Research Article Open Access
Cloning, Sequencing and Expression of Novel Trichloroethylene Degradation Genes from Stenotrophomonas maltophilia PM102: A Case of Gene Duplication
| Piyali Mukherjee and Pranab Roy* | |
| Department of Biotechnology, University of Burdwan, Golapbag more, Burdwan-713104, West Bengal, India | |
| Corresponding Author : | Pranab Roy Department of Biotechnology University of Burdwan, Golapbag more Burdwan-713104. West Bengal, India Tel: +9933037099 E-mail: pranabroy@rediffmail.com |
| Received: November 24, 2012; Accepted: January 15, 2013; Published: January 17, 2013 | |
| Citation:Mukherjee P, Roy P (2013) Cloning, Sequencing and Expression of Novel Trichloroethylene Degradation Genes from Stenotrophomonas maltophilia PM102: A Case of Gene Duplication. J Bioremed Biodeg 4:177. doi:10.4172/2155-6199.1000177 | |
| Copyright: © 2013 Mukherjee P, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
A bacterium capable of growth on trichloroethylene as the sole carbon source was identified as Stenotrophomonas maltophilia PM102 by 16S rDNA sequencing (GenBank acc. No. JQ797560). Genomic DNA of this bacterium was amplified using primers specific for the original todC1 gene of Pseudomonas putida F1. Two PCR products were obtained: 300 bp and 350 bp respectively, designated as tce300 and tce350 genes. These novel genes were separately cloned into p-GEMT Easy vector: PR300 and PR350 and sequenced. The sequences have been deposited at NCBI GenBank under accession nos. JX910450 and JX910451.
| Keywords |
| Stenotrophomonas maltophilia PM102; PR300; PR350; Trichloroethylene; Over expression; Immunoreactivity; Gene duplication |
| Abbreviations |
| TCE: Trichloroethylene; a.a.: Amino acid; mM: Milli Molar; μl: Microliter; U: Units; ml: Milliliter; nm: Nanometers; AgNO3: silver nitrate; BCIP/NBT: 5-Bromo,4-Chloro,3-Indolyl phosphate/Nitrobluetetrazolium; Ab: Antibody; IPTG: Isopropyl-β-D-thio-galacto-pyranoside; X-gal: 5-bromo-4-chloro-indolyl-β-Dgalactopyranoside; acc no.: Accession Number; bp: Base Pair. |
| Introduction |
| Trichloroethylene is one of the major groundwater pollutants throughout the world [US EPA]. From the extensive literature available on trichloroethylene degradation, it is evident that oxygenases like: soluble methane monooxygenase from Methylosinus trichosporium OB3b [1,2] toluene 2-monooxygenase from Pseudomonas cepacia G4 [3] toluene 4-monooxygenase from Pseudomonas mendocina [4], and toluene dioxygenase from P. putida F1 [5], play an important role in the breakdown of TCE. Oxygenase enzymes are more preferable due to the harmless byproducts formed in comparison to the byproducts of anaerobic degradation like vinyl chloride [6,7]. The toluene dioxygenase system of Pseudomonas putida F1, has been shown to be involved in degradation of TCE to formic acid and glycolic acid [8].Pseudomonas putida F1 can degrade benzene, toluene and ethylbenzene via the toluene degradation (tod) pathway [9,10]. The tod pathway is initiated by a multicomponent toluene dioxygenase (encoded by todC1C2BA) [11]. todC1 (EMBL acc no ABA10809.1) is the most important gene in this pathway involved in TCE cooxidation [12], that encodes a 450a.a. protein: TodC1 (toluene dioxygenase large subunit-EMBL acc. no.Q3LWS6). |
| Recently, we reported the isolation, characterization and molecular identification of a bacterial isolate: Stenotrophomonas maltophilia PM102 that was capable of growth on TCE as the sole carbon source [13]. The PM102 isolate was found to degrade TCE efficiently. Aim of this research work was to identify and characterize novel genes involved in degradation of trichloroethylene from the PM102 isolate. Two genes: tce300 and tce350 from the PM102 isolate were found to have important role in the catabolism of TCE. Role of these genes in TCE degradation have been examined after cloning and IPTG induced expression of these genes in natural soil bacteria. It was also shown that the IPTG induced proteins of the recombinant E.coli reacted with specific antibody raised against TCE induced proteins of the PM102 isolate. Evolution by gene duplication is a mechanism of Natural Selection which results in novel features of living organisms. When Charles Darwin wrote The Origin of Species, there was no existing data to inform him of the molecular nature of genetic variation that fuels evolutionary change. Today the existence of sequences of entire genomes and the ability to compare related sequences allows identification and characterization of sources of genetic variation. The comparative analysis of sequences of related and unrelated bacteria has filled out our understanding of some of these mechanisms of evolution. The tce300 and tce350 genes were aligned with the todC1 gene of Pseudomonas putida, tce1 gene of Bacillus cereus [14] and todC1 genes discovered in other microbes and found to be different from these genes. The tce350 deduced a.a sequence was found to belong to the dioxygenase family after secondary structure analysis. |
| Materials and Methods |
| Strains and growth condition |
| Stenotrophomonas maltophilia PM102 was isolated in our laboratory, from soil samples collected from Asansol and Dhanbad industrial belt that is heavily polluted with TCE. The isolate was capable of growth in minimal medium with 0.2% TCE. The minimal medium composition was: Na2HPO4 – 1 g/l, K2HPO4 – 3 g/l, NH4Cl – 1 g/l, MgSO4.7H2O – 0.4 g/l. The plasmid p-GEMT Easy was obtained from Promega and E.coli strain DH5α was used as host for expression of recombinant plasmids. |
| Nucleic acid isolation |
| Genomic DNA was isolated from the PM102 strain by the method of Sambrook and Russell [15]. Plasmid DNA was isolated from transformed E.coli DH5α by the alkaline lysis method. |
| Isolation of novel genes by PCR amplification |
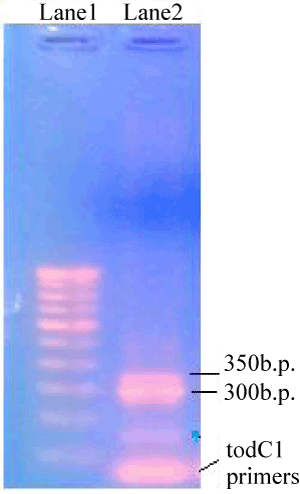
| The genomic DNA of strain PM102 was amplified using toluene dioxygenase C1 (todC1) gene specific primers from P. putida F1 [16] synthesized by Sigma-Aldrich Co, USA: todC1 F: 5’-GCGAGATGAAGCGCTCTTTG-3’. todC1 R: 5’-GTATTGATACCTGGGAGGAGG-3’. The reaction was carried out with PCR amplification kit (Bangalore Genei) as follows: 5 μl 10 X buffer with 1.5 mM MgCl2, 1 μl of 10 mM dNTP mix, 2 μl each of the two primers (20 pmol/μl), 1 μl Taq pol (5 U/μl) and 100 ng template DNA for 50 μl master mix. The amplification conditions were : 94° for 3 min for the first step, 30 cycles comprising of 94° for 1 min, 52° for 45 sec, 72° for 1 min and the final extention step of 72° for 3 min. Two PCR products were obtained of 300 bp and 350 bp respectively as checked by 1.2% agarose gel in the gel documentation system. |
| Cloning and sequencing |
| The PCR products were purified by spin column (Sigma Aldrich USA) and set up in a overnight ligation reaction with 50 ng p-GEMT Easy vector (Promega) and 3U T4 DNA ligase (Sigma Aldrich USA). The vectors with cloned insert: PR300 and PR350 were transformed into E.coli host DH5α (BioBharati LifeScience Pvt Ltd, Kolkata, India). Recombinant E. coli was screened on Luria Bertani (LB) agar containing Ampicillin (100 μg/ml), IPTG (50 μM) and X-gal (40 μg/ml) plate for blue-white selection. White colonies were picked for plasmid isolation and presence of insert was confirmed by PCR amplification of the recombinant plasmids: PR300 and PR350 with todC1 primers as before. As the PCR products were separately cloned into the EcoR1 site of p-GEMT Easy vector, the inserts could also be released by EcoR1 digestion of PR300 and PR350 recombinant plasmid DNA. The cloned PCR products were sequenced by Sanger’s dideoxy chain termination method (Xcelris Labs Ltd, Ahmadabad, India) using p-GEMT specific T7 and SP6 primers. The sequences were assembled through DNA Baser software v 3.5 and screened with Vec-screen program (NCBI) to remove any contaminating vector sequences. |
| IPTG induced over-expression of cloned genes |
| Recombinant E.coli harboring PR300 and PR350 plasmids were grown in Luria Bertini broth with 100 μg/ml ampicillin. When the cultures reached an O.D600 nm of 0.5, IPTG was added at concentrations 0 mM, 1 mM, 1.5 mM, 2 mM, 2.5 mM and 3 mM respectively and cultured for another 4 hours. The cells were harvested by centrifugation and equal concentration of proteins extracted by SDS lysis was resolved in a 12% SDS PAGE after measuring protein quantity by Bradford assay [17]. |
| Immunoblotting |
| Polyclonal Ab was raised by injecting rabbit with the total protein extracted from PM102 strain grown in minimal medium with 0.2% TCE. This Ab was preadsorbed on nitrocellulose membrane spotted with proteins extracted from PM102 cells grown in minimal medium with 0.2% peptone. Thus the antibodies reacting to the common antigens found in both peptone grown and TCE grown cells were removed. The unstained SDS PAGE profile of proteins extracted from recombinant E.coli containing PR300 and PR350, induced with 2 mM IPTG and non transformed DH5α was electro-blotted on nitrocellulose membrane (Sigma Aldrich, USA) at 65 volts for two hours. The membrane was blocked with 3% milk powder and incubated in the 1:100 dilution of preadsorbed Ab overnight, at 4°C. Then the membrane was incubated in 1:15,000 dilutions of Goat antirabbit IgG coupled to alkaline phosphatase (Sigma Aldrich USA) for 2 hours at room temperature. The membrane was stained with BCIP/NBT in alkaline phosphatase buffer and kept in the dark. After each step, the membrane was washed thrice with buffer A (10 mM tris HCl pH 8, 1 mM EDTA pH 8, 0.05% tween 20 and 0.9% NaCl). |
| TCE degradation studies with recombinant E.coli |
| Fujiwara reaction: In the Fujiwara reaction, when trichloro compounds are heated in presence of pyridine and alkali, a red color compound is formed, nature of which is not fully elucidated but some insights into the reaction mechanism is available [18]. E.coli DH5α carrying PR350, DH5α carrying PR300, non transformed DH5α and S.maltophilia PM102 cells were separately cultured in 20 ml minimal medium with 0.2% TCE and 0.2% peptone. Recombinant E.coli cultures were induced with 2 mM IPTG and 100 μg/ml ampicillin. 2 ml culture was harvested by centrifugation at 0 hrs (just after inoculation), 12 hrs and 24 hrs respectively. The supernatant (2 ml) was treated with 2 ml pyridine and 2 ml of 5 N NaOH and heated at 80°C for 2 minutes. Absorbance of the upper aqueous phase was measured at 470 nm. |
| Chloride release: when chloride containing medium containing few drops of potassium chromate is titrated against silver nitrate, a white precipitate of silver chloride is formed. End point is marked by reddish brown color formed due to the formation of silver chromate, when free chloride is no longer present in medium: |
| Ag+ + Cl– = AgCl |
| 2Ag+ + CrO4– = Ag2CrO4 |
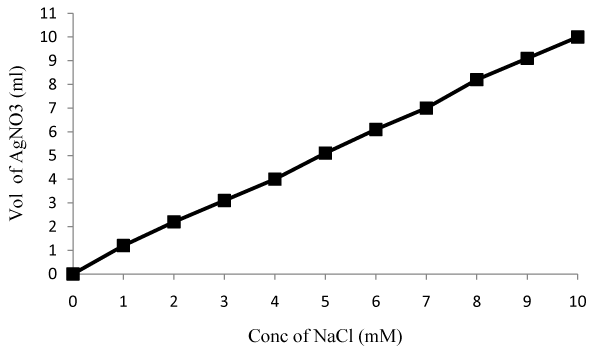
| PM102 cells, DH5α cells and recombinant DH5α carrying PR300 and PR350 were separately grown in 20 ml chloride free minimal medium (K2HPO4—3 g/l, Na2HPO4—1 g/l, (NH4)2SO4—1 g/l, MgSO4·7H2O—0.4 g/l) with 0.1% peptone and 0.2% TCE. Recombinant E.coli cultures were induced with 2 mM IPTG and 100 μg/ml ampicillin. PM102 cells and PR350 containing E.coli were also grown in the same medium with 1 mM Fe (Ferric citrate) and 1 mM Cu (Copper sulfate) in separate flasks. 10 ml of the cultures were harvested after centrifugation and free chloride present in the supernatant was measured after 24 hrs and 48 hrs. A standard curve was plotted by varying NaCl concentration from 1 mM to 10 mM and concentration of chloride released by the mineralization of 0.2% TCE by the cultures, at the respective time intervals, was obtained from the standard curve. |
| Sequence analysis: Homology between the tce300 and tce350 gene sequences was determined using BLAST similarity search tool at NCBI. The todC1 genes present in other microorganisms were retrieved from NCBI GenBank and compared with tce300 and tce350 gene sequences using ClustalX and Mega5 software. Phylogenetic tree was constructed using neighbor joining model. |
| The tce300 and tce350 sequences were analyzed with ORF finder (NCBI) software to look for the 6 possible reading frames. Tce300 ORF was translated into 76a.a while the tce350 ORF was found to code for 102a.a. Theoretical molecular weight and pI of the deduced a.a. sequences were computed with Compute pI/MW tool available at http://www.expasy.org/tools/ [19]. Hydropathy profile was generated with Kyte-Doolittle plot using default window of 7. Secondary structure was revealed with CFSSP (Chou and Fasman secondary structure prediction server) [20,21] and first attempted model was generated through SAM_T08 (Sequence Alignment and Modeling System) [22,23]. |
| Results |
| Isolation and cloning of novel genes |
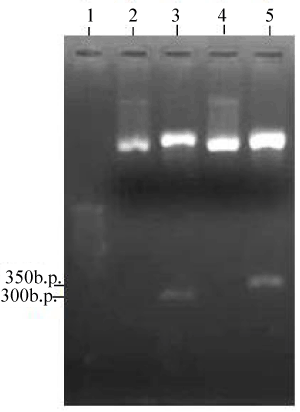
| Two PCR products were obtained when PM102 genomic DNA was amplified with primers specific for the todC1 gene of Pseudomonas putida. 1.2% agarose gel of the PCR products revealed two bands at 300 bp and 350 bp respectively (Figure 1). The two PCR products were separately cloned into p-GEMT Easy vector and sequenced. Presence of inserts could be confirmed by EcoR1 digestion of the recombinant plasmids designated as PR300 and PR350 (Figure 2). It was found that the tce300 and tce350 genes were different from the original todC1 gene of Pseudomonas putida or any other todC1 sequences available in the database. |
| GenBank submission |
| The partial sequence of the tce300 and tce350 genes were deposited at NCBI GenBank under accession numbers: PR300-JX910450 and PR350-JX910451, through the online sequence submission tool Bankit. |
| Protein profile of tce300 and tce350 gene over expression in DH5α |
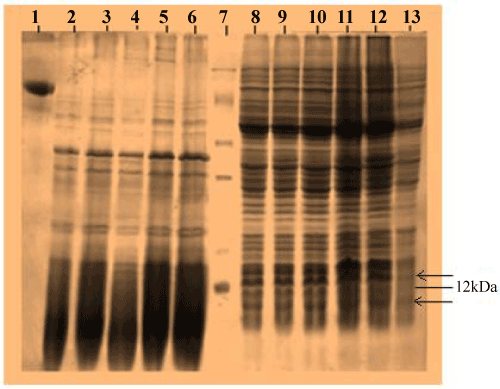
| Recombinant E.coli DH5α carrying the PR300 and PR350 plasmids were induced under the lac promoter by varying concentrations of IPTG. When proteins extracted from these induced cells were analyzed in a 12% SDS PAGE, a band could be seen at 12 kDa for PR350 proteins that seems to be over induced in presence of IPTG but is absent in the control ( Recombinant E.coli DH5α carrying PR350 in the absence of IPTG). For the PR300 induced proteins, no induced band could be detected as such (Figure 3). The theoretical molecular weights of the deduced amino acid sequences were predicted to be 11.74 kDa (PR350) and 8.86 kDa (PR300) with the Compute pI/MW tool (ExPasy). |
| Western blot |
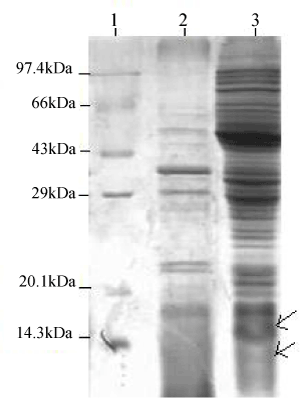
| Proteins were extracted by SDS lysis from 2 mM IPTG induced recombinant E.coli DH5α containing PR300 and PR350 (Figure 4a). The 2 mM IPTG induced protein of PR350 (over expressed in E.coli DH5α) was found to react with the antibody specific for TCE induced antigens in the western blot analysis. However, no reaction was obtained for the 2 mM induced protein of PR300 (over expressed in E.coli DH5α) with this specific antibody (Figure 4b). |
| TCE degradation by recombinant E.coli DH5α clones |
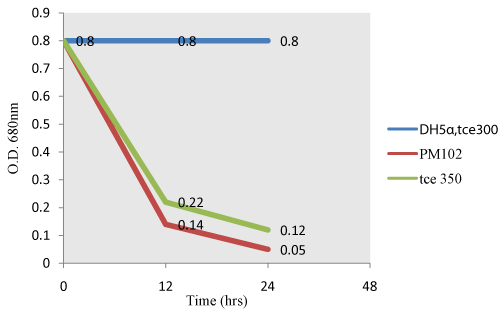
| Fujiwara test: When TCE is present in the supernatant, it reacts with pyridine under alkaline condition to give a red color (Fujiwara reaction). As TCE gets degraded by PM102 cells or recombinant E.coli carrying PR350, intensity of the red color decreases resulting in a corresponding decrease in O.D (Figure 5). Non transformed E.coli carrying PR300 does not exhibit TCE degradation activity as evident from the parallel graph indicating no change in intensity of the red color. |
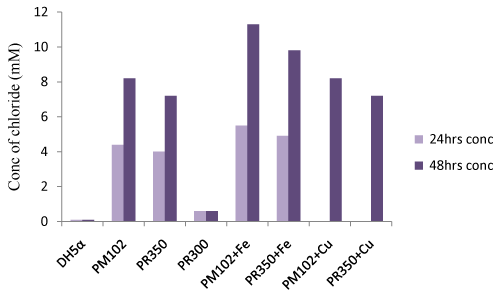
| Titration with silver nitrate: Standard curve of chloride release was plotted by varying NaCl concentration (Figure 6a). The PM102 isolate was found to release 8.8 mM chloride after 48 hrs that got increased to 11.9 mM on addition of 1 mM Fe (as calculated from the standard curve). No increment in chloride release was obtained in presence of 1mM Cu. Recombinant E.coli containing PR350 mineralized 0.2% TCE to 7.8 mM free chloride that was also found to increase to 10.4 mM chloride in presence of 1 mM Fe. No increment was seen in presence of Cu as also noted for the PM102 cells. Also, no growth was seen in presence of 1 mM Cu after 24 hrs. Hence, for cultures containing 1 mM Cu, chloride was measured only after 48 hrs. For recombinant E.coli containing PR300, only 1.2 mM chloride was estimated while nontransformed E.coli gave 0.8 mM chloride content (Figure. 6b). 0.6 mM chloride was found to be initially present in the peptone containing chloride free minimal medium without inoculation. Thus, 0.6 mM was subtracted from all the readings to get the correct value of chloride concentration actually released from the mineralization of 0.2% TCE. |
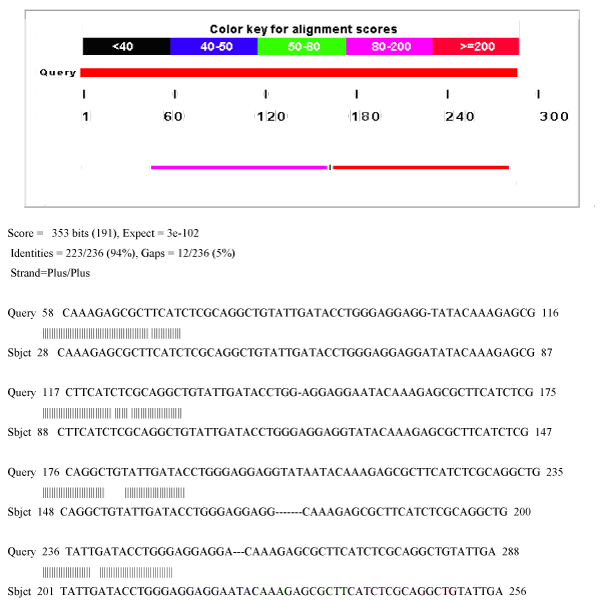
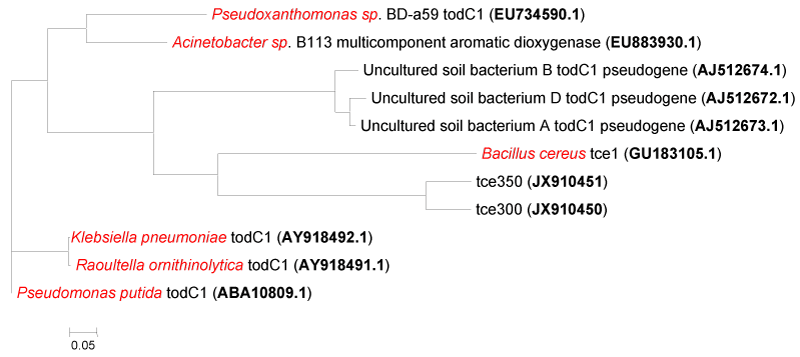
| Bioinformatics studies: 80% homology was seen between tce300 and tce350 gene sequences (Figure 7). Phylogenetic tree constructed with Mega5 shows that tce300 and tce350 genes have no significant similarity with the todC1 gene of Pseudomonas putida (Figure 8). The tce300 and tce350 genes were found to code for 76a.a and 102a.a by ORF finder program (NCBI) (Figure 9a and Figure 9b). Theoretical pI and molecular weight of the deduced amino acid sequences were predicted with Compute pI/MW Expasy proteomics tool as follows: TCE300 protein: pI/Mw: 9.10 / 8862.25; TCE350 protein: pI/Mw: 8.58 / 11741.44. |
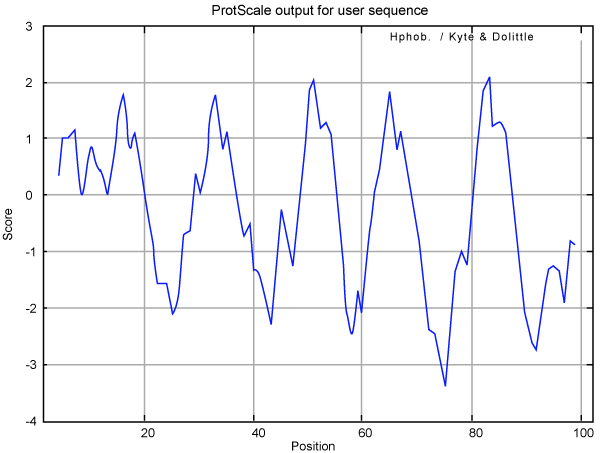
| TCE350 functional protein was further analyzed with protscale tool from Expasy [24]. Kyte-Doolittle plot is a widely applied scale for studying the hydrophobic regions of a protein. Regions with values above 0 are hydrophobic in character, while regions below 0 are hydrophilic. Hydrophilic character of the TCE350 protein was dominant over the hydrophobic character as score of hydrophilicity was greater than -3 while maximum core of hydrophobicity was 2 (Figure 10). Also, region of this protein from residue 60 to 80 had the maximum hydrophilicity. |
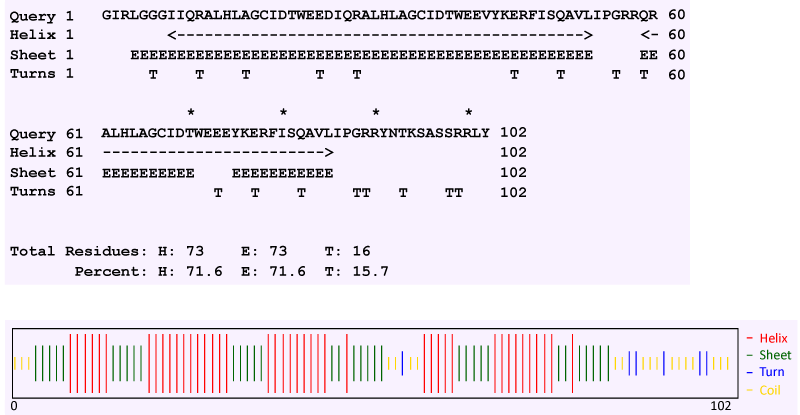
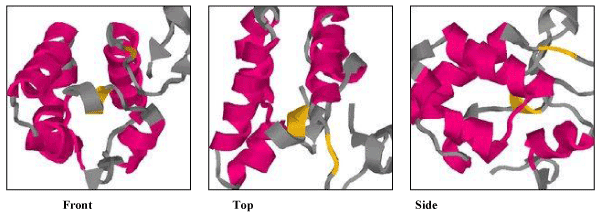
| Secondary structure prediction by the Chou and Fasman method revealed that TCE350 protein consists of alternating α helix and β strand (71.6% each) with 15.7% turn and very small amount of random coil (Figure 11). Figure 12 shows the first attempted model of TCE350 protein using SAM_T08 server. SAM_T08_server (ExPASy bioinformatics resource portal) finds similar protein sequences in NR and aligns them, providing sequence logos that show relative conservation of different positions. Local structure predictions are done with neural nets for several different local structure alphabets, and hidden Markov models are created. Fold recognition and alignment to proteins in the Protein Data Bank are done, and a full three-dimensional model is constructed. |
| Discussion |
| There are reports on the identification and functionality of a number of biodegradation genes for TCE, toluene, benzene, xylene, polycyclic aromatic compounds, etc. from different microbes based on cloning and expression studies in literature [25-28]. A linear rate of TCE degradation was observed with E.coli containing cloned toluene dioxygenase genes of Pseudomonas putida F1 [29]. The todC1 gene of P. putida F1 has also been separately cloned and expressed in E.coli [30]. All these studies deal with genes involved in cometabolism of TCE in presence of some other primary carbon source but the genes isolated from PM102 strain enabled TCE mineralization under conditions where TCE was available as the sole carbon source to the bacterium. We cloned and characterized from Stenotrophomonas maltophilia PM102, two genes corresponding to the toluene dioxygenase that catalyzes the first reaction in the degradation of trichloroethylene by P. putida F1 [31]. The genes were cloned into the EcoR1 site of p-GEMT Easy vector. Sequence analysis revealed that the tce300 and tce350 sequences were different from that of the original todC1 gene of P.putida. Furthermore, 80% homology was noted between the tce300 and tce350 sequences. |
| In cases of gene duplication, both copies of the gene might accumulate mutations that for instance reduce the functional efficiency of the encoded proteins without inhibiting this function altogether. In such a case, the molecular function (e.g. protein/enzyme activity) would still be available to the cell at least to the extent that was available before duplication. However, the accidental loss of one gene copy might then be detrimental, since one copy of the gene with reduced activity would almost certainly lie below the activity that was available before duplication [32]. A similar pattern was observed for the tce300 sequence reported in this study. The tce300 sequence derived from the functional tce350 gene has been rendered nonfunctional by mutations. This was experimentally proved by the observation that tce300 gene did not over express any protein on IPTG induction and also did not react to antibody specific for TCE inducible antigens. Further, recombinant E.coli carrying PR300 did not degrade TCE as observed by Fujiwara test and further confirmed by chloride release experiment. Thus, tce300 can be termed as a pseudo gene. |
| From the Fujiwara reaction, E.coli carrying PR350 was found to degrade TCE almost as efficiently as PM102 strain. This was further confirmed by chloride release experiment by titration with 10 mM silver nitrate. As chloride present in minimal medium interferes with this experiment, chloride free minimal broth was formulated. Still, this chloride free broth without inoculation was found to contain 0.6 mM chloride that may be from the peptone that was added. After 48 hours, PM102 cells released 8.2 mM (8.8-0.6) free chloride by mineralizing 0.2% TCE added initially while E.coli carrying PR350 released 7.2 mM (7.8-0.6) free chloride. On adding 1 mM Fe, chloride release was enhanced to 11.3 mM (11.9-0.6) by PM102 cells and to 9.8 mM (10.4- 0.6) by PR350 containing DH5α. The theoretical amount of chloride present in 0.2% TCE is 15.22 mM. Thus, PM102 strain can mineralize 74% TCE and DH5α harboring PR350 mineralizes TCE by 64%, in presence of 1mM iron. Thus, this tce350 gene mineralized TCE when it was present as the sole carbon source to the PM102 isolate. |
| The tce350 gene was found to over express a 12 kDa polypeptide as noted in the SDS PAGE analysis. Theoretical pI and MW values were 8.58 and 11.74 kDa respectively. In Western blot analysis, with antibodies specific for TCE inducible proteins, another 16.4 kDa band was observed in addition to the desired 12 kDa band. This may be due to the observation that specificity of antibodies is hard to predict as antibodies may cross-react to unrelated molecules. E.coli harboring tce350 exhibited TCE degradation activity. This is the first report of a TCE degrading gene isolated from the genus Stenotrophomonas that can grow on TCE as the sole carbon source. |
| Base 58 to 288 of tce300 sequence was 100% homologous to base 28 to 256 of tce350 gene sequence (Figure. 7). This suggests that tce300 sequence has resulted from deletion of 50 bases from 3’ end of tce350 gene; which means the functional activity of tce350 gene might lie at its 3’ end. |
| Analysis of the deduced amino acid sequence of the tce350 gene revealed presence of a conserved region of Xaa6-Gly (where Xaa is any amino acid) predicted to bind NAD+ or FAD [33]. Dioxygenase families of proteins are heteromultimers comprising of α and β subunits [34]. Secondary structure prediction of the deduced protein sequence of tce350 gene showed a predominance of α helix and β strand (71.6% each) and hence, can be classified under the dioxygenase family of proteins. |
| Acknowledgement |
| The authors thank The Department of Science and Technology (DST), New Delhi, India for the grant of INSPIRE fellowship (Innovation in Science Pursuit for Inspired Research). |
References
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4a | Figure 4b |
 |
 |
 |
 |
 |
| Figure 5 | Figure 6a | Figure 6b | Figure 7 | Figure 8 |
 |
 |
 |
 |
 |
| Figure 9a | Figure 9b | Figure 10 | Figure 11 | Figure 12 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14831
- [From(publication date):
February-2013 - Nov 13, 2025] - Breakdown by view type
- HTML page views : 10088
- PDF downloads : 4743
