
Page 113
Notes:
conferenceseries
.com
Volume 8, Issue 2 (Suppl)
Chem Sci J 2017
ISSN: 2150-3494 CSJ, an open access journal
Euro Chemistry 2017
May 11-13, 2017
May 11-13, 2017 Barcelona, Spain
4
th
European Chemistry Congress
Cigdem Yagci et al., Chem Sci J 2017, 8:2(Suppl)
http://dx.doi.org/10.4172/2150-3494-C1-009Novel organosoluble phthalocyanines bearing 2-(Benzylthio)ethoxy units: synthesis and characterization
Cigdem Yagci
1
,Bilgin, Ahmet
1
, Acıerik, Nagihan
2
and
Kadi, Cigdem
2
1
Kocaeli University, Faculty of Education, Kocaeli, Turkey
2
Karabük University, Faculty of Scienc, Karabük, Turkey
P
hthalocyanines have been employed extensively as subunits for the construction of functional materials since they exhibit
special optical and electronic properties and self-organizing abilities to form columnar mesophases, Langmuir–Blodgett (LB)
multilayers and aggregates in solution, or in the solid state.1,2 Applications of peripherally unsubstituted phthalocyanines are
restricted because of their insolubility in common solvents. Phthalocyanines own an extended π-conjugated electron system which
allows π stacking between planar macrocycles, provided the distance between the macrocycles is small. By the peripheral attachment
of alkyl, alkoxy or alkylthio groups to the macrocycle, metal phthalocyanine complexes can be made soluble in common organic
solvents.3 Heteroatom-functionalized substituents can be introduced onto the periphery of the phthalocyanine nucleus. These
peripheral groups are capable of binding soft or hard metal cations and provide donor sites for binding different metal ions. We
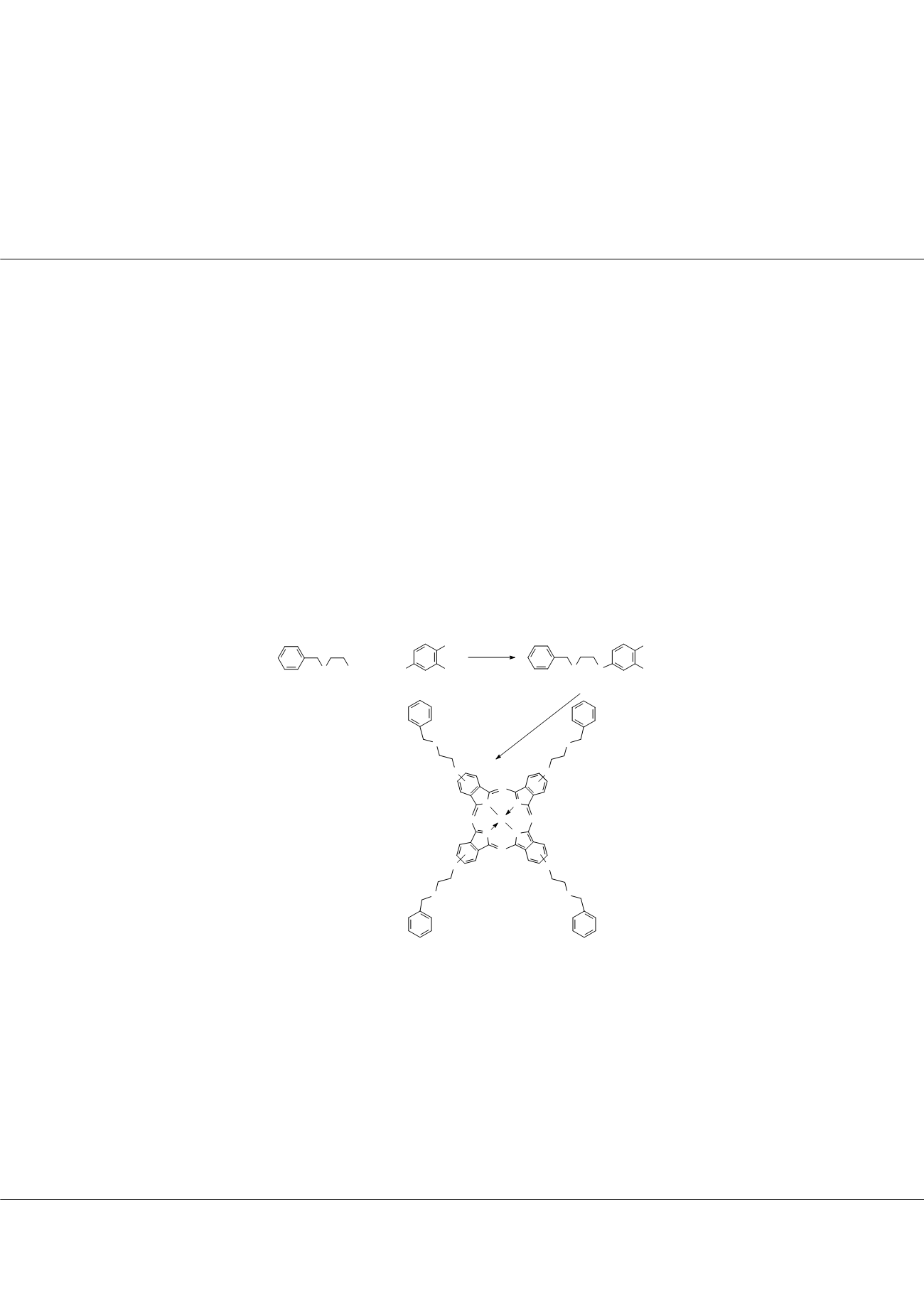
presented synthesis and characterization of new organosoluble phthalocyanines bearing 2-(benzylthio)ethoxy units in this work. For
this purpose 4-[2-(benzylthio)ethoxy]phthalonitrile was prepared with the reaction of 4-nitrophthalonitrile with the hydoxyl end
group of 2-(benzylthio)ethanol. Metal-free and metallo phthalocyanines (4-8) were synthesized by cyclotetramerization reaction of
the novel phthalonitrile derivative under suitable conditions. Aggregation and metal binding properties of ZnPc (5) was investigated.
All the novel compounds have been characterized by FT-IR, NMR, DSC, TGA techniques.
cyagci@kocaeli.edu.trN
N
N
N
N
N
N
N
M
M: 2H, Zn, Ni, Co, Cu
4 5
6 7 8
O
S
O
S
O
S
O
S
S OH
CN
CN
O
2
N
+
Dry K
2
CO
3
80 °C
Ar(g)
Dry DMF
S O
CN
CN
1
2
3
















