
Page 102
Notes:
conferenceseries
.com
Volume 8, Issue 2 (Suppl)
Chem Sci J 2017
ISSN: 2150-3494 CSJ, an open access journal
Euro Chemistry 2017
May 11-13, 2017
May 11-13, 2017 Barcelona, Spain
4
th
European Chemistry Congress
Dila Ercengiz et al., Chem Sci J 2017, 8:2(Suppl)
http://dx.doi.org/10.4172/2150-3494-C1-009Theoretical IR, UV,
1
H and
13
C-NMR spectra of certain Schiff bases derived substituted-2-aminophenol
and hydroxyl benzaldehyde
Dila Ercengiz
1
, Halil Berber
2
and
Ulku Dilek Uysal
2
Anadolu University, Turkey
S
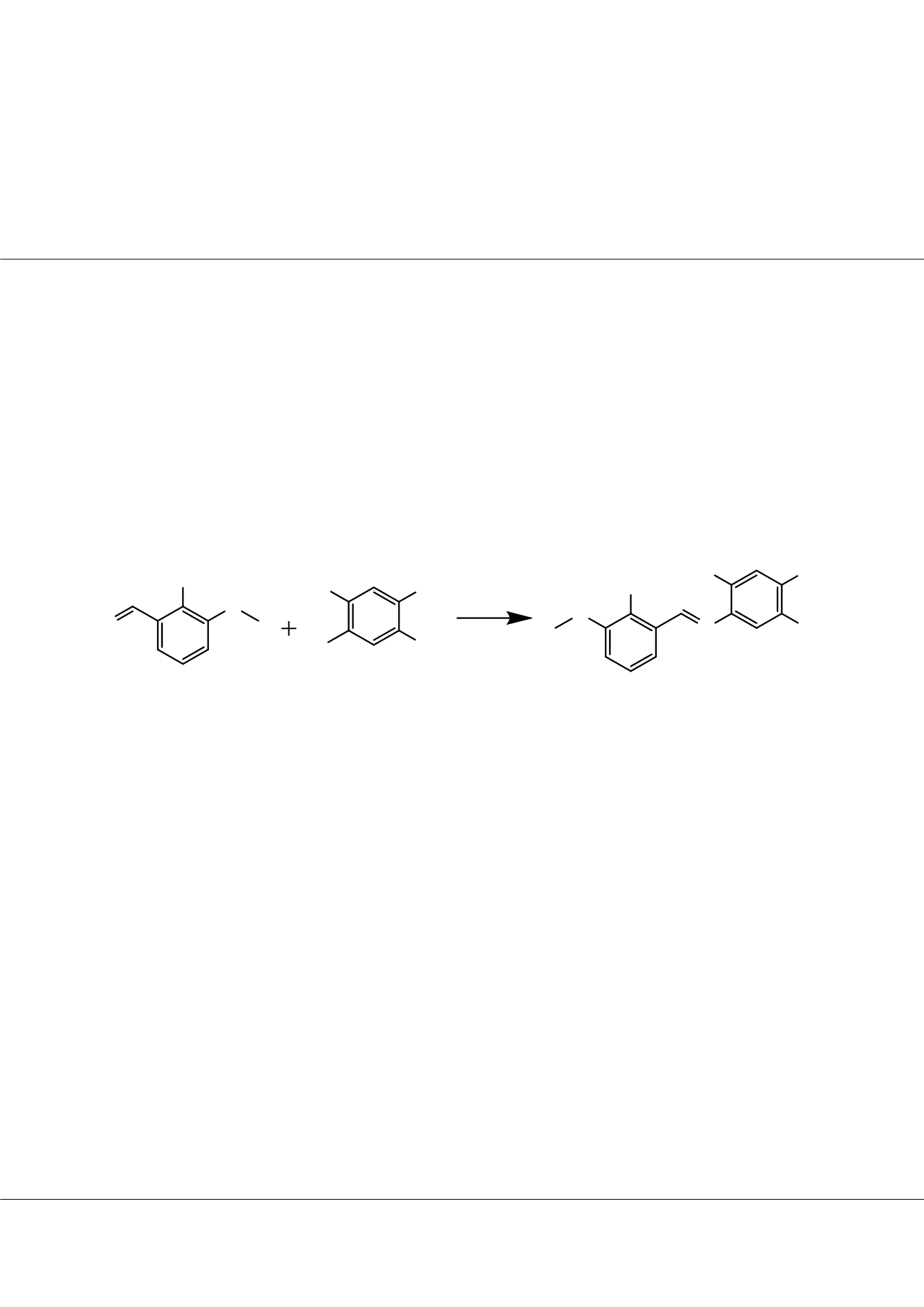
chiff bases are compounds formed by the condensation of an active carbonyl group with primary amine or N-substituted imine
containing an imino group (R-C=N-). They have been used as ligands, liquid crystals, heterogeneous catalysts, high-performance
organic light emitting diodes (OLED), and to design molecular ferromagnet, in catalysis and biological applications [1]. In this study,
five Schiff bases (Figure 1) have been synthesized and characterized with
1
H and
13
C-NMR. These Schiff bases’ Gibbs Free Energies,
Dipole moments, HOMO-LUMO values, theoretical IR, UV,
1
H and
13
C-NMR spectra have been researched by DFT method
with Gaussian09 program (B3LYP/6-311++G(d,p)) [2] and compared than those with experimental values. Structure- reactivity
relationship for these molecules was also searched.
Figure 1
. The studied Schiff bases.
Biography
Dila Ercengiz has completed her Bachelor’s degree in 2015 from Anadolu University. She is student of Anadolu University at Graduate School of Sciences.
dercengiz@anadolu.edu.trOH
O
N
HO
R
1
R
2
O
O
OH
R
2
NH
2
OH
R
1
=H; R
2
=Cl, CH
3,
NO
2
R
2
H; R
1
=Cl, CH
3
R
1
















