
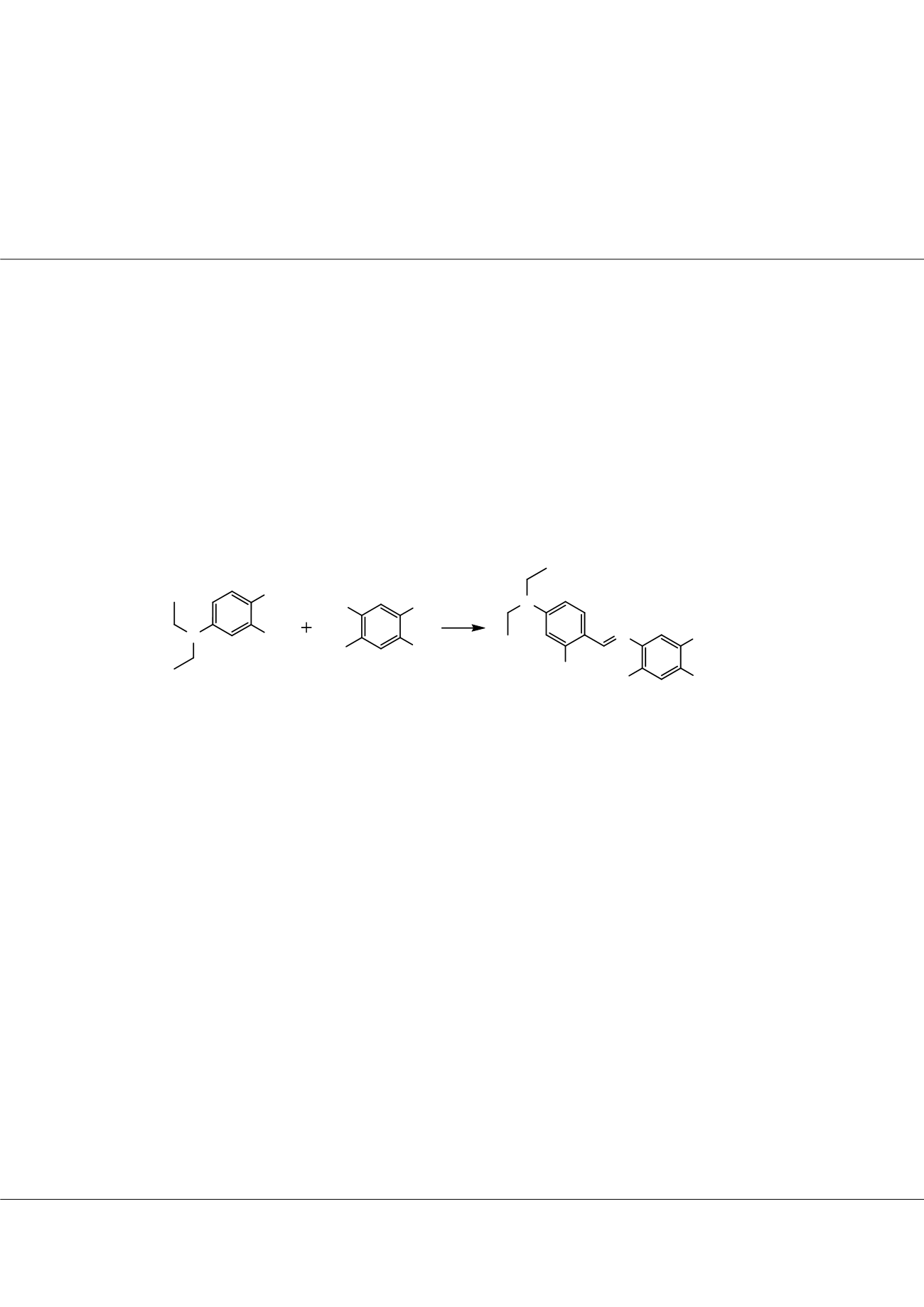
Page 98
Notes:
conferenceseries
.com
Volume 8, Issue 2 (Suppl)
Chem Sci J 2017
ISSN: 2150-3494 CSJ, an open access journal
Euro Chemistry 2017
May 11-13, 2017
May 11-13, 2017 Barcelona, Spain
4
th
European Chemistry Congress
Ayse Aydogdu et al., Chem Sci J 2017, 8:2(Suppl)
http://dx.doi.org/10.4172/2150-3494-C1-009Newly synthesized Schiff bases: Structure analysis, theoretical IR, UV,
1
H,
13
C-NMR spectra and structure-
activity relationship
Ayse Aydogdu
1
, Ulku Dilek Uysal
2
and
Halil Berber
2
1,2
Anadolu University, Turkey
S
chiff bases are most widely used organic compounds. They are used as pigments and dyes, catalyst, intermediates and polymer
stabilizer. They exhibit a biological activities, including antifungal, antibacterial, antimalarial, antiproliferative, anti-inflammatory,
antiviral, antipyretic,and herbicide properties. Furthermore, they have anti-tumor activity. These are widely applicable in analytical
determination, using complex formation reactions, utilizing the variation in their spectroscopic characteristics following changes in
pH and solvent. They have been used for manufacturing organic light emitting diodes having significant applications in night-vision
readable displays, optical communications, laser technology and optical sensors recently [1]. To understand the mechanism of these
properties, we need to some physicochemical properties of the five Schiff bases (Figure 1) synthesized by our group. Their structures
were elucidated by 1H-NMR, 13C-NMR. Two out of the Schiff bases are original. Their properties have been searched DFT method
Biography
Ayse Aydogdu has taken her Bachelor’s degree in 2015 from Anadolu University. She is student of Anadolu University at Graduate School of Sciences.
a.aydogdu@anadolu.edu.trR
1
=H, R
2
=Cl, CH
3,
NO
2
R
2
=H, R
2
=Cl, CH
3,
CHO
OH
NH
2
OH
R
1
R
2
N
N
OH
HO
N
R
1
R
2
















