Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Google Scholar citation report
Citations : 1089
Clinical Pharmacology & Biopharmaceutics received 1089 citations as per Google Scholar report
Clinical Pharmacology & Biopharmaceutics peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Editorial Board
Submit Manuscript
Journal Impact Factor 1.91*
Submit manuscript at or send as an e-mail attachment to the Editorial Office at manuscript@omicsonline.org
If you are interested in publishing with us or have any questions, please feel free to contact us directly on WhatsApp .
Table of Contents
About the Journal
Index Copernicus Value: 62.89
Clinical Pharmacology and Biopharmaceutics is an open access journal that provides an advanced forum for the science and technology of pharmacology and biopharmaceutics. It deals with the study of chemical and physical properties of pharmaceuticals, their components and their activities in living organisms. Topics include pharmacokinetics, pharmacodynamics, pharmacogenetics, pharmacogenomics, and pharmaceutical formulation, toxicology.
The aim of Clinical Pharmacology and Biopharmaceutics Journal is to encourage scientists to publish their experimental and theoretical records in as much detail as reviews, regular research papers, communications, and short notes. This Journal provides an efficient and fair peer review using Editorial managing system. Clinical pharmacology & Biopharmaceutics Peer Reviewed Journal is proficiently supported by universally prominent Editorial Board members.
Clinical Pharmacology & Biopharmaceutics Journals impact factor is mainly calculated based on the number of articles that undergo a single blind peer review process by competent Editorial Board so as to ensure excellence, essence of the work and number of citations received for the same published articles.Abstracts and full texts of all articles published by Clinical Pharmacology & Biopharmaceutics Open Access Journals are freely accessible to everyone immediately after publication.
Submit manuscript electronically at Online Manuscript Submission System and regarding general queries/detailed information, also for E-mail manuscript submissions send it directly to the Editorial office at manuscript@omicsonline.org
Clinical Pharmacology
Clinical Pharmacology is the study of drugs and the interactions of chemical substances with living beings, with a view to understanding the properties and their actions, including the interactions between drug molecules drug receptors and how these interactions induce an effect.
Applied Biopharmaceutics
Biopharmaceutics process validation is the most important and recognized parameters of CGMPs.The requirement of process validation appears of the quality system (QS) regulation. The goal of a quality system is to consistently produce products that are fit for their intended use. Process validation is a key element in assuring that these principles and goal are met. The process validation is standardization of the validation documents that must be submitted with the submission file for marketing authorization.
Journals related to Biopharmaceuticals process validation
Drug Designing: Open Access, Advances in Pharmacoepidemiology & Drug Safety, Journal of Drug Metabolism & Toxicology, Clinical Pharmacology & Biopharmaceutics, Process Validation for Biopharmaceuticals, Validation of Biopharmaceutical Manufacturing Processes, Drug Development and Industrial Pharmacy, Pharmacogenomics & Pharmacoproteomics, International Journal of Pharmaceutical Sciences and Research, Journal of Advanced Pharmaceutical Technology & Research.
Clinical Drug Trials
A clinical trials is a study that is purposely designed to test the points of interest and threats of a specific therapeutic treatment or intercession, such as new drug or a behaviour change.
Journals related to Clinical drug trials
Journal of Developing Drugs, Drug Designing: Open Access, Journal of Clinical Trials, Contemporary Clinical Trials, International Journal of Clinical Trials, Open Access Journal of Clinical Trials, Journal of Clinical Trials, PLOS Clinical Trials, Journal of Cancer Clinical Trials, Drug Development and Industrial Pharmacy, Journal of Clinical Medicine Research, Controlled Clinical Trials.
Methods in Clinical Pharmacology
Clinical pharmacology often uses the methods that have been developed by other disciplines, such as analytical chemistry ,biochemistry/molecular biology, physiology, psychology. Drug Discovery and Evaluation has become a more and more difficult, expensive and time-consuming process. The effect of a new compound has to be detected by in vitro and in vivo methods of pharmacology.
Journals related to methods in clinical pharmacology
Clinical Pharmacology & Biopharmaceutics, Clinical & Experimental Pharmacology, Journal of Pharmaceutical Sciences & Emerging Drugs, Biochemistry & Pharmacology: Open Access, The Journal of Clinical Pharmacology, International Journal of Basic & Clinical Pharmacology, Journal of Anaesthesiology Clinical Pharmacology, Clinical Pharmacology: Advances and Applications, Pharmacology and Toxicology, International Journal of Basic & Clinical Pharmacology.
Biopharmaceutics and Drug Disposition
In preclinical and early clinical stages of drug designing, drug disposition is used to predict drug interaction potential, evaluation and understand population pharmacokinetic variability, and select doses for clinical trials. Drug disposition involves the absorption, Distribution, excertion metabloism of drugs in living organism in called drug disposition.
Journals related to Biopharmaceutics and drug disposition
Clinical Pharmacology & Biopharmaceutics, Clinical & Experimental Pharmacology, Journal of Pharmaceutics & Drug Delivery Research, Journal of Molecular Pharmaceutics & Organic Process Research, Journal of Molecular Pharmaceutics & Organic Process Research, International Journal of Pharmaceutics, Indian Journal of Pharmaceutical Sciences, Journal of Pharmaceutics, International Journal of Applied Pharmaceutics, European Journal of Pharmaceutics and Biopharmaceutics, Journal of Pharmaceutics & Drug Delivery Research.
Biopharmaceuticals Process Validation
Biopharmaceutics process validation is the most important and recognized parameters of CGMPs.The requirement of process validation appears of the quality system (QS) regulation. The goal of a quality system is to consistently produce products that are fit for their intended use. Process validation is a key element in assuring that these principles and goal are met. The process validation is standardization of the validation documents that must be submitted with the submission file for marketing authorization.
Journals related to Biopharmaceuticals process validation
Drug Designing: Open Access, Advances in Pharmacoepidemiology & Drug Safety, Journal of Drug Metabolism & Toxicology, Clinical Pharmacology & Biopharmaceutics, Process Validation for Biopharmaceuticals, Validation of Biopharmaceutical Manufacturing Processes, Drug Development and Industrial Pharmacy, Pharmacogenomics & Pharmacoproteomics, International Journal of Pharmaceutical Sciences and Research, Journal of Advanced Pharmaceutical Technology & Research.
Pharmacoeconomics
Pharmacoeconomics identifies, measures, and compares the costs and consequences of drug therapy to healthcare systems and society. Economic, humanistic, and clinical outcomes should be considered and valued using pharmacoeconomic methods.
Journals related to pharmacoeconomics
Pharmacoeconomics: Open Access, Journal of Pharmacovigilance, Journal of Pharmacological Reports, Journal of Pharmacognosy & Natural Products, Expert Review of Pharmacoeconomics & Outcomes Research, The Open Pharmacoeconomics & Health Economics Journal, ClinicoEconomics and Outcomes Research, Pharmacoeconomics and Pharmaceutical Policy, Journal of Pharmacoeconomics and Pharmaceutical Management, C.V.P. Pharmacoeconomics.
Clinical Trials Databases
Clinical Trials Database is providing to the public a listing of specific information relating to phase I, II and III clinical trials in patients. It provides a source of information about clinical trials involving human pharmaceutical and biological drugs. Patients can access the database to determine if a clinical trial has met the regulatory requirements.
Journals related to Clinical trials databases
Journal of Clinical Trials, Journal of Cancer Clinical Trials, Clinical & Experimental Pharmacology, Clinical Pharmacology studies, Trials, Journal of Clinical Medicine Research, Controlled Clinical Trials, Contemporary Clinical Trials Journals, International Journal of Clinical Trials.
Medical Trails/ Drug Medical Trails
Medical trials are exploration mulls over that investigate whether a medical strategy, treatment, or device is protected and viable for humans. The main purpose of clinical trials is examination, so the studies take after strict logical guidelines. These measures ensure patients and help produce reliable study results.
Journals related to Medical trails/ drug medical trails
Clinical Trials: Journal, Open Access Journal of Clinical Trials, Contemporary Clinical Trials, Journal of Clinical Trials, International Journal of Clinical Trials, Contemporary Clinical Trials, PLOS Clinical Trials, International Journal of Clinical Trials, Journal for clinical studies.
Clinical Pharmacists
Clinical pharmacist is educated and trained in direct patient care environments, including medical centers, clinics, and a variety of other health care settings. Clinical pharmacists are frequently granted patient care privileges by collaborating physicians or health systems that allow them to perform a full range of medication decision-making functions as part of the patient’s health care team.
Journals related to clinical pharmacists
Journal of Clinical Trials, Journal of Cancer Clinical Trials, Clinical & Experimental Pharmacology, Journal of Clinical Pharmacy and Therapeutics,International Journal of Clinical Pharmacy, Journal of Basic and Clinical Pharmacy, European Journal of Clinical Pharmacy, Journal of Basic and Clinical Pharmacy, International Journal of Clinical Pharmacy, Indian Journal of Pharmacy Practice, Reswearch & Reviews: Journal of Hospitality and Clinical Pharmacy.
Preclinical Safety Evaluation of Biopharmaceuticals
Pharmaceutical products are evaluated in humans, it is essential that they undergo a rigorous safety assessment using in vitro models and studies in preclinical species. Once products progress into the clinic, additional preclinical studies are needed to support further clinical testing. Although regulatory guidelines provide a good framework for the types of studies that should be performed, there are some areas where it is unclear how these should be applied to microbicides, what study designs should be used, whether certain tests are relevant or if additional assays are appropriate.
Journals related to Preclinical safety evaluation of biopharmaceuticals
Clinical Pharmacology & Biopharmaceutics, Biochemistry & Pharmacology: Open Access, Advances in Pharmacoepidemiology & Drug Safety, Journal of Molecular Pharmaceutics & Organic Process Research, Biopharmaceutics & Drug Disposition, Pharmaceutical Sciences & Emerging Drugs, Journal of Pre-Clinical and Clinical Research, Austin Journal of Cancer and Clinical Research, European Journal of Clinical Pharmacy, Journal of Basic and Clinical Pharmacy.
Biopharmaceuticals Manufacturing and Industry
Biopharmaceuticals manufacturing and industry gives an overview of biotech manufacturing processes used to make biopharmaceuticals. the most commonly used manufacturing processes, including cell culture and fermentation; harvest and recovery; viral removal and inactivation; and purification processes such as tangential flow filtration, centrifugation, and size exclusion and adsorptive chromatography.
Journals related to Biopharmaceuticals manufacturing and industry
Journal of Applied Pharmaceutical Science, Indian Journal of Pharmaceutical Education, Molecular Pharmaceutics journal, Molecular Pharmaceutics, Pharmaceutics & Drug Delivery Research, Molecular Pharmaceutics & Organic Process Research, Journal of Pharmacokinetics and Pharmacodynamics.
Pharmacology Drug Classes
A drug may be classified by the chemical type of the active element or by the way it is used to treat a particular condition. Each drug can be classified into one or more drug classes.
Journals related to Pharmacology drug classes
International Journal of Drug Policy, Journal of Drug Delivery, International Journal of Drug Development and Research, Journal of Drug Metabolism & Toxicology, Drug Design, Development and Therapy, Journal of Developing Drugs, International Journal of Drug Delivery, Journal of Drug Education
Psychopharmacology
Psychopharmacology is the study of the use of medications in treating mental disorders. The complexity of this field requires continuous study in order to keep current with new advances. Psychopharmacologists need to understand all the clinically relevant principles of pharmacokinetics and pharmacodynamics. This includes an understanding of Protein binding, Half-life, Polymorphic genes, Drug-to-drug interactions.
Journals related to Clinical Pharmacology studies
Clinical Pharmacology & Biopharmaceutics, Biochemistry & Pharmacology: Open Access, Clinical & Experimental Pharmacology, Journal of Pharmacokinetics and Pharmacodynamics, International Journal of Applied Pharmaceutics, Journal of Pharmacokinetics and Pharmacodynamics, Molecular Pharmaceutics
Gold standard drug database
The next generation drug database and drug decision support engine, which fulfills the complex and evolving drug information needs throughout the healthcare system. Healthcare professionals are able to quickly make fully informed decisions on drug prescribing, dispensing, adjudication and analysis.
Lyophilisation of biopharmaceuticals
Lyophilization is a method for drying, accomplished by solidifying the wet substance and bringing on the ice to great straightforwardly to vapor by exposing it to a low pressure of water vapour. For drug manufacturers, the challenge may at first appear daunting, but it is one that must be met if the compounds being developed are to be successful.
Clinical softwares and data management
Clinical software & data management is a practice of acquiring, analyzing and protecting digital medical information vital to providing quality patient care. With the widespread computerization of health records, traditional (paper-based) records are being replaced with Electronic Health Records.
OMICS International through its Open Access Initiative is committed to make genuine and reliable contributions to the scientific community. OMICS International hosts over 700 leading-edge peer reviewed Open Access Journals.
OMICS international is hosting 3rd International Summit on Clinical Pharmacy during December 07-09, 2015 in Atlanta, USA. Clinical Pharmacy International Conference 2015 will be organized around the theme "Creating Tremendous Challenges and Development in Clinical Pharmacy".
Pharmacogenomics
Pharmacogenomics is a field of medicine that examines how an individual's genetic makeup influences their response to drugs. It combines pharmacology and genomics to tailor drug treatments based on genetic profiles, aiming to optimize therapeutic efficacy and minimize adverse effects.
Pharmacogenomics helps personalize medical treatments by identifying genetic variants that affect drug metabolism, efficacy, and safety. This approach enables healthcare providers to predict patient responses to medications, adjust dosages accordingly, and choose the most effective drugs, ultimately improving treatment outcomes and reducing the risk of adverse drug reactions.
Biomarker Discovery
Biomarker discovery involves identifying biological markers—molecules found in blood, other body fluids, or tissues—that indicate the presence or progression of a disease, or the effects of a treatment. These biomarkers can help in diagnosing diseases, predicting outcomes, and monitoring therapeutic responses.
Biomarker discovery is crucial for advancing precision medicine by providing insights into disease mechanisms and treatment effects. It involves techniques such as genomics, proteomics, and metabolomics to find novel biomarkers that can be used for early diagnosis, personalized treatment plans, and monitoring disease progression.
Precision Medicine
Precision medicine is an approach to medical treatment and diagnosis that tailors interventions based on an individual’s genetic, environmental, and lifestyle factors. It aims to provide personalized healthcare by considering the unique characteristics of each patient to optimize treatment outcomes.
Precision medicine moves away from a one-size-fits-all model by using detailed information about a patient's genetic makeup, environment, and lifestyle to make more informed decisions about their care. This approach allows for customized treatment plans that improve the effectiveness of therapies, minimize side effects, and enhance overall health outcomes.
DMPK (Drug Metabolism and Pharmacokinetics)
It refers to the study of how drugs are absorbed, distributed, metabolized, and excreted by the body. This field involves understanding the fate of pharmaceuticals within the organism and how their efficacy and safety are influenced by various biological processes. Absorption How a drug enters the bloodstream. Distribution How the drug spreads throughout the body's tissues and organs. Metabolism How the drug is chemically altered by the body, primarily in the liver. Excretion How the drug is eliminated from the body, usually through urine or faeces
Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
Itis a method used to describe and predict the relationship between drug concentrations in the body and their pharmacological effects. It combines two key aspects. Pharmacokinetics (PK) How a drug is absorbed, distributed, metabolized, and eliminated in the body.Pharmacodynamics (PD) The drug's biological effects and the relationship between drug concentration and effect.PK-PD modeling integrates these aspects to predict how drug concentrations correlate with therapeutic and adverse effects, guiding dose optimization and improving drug efficacy and safety.
*2024 Journal Impact Factor was established by dividing the number of articles published in 2022 and 2023 with the number of times they are cited in 2024 based on Google Scholar Citation Index database. If 'X' is the total number of articles published in 2022 and 2023, and 'Y' is the number of times these articles were cited in indexed journals during 2024 then, journal impact factor = Y/X
Fast Editorial Execution and Review Process (FEE-Review Process):
Clinical Pharmacology & Biopharmaceutics is participating in the Fast Editorial Execution and Review Process (FEE-Review Process) with an additional prepayment of $99 apart from the regular article processing fee. Fast Editorial Execution and Review Process is a special service for the article that enables it to get a faster response in the pre-review stage from the handling editor as well as a review from the reviewer. An author can get a faster response of pre-review maximum in 3 days since submission, and a review process by the reviewer maximum in 5 days, followed by revision/publication in 2 days. If the article gets notified for revision by the handling editor, then it will take another 5 days for external review by the previous reviewer or alternative reviewer.Acceptance of manuscripts is driven entirely by handling editorial team considerations and independent peer-review, ensuring the highest standards are maintained no matter the route to regular peer-reviewed publication or a fast editorial review process. The handling editor and the article contributor are responsible for adhering to scientific standards. The article FEE-Review process of $99 will not be refunded even if the article is rejected or withdrawn for publication.
The corresponding author or institution/organization is responsible for making the manuscript FEE-Review Process payment. The additional FEE-Review Process payment covers the fast review processing and quick editorial decisions, and regular article publication covers the preparation in various formats for online publication, securing full-text inclusion in a number of permanent archives like HTML, XML, and PDF, and feeding to different indexing agencies.
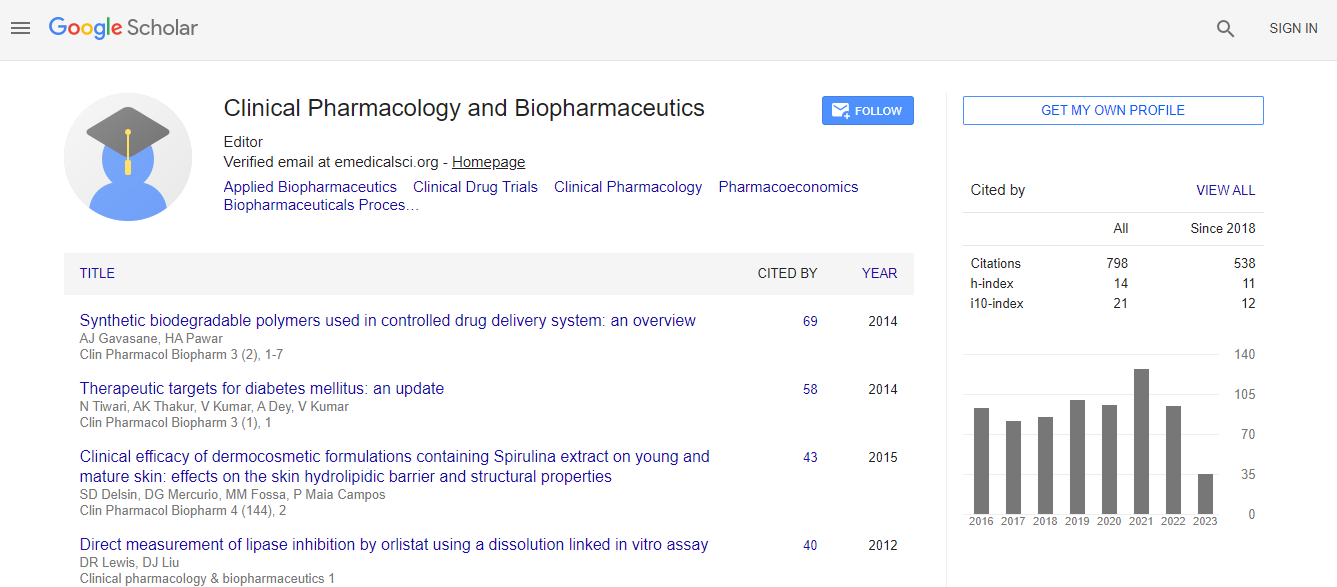
h-index
Articles published in Clinical Pharmacology & Biopharmaceutics have been cited by esteemed scholars and scientists all around the world. Clinical Pharmacology & Biopharmaceutics has got h-index 14, which means every article in Clinical Pharmacology & Biopharmaceutics has got 14 average citations.
Recently Published Articles
-
The Uses and Side Effects of Azithromycin
Paraskev Katsakori -
A Note on Biochemical Pharmacology
Mohamed Sayed -
Formulation and Development of Herbal Sanitizer
PT Chavanke -
Insulin: The medication of Type 1 Diabetes
Mohamed Sayed -
A Brief Note on Trials of Drugs
Xiaoya Zhang -
Micronutrients, N-Acetyl Cysteine, Probiotics and Prebiotics, a Review of Effectiveness in Reducing HIV Progression
James A Uchizono

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi