effect of reaction temperature and oil to methanol molar ratio were studied and compared.
Materials and Methods
Chemicals and equipment
The analytical grade methanol and ethanol were purchased from Fisher Scientific. Hexane (GC grade) was obtained from Fisher Scientific. The shaking incubator was supplied by Ecotron (Switzerland). The gas chromatography was supplied by Agilent tech (Creek Blvd Santa Clara CA USA). Flasks were used from Schott (Germany).
Palm oil pre-treatment and crude enzyme
The crude palm oil (1L) was collected from LKKP (Lembaga Kemajuan Perusahaan Pertanian Negeri Pahang) Corporation Sdn. Bhd Malaysia LKPP Corporation Sdn. Bhd. (LCSB) was established in 1994 with an authorised capital of RM 50 million. It is a subsidiary company wholly owned by Lembaga Kemajuan Perusahaan Pertanian Negeri Pa- hang (LKPP). The crude palm oil was filtered at reduced pressure with filter paper to eliminate the impurities. The crude enzymes were produced from bacteria which were isolated from soils of palm oil plantation the bacteria has been reserved in the laboratory. The three isolates were Pseudomonas qeniculata and setorophomonas maltophilia and Bacillus pseudomycoides strains.
Reaction conditions and optimization
The enzymatic transesterification reactions were carried out in a 50 ml conical flask and optimized with respect to temperature and the molar ration of methanol to palm oil in an orbital shaker. A standard set of conditions were used as the baseline in the optimization studies. The initial conditions set were 6 ml palm oil 2.4-3.0 ml methanol (methanol to oil molar ratio of 3 or 4) 10% volume crude lipases 40°C 150 rpm and 5 h reaction time. During optimization studies each parameter was varied one at a time. For example when the effect of methanol to palm oil ratio was investigated the remaining reaction conditions were unchanged at 6 ml palm oil 10% V/V crude lipases 40°C 150 rpm and 5h reaction time [10]. In order to compare which is better acyl-acceptors between methanol and ethanol ethanol was also run same reaction under same conditions with methanol ethanol will replace methanol in reaction.
Analytical method
The analysis of palm oil (Fatty Acid Methyl Ester) content in the samples was carried out using Gas Chromatography (GC) by means of Inert DP WAX capillary column ( 30m x 0.25 mm I.D. 0.25 um). Helium was used as the carrier gas. Oven temperature program was as follows: 155°C for 1 min and programmed from 155 to 180°C at a rate of 2°C/min kept for 2 min and finally raised to 220°C at 4°C/min and maintained for 6 min. The injector was set up for 250°C and the FID detector at 260°C.
Results and Discussion
Activity of crude enzymes from bacteria
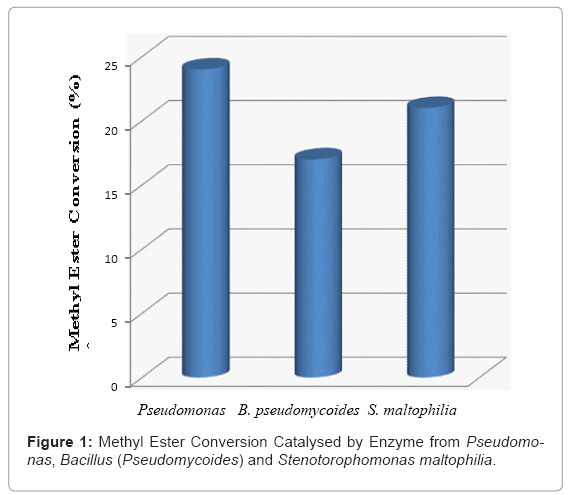
All these crude enzymes were screened for biodiesel synthesis from crude palm oil and methanol. The catalytic activities of three lipases on transesterification were compared in Figure 1 all three lipases show conversion activity during the transesterification from palm oil to methyl esters. The methyl ester conversion of oil by using lipase from Pseudomonas qeniculatais around 24% and methyl ester conversion of lipase from Stenotorophomonas maltophilia showed comparable higher catalytic activity around 21% as compared to lipase from Bacillus (Pseudomycoides) around 17% .
It was reported that the transesterificatin of several vegetable oils by bacterial lipases including Pseudomonas lipase showed a stronger activity compared to fungal lipases such as Lipozyme TL IM [6]. In this study since the final results of methyl ester conversation rate crude enzymes from Pseudomonas aeruginosa and lipase from Stenotorophomonas maltophilia were considered as the most suitable lipase for transesterification reaction of crude palm oil and methanol to methyl ester.
Effect of temperature on the transesterification reaction
Enzyme catalytic reaction temperature is an important parameter and the selection of the appropriate reaction temperature not only enhances the reaction rate but also helps extend the life of the enzyme. At certain temperature range temperature reactant collision frequency increases causing the enzyme reaction to increase in speed; but when the temperature exceeds a certain range the enzyme protein denatures enzyme activity to decrease which cause the enzyme reaction rate to decrease.
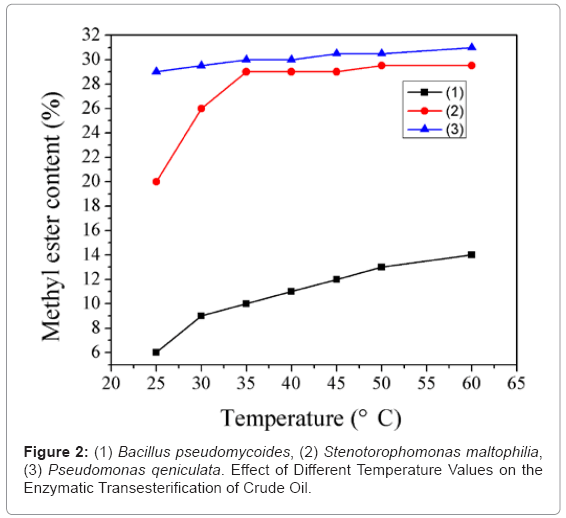
Figure 2 shows that in the studied temperature range (20-65°C) the enzymatic reaction speed increases with increasing temperature up to the maximum at the temperatures of 50 ~ 60°C where the product of the methyl ester at this degree was the highest. At lower temperatures given a longer reaction time the methyl ester content (%) produced could reach the same level as same as the optimum degree.
Pseudomonas B. pseudomycoides S. maltophilia
This finding is correspond with the work of Wu and Zong (2004) who concluded that after taking into account the speed the reaction temperature in the range of 35 ~ 40°C was more appropriate.
Effect of methanol to oil molar ratio
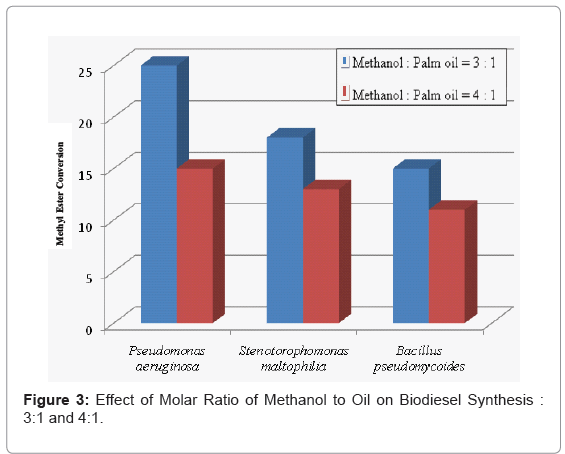
The methanol to oil molar ratio is one of the most important parameters in methyl ester production. Experiments were performed to optimize the synthesis of biodiesel by varying the molar ratio of methanol to palm oil. Optimum methanol requirements were determined as shown in Figure 3. The amount of methanol added was varied from 3 to 4 molar ratios to crude palm oil.
The three bacterial strains Pseudomonas qeniculata Stenotorophomonas maltophilia and Bacillus pseudomycoides yield of biodiese at 4 molar methanol / palm oil ratio was lower than that at 3 molar ratio. This could be due to the inhibitory effect of high amount of methanol on the activity of the enzyme. Fatty alcohols in which the carbon lengths are more than 3 completely dissolve in the oil in a stoichiometric amount but the solubility of methanol was only 1/2 of the stoichiometric amount [15]. Therefore lipases could be deactivated by insoluble methanol which exists as drops in the oil. Soumanou and Borscheuer (2003) reported that some Pseudomonas strains developed substantial methanol resistance. They may also be tolerant to methanol.
Although the mixed lipases in this study gave especially high conversion with three molar equivalents of methanol one of the problems faced in the enzyme-based route for methyl ester preparation has been the inactivation of the lipase by exposure to methanol; hence the stepwise addition of methanol was also attempted. It was observed that it made no difference to methyl esters whether the whole of the methanol was added in a single step. In this respect the single step is a good choice since stepwise addition of the substrate does complicate the process design.
Comparison between methanol and ethanol as acyl-acceptors
Alcohols are the most frequently used acyl-acceptors particularly methanol and to a lesser extent ethanol. Other alcohols can be used such as butanol isopropanol and octanol but the cost is much higher. Although the use of different alcohols presents some differences with regard to the reaction kinetics the final yield of esters remains more or less inalterable. Therefore selection of the alcohol is based on cost and performance consideration [2]. Regarding the choice between methanol and ethanol Methanol was dominating in most of the literature reviewed methyl rather than ethyl ester production was moderate because methyl esters are the predominant commercial products methanol is considerably cheaper and more available than ethanol [13] and the downstream recovery of unreacted alcohol is much easier [20].
However ethanol is less toxic and it can be considered more renewable because it can be easily produced from renewable sources by fermentation. In addition to the entirely agricultural nature of the ethanol the extra carbon atom brought by the ethanol molecule slightly increases the heat content and the cetane number. Finally another important advantage in the use of ethanol is that the ethyl esters have cloud and pour points that are lower than the methyl esters [2]. However the utilization of ethanol also presents inconveniences. Also the formation of stable emulsion during ethanolysis is a problem. During the reaction emulsions are usually formed. In the case of methanolysis these emulsions break down quickly and easily to form a lower glycerol rich layer and upper methyl ester rich layer. In ethanolysis these emulsions are more stable and severely complicate the separation and purification of esters [20].
In this study experiments on transesterification reactions were carried out by using palm oil and ethanol under the same conditions as has been done with methanol. The yield showed no significant gap between these two alcohols as shown in the Table 1. In the process the both of two alcohols could mix well with oil and crude lipase. The conversion yields of using two alcohols were very close. So both of the two alcohols could be used as acyl-acceptors and will give a positive result.
| Lipases Resource | Conversion with Ethanol (%) | Conversion with Methanol (%) |
|---|---|---|
| Control without Lipase | 0 | 0 |
| Pseudomonas qeniculata | 24 | 22 |
| Bacillus pseudomycoides | 17 | 16 |
| Stenotorophomonas Maltophilia | 21 | 20 |
Table 1: Comparison of the Conversion Rate with Ethanol and Methanol.
Conclusions
The final results of methyl ester produced by lipase from Pseudomonas qeniculata and setorophomonas maltophilia strains were higher than that from Bacillus pseudomycoides strains or Bacillus sp the former bacteria has a conversion of approximately 24% the second bacteria has an approximately conversion rate of 21% for lipase from bacteria Bacillus pseudomycoides strains or Bacillus sp which has a lower conversion rate around 17%; therefore the lipases from Pseudomonas qeniculata and from Stenotorophomonas maltophilia were considered as the most suitable catalyst in the conversion reaction. Compare with the commercial lipase the chemical method which used alkaline or acid lipase all three bacteria were lower activity because the conversion rate of commercial and chemical lipases could even achieved 90% or 100% according to the previously published research papers and same research using chemical methods.
For the transesterification reactions a number of parameters had effects on the yield of the conversion reactions. In this research focus was given on the effect of reaction temperature the performance of the two different alcohols (methanol and ethanol) as acyl-acceptors the effect of palm oil to methanol ratio and the effect of different lipases concentration. Each of the four parameters had a relatively unique optimum range. The optimum temperature range is 35°C – 40°C. The optimum palm oil to methanol ratio is 1:3. The most suitable alcohol is methanol on the basis of a series of comparisons.
Acknowledgements
This work was funded by University Malaysia Pahang.
References
- Dalmou E, Montesinos JL, Lotti M, Casas C (2000) Effect of different carbon sources on lipase production by Candida rugosa. Enzyme Microb Technol 26: 657-663.
- Encinar JM, Juan F, Gonzalez JF, Rodriguez-Reinares A (2007) Ethanolysis of used frying oils: Biodiesel preparation and Characterization. Fuel Process. Tech 88: 513-522.
- Ferreira C, Maria A, Perolta RM (1999) Production of lipase by soil fungi and partial characterization of lipase from selected strains (Penicillium wortmanii). J Basic Microbiol 39: 11-15.
- Gao XG, Cao SG, Zhang KC (2000) Production properties and application to nonaqueous enzymatic catalysis of lipase from a newly isolated Pseudomonas strain. Enzyme Microb Technol 27: 74-82.
- Kalam MA, Masjuki HH (2002) Biodiesel from palm oil - an analysis of its properties and potential. Biomass 23: 471-479.
- Ketsara T, Benjiamas C, Aran H-Kittikun (2010) Mixed lipases for efficient enzymatic synthesis of biodiesel from used palm oil and ethanol in a solvent-free system. Catalysis 67: 52-59.
- Martin BD, Ampofo SA, Linhardt RJ, Dordick JS (1992) Biocatalytic synthesisof sugar containing poly(acrylate)-based hydrogels. Macromolecules 25: 7081- 7085.
- Mittelbach M (1990) Lipase catalyzed alcoholysis of sunflower oil. J Am Oil Chem Soc 67: 168-170.
- Noble M, Cleasby A, Johnson L, Egmond M, Frenken L (1993) The crystal structure of triacylglycerol lipase from Pseudomonasglumae reveals a partially redundant catalytic aspartate. FEBS Letters 33: 123-128.
- Noureddini H, Gao X, Philkana RS (2005) Immobilized Pseudomonas cepacia lipase for biodiesel fuelproduction from soybean oil. Bioresource Technology 96: 769-777.
- Palekar A, Vasudevan P, Yan S (2000) Purification of lipase: a review. Biocatal Biotransform 18: 177-200.
- Pandey A, Benjamin S, Soccol CR, Nigam P, Krieger N, et al. (1999) The realm of microbial lipases in biotechnology. Biotechnol. Appl Biochem 29: 119-131.
- Pinto AC, Guarieiro LN, Rezende MJ, Ribeiro NM, Torres EA, et al. (2005) Biodiesel: An overview. J Brazil Chem Soc 16: 1313-1330.
- Sharma Y, Singh B (2008) Development of biodiesel from karanjia a tree found in rural India. Fuel 87: 1740-1742.
- Shimada Y, Watanabe Y, Sugihara A, Tominaga Y (2002) Enzymatic alcoholysis for biodiesel fuel production and application of the reaction to oil processing. Journal of Molecular Catalysis B: Enzym 17:133-142.
- Soumanou M, Bornscheuer U (2003) Enzyme from microbial. Microbial Technol 33: 97-103.
- Vicente G, Coteron A, Martinez M, Aracil J (1998) Application of the factorial design of experiments and response surface methodology to optimize biodiesel production. Ind Crops Prod 8: 29-35.
- Vicente G, Martinez M, Aracil J (2007) Optimisation of integrated biodiesel production Part II: A study of the material balance. Bioresource Technol 98: 1754-1761.
- Wu H, Zong M (2004) Transesterification of waster oil to biodiesel in solvent fre system catalyzed by immobilized lipase. Chinese Journal of Catalysis 11: 903- 908.
- Zhou W, Boocock DGB (2006) Phase behavior of the base-catalyzed transesterification of soybean oil. J Am Oil Chem Soc 83: 1041-1045.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 23317
- [From(publication date):
August-2011 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 18365
- PDF downloads : 4952