Review Article Open Access
Claudins, Inflammation and Epithelial-Mesenchymal Transition in Gastric Tissue
Rendon-Huerta E*, Chavarria-Velazquez CO and Montaño LFDepartment of Cell and Tissue Biology, Immunobiology Laboratory, UNAM, Mexico
- *Corresponding Author:
- Rendon-Huerta E
Faculty of Medicine, Department of Cell and Tissue Biology
Immunobiology Laboratory, UNAM, Mexico
E-mail: erendon@bq.unam.mx
Received date: September 26, 2013; Accepted date: October 26, 2013; Published date: November 02, 2013
Citation: Rendon-Huerta E, Chavarria-Velazquez CO, Montaño LF (2013) Claudins, Inflammation and Epithelial-Mesenchymal Transition in Gastric Tissue. J Gastroint Dig Syst 3:149. doi:10.4172/2161-069X.1000149
Copyright: © 2013 Rendon-Huerta E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Gastric cancer is a serious worldwide health burden. It is the fourth most frequently diagnosed cancer with an estimated 1 million new cases per year. The disease is often diagnosed in advanced stages and is associated with a poor prognosis for patients. It exhibits heterogeneity in clinical, biologic, and genetic aspects. Although H. pylori is the best studied risk factor, interleukins associated with chronic inflammation, disruption of tight junctions especially claudins, MERK/ERK signaling pathways and cancer stem cells have key roles in carcinogenesis and progression. An in-depth understanding of the role that inflammation and modified tight junction proteins play in epithelial-mesenchymal transition may improve our understanding of the cancer process and lead to the recognition of new biomarkers for early diagnosis, and possibly, improved therapeutics.
Keywords
Chronic inflammation; Epithelial-mesenchymal transition; Gastric tissue
Introduction
The mammalian gastric mucosa and its glands are formed by simple cylindrical mucus secreting epithelium, and gastric tubular glands reaching the muscular layer, underneath; the structure of these glands differs according to the gastric anatomical area [cardias, body, pylori] [1]. Epithelial cells are attached to each other at their lateral membranes by a complex of intercellular junctions that maintain the cells together. The most apical of them is the occluding junction also known as zonula occludens, or tight junction. This complex is composed of multiple proteins that include transmembrane proteins, cytoplasmic signaling proteins, and adapters that link them to the actin cytoskeleton [2]. The transmembrane proteins mediate the major functions of the tight junction: barrier, pore, and fence. There are single transmembrane domain proteins [JAM, Crb3, CAR], a triple transmembrane domain protein [Bves], and the four-transmembrane domain proteins, the claudin family and TAMP [occludin, tricellulin, MarvelD [3].
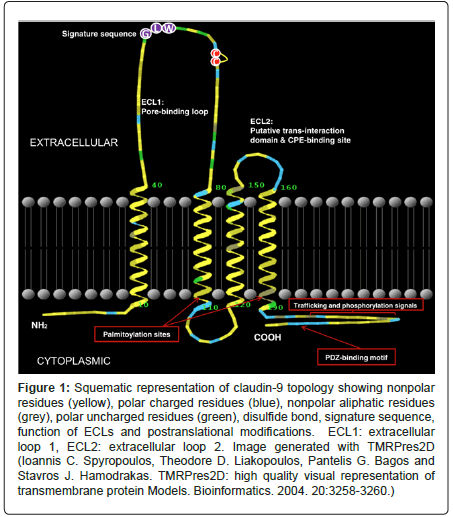
Claudins are barrier-forming proteins. They regulate paracellular permeability, can form pores, especially small pores [-4 A], or enhance water permeability. They are considered the major determinants of the permeability properties of epithelial cells. There are 27 claudins in mammals grouped in eight subgroups [3,4]; they are expressed in a tissue-specific pattern and distributed in all the cell-cell contact areas in epithelia. There is abundant evidence on the function and tissue specificity of claudins [2,5]. Multiple claudin isoforms are expressed simultaneously at the tight junction. Using the serial analysis of gene expression [SAGE] Genie database it has been confirmed that some tissues express a large number of claudins genes whereas others show a more restricted pattern [6]. The arrangement of claudins give raise to two extracellular loops, the first play crucial roles in paracellular charge selectivity whereas the second is important in tight junction strand interactions between claudins located in neighbor cells (Figure 1). Despite the physiological functions of the first and second extracellular loops of claudins, the long and highly divergent C-terminal tail of claudins are related to various important functions. Firstly, the C-terminal is essential for trafficking to the tight junction [7], since its truncation lead to claudin intracellular retention and subsequent degradation; this tail is also vital to determine the protein half-life [8] but it has also been recognized as a regulator of some homeobox genes [9] related to embryo development [10]. Claudin expression can be regulated at the transcriptional, posttranscriptional, and posttranslational levels. The regulators of claudin expression comprise some homeobox transcription factors such as CDX1, CDX2 and HNF- 1a as well as TNF-a/NFkB and TGF-b-Smad/Snail pathways, PPARy, SP1, HNF-4a, GATA-4 and Grhl2.
Figure 1: Squematic representation of claudin-9 topology showing nonpolar residues (yellow), polar charged residues (blue), nonpolar aliphatic residues (grey), polar uncharged residues (green), disulfide bond, signature sequence, function of ECLs and postranslational modifications. ECL1: extracellular loop 1, ECL2: extracellular loop 2. Image generated with TMRPres2D (Ioannis C. Spyropoulos, Theodore D. Liakopoulos, Pantelis G. Bagos and Stavros J. Hamodrakas. TMRPres2D: high quality visual representation of transmembrane protein Models. Bioinformatics. 2004. 20:3258-3260.)
Claudin Expression in Gastric Tissue
As already mentioned, each claudin isoform is expressed in a tissue-specific pattern. In rodent stomachs, claudin-3 is most strongly expressed in the surface epithelial cells along the basolateral membrane, claudin-4 is expressed mainly at the tight junction in proximal gastric glands, and claudin-5 is uniformly expressed from the base of the glands to the surface on the basolateral membrane [11]. Claudin-21, -24 and -25 are expressed in the stomach between day 7 and 17 of mouse embryo development [3].
Developmental changes in claudin expression during human neonatal life have been observed in the jejunum and intestine [12,13]. Claudin-2 epithelial expression at birth decreases markedly over 90 days whereas claudin-19 is no longer expressed 28 days after birth. Claudins-3, -4, and -7 increase its expression in mesodermal-derived tissues during early life, and claudin-15 migrates from the crypts to the jejune surface epithelium [2]. Despite all this, there are reports of nuclear localization of claudins, suggesting a direct role in the regulation of gene expression; however their physiological function remains to be determined.
The mammalian stomach is a histologically complex organ protected with a simple cylindrical epithelium, the mucosa and the socalled gastric fossae. The fossae gastric glands are composed by neck, principal, parietal, enteroendocrine, and non-differentiated stem cells are found. The composition of claudins plays an essential role in the physiological function of the gastric TJ. Claudin-6 is one of the earliest molecules expressed during epithelial differentiation, and its expression is largely confined to embryonic and fetal life [14-16]. Nevertheless, the expression of claudins in human stomach development is not known. Claudin-6 has been detected in human fetal cells isolated from maternal blood [17]. Claudin-18.2 expression appears to be exclusive and restricted to exocrine and endocrine cells of the gastric glands [18]. Our initial results in human fetal gastric samples show that claudin-6 and claudin-9 are temporarily expressed between the 3- and 7-week [data not shown]. Claudins-12, -18.2, and -23 and, are highly expressed in healthy adult human stomach [19]. As claudin expression is tissue specific, a definite knowledge of the claudins normally expressed in the different regions of the stomach, is vital.
Claudins and Epithelial-mesenchymal Transition
Epithelial-mesenchymal transition [EMT] is a developmental process whose essential features are the disruption of intercellular contacts and the enhancement of epithelial cell motility, migratory properties of mesenchymal cells and the acquisition by the latter of stemcell like properties [20-22]. This transition is defined by the progressive loss of epithelial protein markers [E-cadherin, Cytokeratin, Laminin-1, Entactin, Syndecan, MUC1, Desmoplakin, a1[IV] collagen, miR200 family and ZO-1] and gain of mesenchymal markers [N-cadherin, Vimentin, Fibronectin, FTS binding protein FAP, Syndecan-1, a5b1 integrin, miR10b, miR21, FOXC2, LEF-1, ETS, and SIP1]. In this transition transcription factors such as Snail, Slug and Twist play a decisive role. There are different EMT types. Type 1 is associated with implantation and embryonic gastrulation, type 2 is associated with wound healing, tissue regeneration and organ fibrosis, and type 3 occurs in the neoplastic transformation process [23]. Inflammatory cytokines are associated with EMT and carcinogenesis. Nevertheless, the modifications in claudin expression must be subordinated to major modification in the signaling pathway. In EMT intercellular adhesion decrease, cell motility increases, synthesis of extracellular matrix proteins increases, there is a loss of cell polarity and elevated resistance to apoptosis, and all these phenomena require dynamic changes of TJ protein expression. The latter depends, amongst many others, on the phosphorylation level of the claudins [24-26]. MAPK activation inhibits the formation of TJ whereas inhibition of MEK1 signaling permits TJ formation [27]. TJ formation is also induced by the complex formed by the atypical protein kinase C [aPKC], PAR3, and PAR6 [28]. Since claudin determine the ion selectivity of pores, ¿is it essential or necessary for EMT a modification in ion selectivity?
HGF, EGF, PDGF, and TGF-β, appear to be responsible for the induction or functional activation of a series of EMT-inducing transcription factors, notably Snail, Slug, zinc finger E-box binding homeobox 1 [ZEB1], Twist, Goosecoid, and FOXC2 [29,30]. Once expressed and activated, intracellular signaling networks involving, ERK, MAPK, PI3K, Akt, Smads, RhoB, β-catenin, lymphoid enhancer binding factor [LEF], Ras, and c-Fos as well as cell surface proteins such as β4 integrins, α5β1 integrin, and αVβ6 integrin [31] facilitate the transition. Activation of EMT programs is also facilitated by the disruption of cell-cell adherens junctions [23,32]. Therefore the modification of intercellular ion exchange becomes necessary since the cells generated in this epitelial-mesenchymal transition possess stem cell properties. Embryonic stem cells differentially express claudin-4 during early stages of hematopoietic commitment [33]. Claudin-4 is considered as a real marker of EMT because it increases the number of TJ strands and trans-epithelial resistance but at the same time it decreases the permeability of Na+. Claudin phosphorylation associated with tight junction disassembly is also enhanced byEphA1, which is recruited to bind to claudin-4 by forming a complex with ephrin-B1 [34]. Interestingly ephrins induce EMT [35,36]. F9 cells, an embryonic carcinoma cell line, form di novo functional tight junctions expressing claudin-6 and claudin-7 under HNF4alpha induction [37,38]. Stem cells express claudin-6 because it is vital for their survival and selfrenewal [39] and is considered a marker of cancers with a primitive phenotype [40].
The transcription factors beta-catenin/Tcf complex [41] and Cdx homeodomain protein/hepatocyte nuclear factor-1a bind directly to claudin promoters [42], thus regulating TJ activity under physiological and pathological conditions. The transcription repressors E12/E47, ZEB-1, SIP-1, Slug, and Snail binds to E-box motifs in claudin-3, -4 and -7 promoters thus suppressing expression [43].
Up-regulation and modification of tissue claudins may also affect cell signaling pathways via binding domains to ZO-1 that interacts with signaling proteins AF-6, connexin 43, or G proteins [44-46] associated with neoplastic process.
When the epithelial cells are activated and the EMT process is initiated the simultaneous loss of epithelial characteristics and cell-cell adhesion is required so the modified cells gain mobility and ability to cross the basal membrane, therefore loss or modification of cell polarity is a priority [47]. This process possibly fulfills a more physiological role in the carcinogenesis environment. It has been shown that NFkB dependent increase of transcription factors related to the development of cancer stem cell properties, thwarts the irreversible loss of epithelial identity, thus maintaining the carcinogenesis process [48,49]. EMT is also related to repression of some kinases important in the regulation of the structure and function of the tight junction [50]. If the basic concept of epithelial carcinogenesis is that an epithelial cell is becoming dedifferentiated, then the modified cell must express proteins compatible with its new differentiation and functional status, similar or identical to those determined during embryogenesis. Therefore tight junction proteins should contribute to maintain the epithelial integrity of cell layers in the context of a normal developing embryo in accordance to its differentiation markers [51].
Claudins in Cancer
The expression of claudin proteins is altered in neoplastic tissues and their role may be linked to functions unrelated to tight junction formation, essentially survival and invasion of cancer cells [52-54]. Nevertheless, the relationship between claudin expression and development of cancer requires a better understanding of the regulatory network [55]. Epithelial-to-mesenchymal transition [EMT] is central to cell migration and thus invasiveness and metastasis in cancer. Several pathways intertwine to regulate this transition and many of their transcription factors are involved in the regulation of claudins expression. The most important signaling pathways involved in EMT seem to be related to TGF-b, a cytokine that induces de-differentiation of cells [56], but many other transcription factors regulate the expression of claudins. For example, HNF-4a up regulate the expression of claudins-6 and -7 and induces epithelial differentiation through their translocation to the tight junction [37] in embryonal carcinoma cells [57] but at the same time it also inhibits the development of stable epithelial cell layer [58]. Increasing evidence emphasizes the role of up- or downregulation of signaling pathways as well aberrant proteosomal activity in carcinogenesis [59,60]. Claudin-1, -3, -4 and -7 are amongst the most frequently deregulated claudins in various cancers but significant differences have been reported depending the stage and grade of the cancers samples. Claudin-6 is also detected although under-expressed in primary mammary gland tumors [61]. Claudin-7 is expressed in epithelial tissues [62]; acts as a paracellular Cl- pore and form a complex with the epithelial cell adhesion molecule [63] thus regulating cell-cell adhesion, cell motility, and tumor progression. Claudin-7 is also under-expressed in primary breast as well as head and neck tumors [64]. Claudin-9 is mainly expressed in the cochlea [65]. Claudin-18-2 is a major constituent of tight junctions in stomach epithelia [66] and it induces a selective sealing of the tight junction against H+ [67] thus protecting the epithelium against low pH. Claudin-23 has also been detected in stomach and it appears to be deregulated in gastric cancer [68].
Claudins in Gastric Cancer
Of the great myriad of cancers affecting the population, gastric cancer is still the fourth most common cancer and the second most common cause of cancer death in the world. The incidence is higher among men and the prognosis is poor. Nearly 1 million new cases of gastric cancer are diagnosed worldwide annually. Risk factors include Helicobacter pylori infection, smoking, high intake of salt-preserved food, dietary nitrite, low intake of fruit and vegetables, family history of cancer, and gender [69]. Around 95% of gastric cancers are adenocarcinomas, which are classified into “intestinal”, associated with a history of atrophic gastritis and better survival rates, and “diffuse”, which are more common and have worse survival rates. The remaining 5% include lymphomas and leiomyosarcomas.
The gastric mucosa and its glands, both of endodermal origin, show continuous bidirectional self-renewal via differentiation from stem and progenitor cells. This process implies extremely frequent tight junction re-arrangement so the presence of risk factors [drugs, stress, food] during this process may alter normal mucosa dynamics.
Deregulated gastric self-renewal can evolve to intestinal metaplasia through abnormal trans-differentiation of some of the constitutive cell lineages such as the mucous neck-zymogen cell lineage [70,71] that are considered long-lived progenitors that can give rise to the gastric epithelial chief cell [72]. The chief cell lineage can itself transdifferentiate into a mucous SPEM [Spasmolytic Polypeptide Expressing Metaplasia] cell metaplasia [73]. Under pathophysiological loss of the acid-secreting parietal cells [i.e. secondary to chronic Helicobacter infection], these are replaced with mucous cell metaplasia [74,75]. In the presence of chronic and exuberant inflammation deregulated differentiation processes occur and the chief cell lineage regains proliferative capacity and may further differentiate into SPEM, and cancer [76]. The mucosa surrounding intestinal type gastric cancers are SPEM [77]. Nevertheless, there is evidencing suggesting that SPEM and intestinal metaplasia might arise independently from trans differentiating chief cells during oxyntic atrophy [78].
Modifications on the expression patterns of claudin in gastrointestinal tract cancer have been characterized at the histological and molecular level. The results have been inconclusive and controversial. For instance, Resnick et al. [79] reported moderate to strong staining of claudins 1, 3 and 4 in 48-74% biopsies of intestinal type adenocarcinoma and 24- 46% in the diffuse type. Diminished expression of occludin in poorly differentiated gastric adenocarcinoma, claudin 23 down-regulations in intestinal type adenocarcinomas, better outcome in patients with a strong expression of claudin 3 in the intestinal type adenocarcinoma or loss of claudin 1, 3 and 4 in diffuse type adenocarcinoma [80-82], accentuate the puzzle.
As far as gastric carcinogenesis is concerned we will focus on claudins-6, -7, and -9, because we clearly demonstrated that these claudins are expressed in gastric adenocarcinoma [54,83,84] and that their presence anticipate a bad prognosis.
EMT and Inflammation in Gastric Cancer
A major concern in relation to gastric carcinogenesis would be the function that inflammation might play in the process. Chronic inflammatory responses are associated with various types of cancer. To maintain homeostatic tissue function, innate immune system recognizes altered levels of self-molecules as well as foreign molecular structures. A simplified pathway would be the activation of a group of multimeric protein complexes called the inflammasome complex. Inflammasomes promote inflammation and inflammatory cell death through the activation of cysteine protease caspase-1. Two steps activate Inflammasomes. Initially, the expression of pro-IL-1β and pro-IL-18 [a cytokine structurally related to IL-1b] is induced through the activation of NF-kB [85]. NF-kB can be activated by TNF-α and IL-1 or by sensing of “danger signals” [PAMPs and DAMPs] by TLRs or NOD1/2. Inflammasome also sense PAMPs and DAMPs by NLRs [Nod-like receptors] or AIM2 [absent in melanoma 2]. Activation of NLRs result in the activation of pro-caspase-1, which once cleaved can catalyze the activation of IL-1β and IL-18. [86,87]. Cancers of the gastrointestinal tract are frequently associated with chronic inflammation, especially gastric cancer where the link between Helicobacter pylori infection and chronic gastritis is well documented. There is evidence linking inflammasome and their IL-1b and IL-18 products to gastric cancer [88]. In fact, IL-1b in stomach induces tumorigenesis [89] through the induction of epithelial to mesenchymal [EMT] transition [90]. EMT is a process involved in normal embryonic development and repair of epithelial injury but it is also implicated in carcinogenesis [91]. IL-1b and IL-18 utilizes Myd88 as an adaptor protein that stimulate the ILR1 and IL-18R signaling pathway that required for NF-kB and MAPK activation. The Toll/IL-1R domain of MyD88 interacts with the TIR domains of IL-1R1 and also with IRAK1 and IRAK2. The role of Myd88 signaling in the regulation of inflammation during cancer progression of the intestine [92] has been documented. The MyD88 signaling pathway control inflammation in tissue repair [93,94] but upon injury the MyD88 signaling pathway is enforced. It has been suggested that tumor development is a continuous and unregulated state of tissue repair and stem cell renewal [95] secondary to abnormal homeostasis. An interesting question is whether the process derives from tissue stem cells or from cancer stem cells. Cancer stem cells might be derived from de-differentiated of progenitors that undergo genetic or epigenetic changes. Experiments in Drosophila testis have shown that germinal stem cells are highly dependent of the Jak-STAT signaling pathway [96]. Wingless [Wnt] signaling pathways, important in tissue regeneration and cancer growth [95], target claudin1 gene [97]. Wnt signaling and Cdx transcriptional activation regulate claudin-2 promoter activity [98]. Similarly, it has been shown that H pylori CagA+ strains increase intestinal cell proliferation by Wnt pathway activation [99] and inflammation stimulates epithelial cells to promote Wnt signaling activity [100]. The cancer stem cells possess features of the so-called EMT cells and through Snail-1 transcription factor, an inducer of EMT cells, these cells have highly activated NF-kB/MAPK signaling pathways in the presence of IL-1b and IL-8 [101] and have enhanced Wnt activity.
A serious concern is how does the inflammatory environment influences these signaling pathways and transcription factors related to modifications in claudin expression. In the majority of H. pylori infected individuals an asymptomatic chronic gastritis develops, the symptomatic disease occurs in approximately 10% of infected individuals. This difference may reside in H. pylory strain-specific virulence factors, genetic predisposition and/or a higher inflammatory response. Nevertheless, H. pylori and particularly CagA+ strains, is the only bacterium clearly associated with development of cancer [102]. The cytotoxin-associated gene A, activates ERK-MAPK pathway [103,104], induce the “hummingbird” phenotype [105], is associated with an increase in cell proliferation, EMT [106], inhibition of apoptotic signals [107], activation of the IL-6/gp130 receptor [108], activation of NF-kB and IL-8 [109,110], angiogenesis [111], regulation of stem cell differentiation [112], and disruption of the innate and Th1, Th17 and Treg balance [113].
Chronic H. pylori infection increases the epithelial expression of TLR-2, -4, -5 and 9 as well as IL-8, IL-10 and TNF-a, in gastric mucosa [114]. This chronic production of the inflammatory cytokines initiates a negative feedback of NF-kB, AP-1 and CREB-1 activation thus perpetuating the synthesis and secretion of the inflammatory cytokines. We have been evaluating the effect of H. pylori CagA+ and CagA- strains on human gastric adenocarcinoma [AGS] cells ability to secrete inflammatory cytokines. The results show that CagA+ strains are powerful inducers of IL-1b and IL-8 secretion whereas CagA- strains have a weak effect. Obviously, when various strains were evaluated there were no differences in the quantity of secreted cytokine but in the exposure time needed to exercise the inductor effect. We attribute the differences to the presence of other infectious toxins such as VacA in the H. pylori strains we evaluated. In co-activation experiments we also found that AGS cells grown in a cytokine milieu representative of a chronic inflammatory status secrete IL-8 in extremely high concentrations [<3,000 pg/ml]. As a consequence of these results we evaluated the effect of these pro-inflammatory cytokines upon claudin expression in AGS cells. The results showed that the expression of claudins-5, -6 and -7 was up-regulated by this cytokines but most interestingly, we found that the expression of claudin-4 was strongly upregulated when IL-8 concentrations above 2,500 pg/ml were used. It has been recently shown that in co-cultures of fibroblasts with malignant epithelial cells IL-8 is secreted to the culture medium and that this cytokine contributed to the maintenance of a low-differentiation phenotype of the epithelial cells [115]. A similar observation has been recently reported in the mammary gland epithelial cell line MCF10A [116].
Clinical Implications of Claudins, EMT and Inflammation
Claudin modification has been clearly established in several cancers including gastric cancer [117-119] and the loss of cell polarity, a function clearly regulated by tight junction proteins [120], is considered a hallmark of EMT. Interleukin 8 participation in oncogenesis has been clearly established [121], therefore in the frame of all these considerations it is clear that the sole prophylaxis of H. pylori infection with antibiotic therapy might not be sufficient to avoid gastric carcinogenesis [122] especially when pre-malignant lesions are already present. Serious efforts to develop an H. pylori vaccine are underway but information regarding possible host advantages in the host life is emerging [123,124].
Efforts to modulate the expression of claudin are underway. It has recently been shown that pegylated interferon and ribavirin modulate claudin and E-cadherin expression in HCV-infected cell lines [125], an infection strongly associated with cancer development. Similarly, Histone deacetylase inhibitors are being considered as promising anticancer drugs due to its effect on claudin-1 regulation [126]. Information regarding the inhibition of EMT in various cancers is emerging. Resveratrol, a compound found in grapes and red wine inhibits EMT in lung cancer [127] similarly to Celastrol, also known as tripterine, that also has inhibitory effects [128]. In the early stages of gastric neoplastic transformation the overexpression of gastrokine 1 might protect against MET [129]. The use of anti-HER2 drugs is promising but apparently only in HER2-positive gastric cancer [130].
The spectrum of possible therapeutic agents is promising, but our aim ought to be focused in determining cell markers that accurately point to the early changes associated with carcinogenesis. The presence of claudins associated with cell de-differentiation, or other markers associated to severe inflammatory processes or early EMT, such as claudins 4, 6, 7 or 9, CD44, Erb-2, E-cadherin or N-cadherin in the gastric biopsies should warn the clinician. We strongly believe that to routinely include the search of these markers will favor the early detection of cancer increasing survival rates and reducing medical costs.
Conclusive Paragraph
Gastric carcinogenesis is a multifactorial process. Genetic predisposition and risk factors have a triggering effect but currently one of the major associations is H. pylori CagA+ infection. The majority of well recognized gene associations are related to inflammation, matrix metalloproteinases, polymorphisms of TLR´s or miRNA´s but the complexity involved in gastric carcinogenesis is reflected in the heterogeneity in clinical, biologic and genetic aspects. Among the great variety of processes in the progression of a normal epithelium to gastric cancer it appears that inflammation and modification of tight junction proteins, provoked by bacteria, diet, tobacco, drugs and others, induce signaling pathways that promote epithelial-mesenchymal transition. This review highlights the relation that apparently unrelated phenomena such as claudin modification – inflammation – and EMT, has in gastric carcinogenesis, and emphasizes the role that claudins might have in the early detection of gastric cancer. Nevertheless, a better understanding of these phenomena is clearly needed.
Acknowledgements
The authors would like to recognize the financial support of PAPIIT-UNAMMéxico through grants IN-210813 and IN-211113, and CONACyT-México through grant CB-177678. Chavarria-Velazquez C.O. is a PhD student at UNAM´s Biomedical Sciences Postgraduate Program.
References
- Gartner LP, Hiatt JL (2001) Color Textbook of Histology.
- Günzel D, Yu AS (2013) Claudins and the modulation of tight junction permeability. Physiol Rev 93: 525-569.
- Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, et al. (2011) Predicted expansion of the claudin multigene family. FEBS Lett 585: 606-612.
- Günzel D, Fromm M (2012) Claudins and other tight junction proteins. Compr Physiol 2: 1819-1852.
- Morita K, Furuse M, Fujimoto K, Tsukita S (1999) Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 96: 511-516.
- Hewitt KJ, Agarwal R, Morin PJ (2006) The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer 6: 186.
- Arabzadeh A, Troy TC, Turksen K (2006) Role of the Cldn6 cytoplasmic tail domain in membrane targeting and epidermal differentiation in vivo. Mol Cell Biol 26: 5876-5887.
- Van Itallie CM, Colegio OR, Anderson JM (2004) The cytoplasmic tails of claudins can influence tight junction barrier properties through effects on protein stability. J Membr Biol 199: 29-38.
- Holland PW, Booth HA, Bruford EA (2007) Classification and nomenclature of all human homeobox genes. BMC Biol 5: 47.
- Simard A, Di Pietro E, Young CR, Plaza S, Ryan AK (2006) Alterations in heart looping induced by overexpression of the tight junction protein Claudin-1 are dependent on its C-terminal cytoplasmic tail. Mech Dev 123: 210-227.
- Rahner C, Mitic LL, Anderson JM (2001) Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 120: 411-422.
- Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM (2006) Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns 6: 581-588.
- Ozden O, Black BL, Ashwell CM, Tipsmark CK, Borski RJ, et al. (2010) Developmental profile of claudin-3, -5, and -16 proteins in the epithelium of chick intestine. Anat Rec (Hoboken) 293: 1175-1183.
- Abuazza G, Becker A, Williams SS, Chakravarty S, Truong HT, et al. (2006) Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am J Physiol Renal Physiol 291: F1132-1141.
- Hashizume A, Ueno T, Furuse M, Tsukita S, Nakanishi Y, et al. (2004) Expression patterns of claudin family of tight junction membrane proteins in developing mouse submandibular gland. Dev Dyn 231: 425-431.
- Turksen K, Troy TC (2001) Claudin-6: a novel tight junction molecule is developmentally regulated in mouse embryonic epithelium. Dev Dynamics 222: 292-300.
- Brinch M, Hatt L, Singh R, Møller K, Sommer S, et al. (2012) Identification of circulating fetal cell markers by microarray analysis. Prenat Diagn 32: 742-751.
- Türeci O, Koslowski M, Helftenbein G, Castle J, Rohde C, et al. (2011) Claudin-18 gene structure, regulation, and expression is evolutionary conserved in mammals. Gene 481: 83-92.
- Lameris AL, Huybers S, Kaukinen K, Mäkelä TH, Bindels RJ, et al. (2013) Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol 48: 58-69.
- Guarino M, Rubino B, Ballabio G (2007) The role of epithelial-mesenchymal transition in cancer pathology. Pathology 39: 305-318.
- Radisky DC, LaBarge MA (2008) Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell 2: 511-512.
- Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, et al. (2008) Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 3: e2888.
- Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420-1428.
- Findley MK, Koval M (2009) Regulation and roles for claudin-family tight junction proteins. IUBMB Life 61: 431-437.
- Turner JR, Angle JM, Black ED, Joyal JL, Sacks DB, et al. (1999) PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC kinase. Am J Physiol 277: C554-562.
- Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, et al. (2005) Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 85: 1139-1162.
- Schneeberger EE, Lynch RD (2004) The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286: C1213-1228.
- Matter K, Balda MS (2003) Signalling to and from tight junctions. Nat Rev Mol Cell Biol 4: 225-236.
- Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2: 442-454.
- Medici D, Hay ED, Olsen BR (2008) Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell 19: 4875-4887.
- Tse JC, Kalluri R (2007) Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem 101: 816-829.
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, et al. (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704-715.
- Stankovich BL, Aguayo E, Barragan F, Sharma A, Pallavicini MG (2011) Differential adhesion molecule expression during murine embryonic stem cell commitment to the hematopoietic and endothelial lineages. PLoS One 6: e23810.
- Tanaka M, Kamata R, Sakai R (2005) EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J Biol Chem 280: 42375-42382.
- Pasquale EB (2005) Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol 6: 462-475.
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S (2003) Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci 116: 1959-1967.
- Chiba H, Gotoh T, Kojima T, Satohisa S, Kikuchi K, et al. (2003) Hepatocyte nuclear factor (HNF)-4 triggers formation of functional tight junctions and establishement of polarized epithelial morphology in F9 embryonal carcinoma cells. Exp Cell Res 286: 288-297.
- Santangelo L, Marchetti A, Cicchini C, Conigliaro A, Conti B, et al. (2011) The stable repression of mesenchymal program is required for hepatocyte identity: a novel role for hepatocyte nuclear factor 4a. Hepatology 53: 2063-2074.
- Ben-David U, Nudel N, Benvenisty N (2013) Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat Commun 4: 1992.
- Ushiku T, Shinozaki-Ushiku A, Maeda D, Morita S, Fukayama M (2012) Distinct expression pattern of claudin-6, a primitive phenotypic tight junction molecule, in germ cell tumours and visceral carcinomas. Histopathology 61: 1043-1056.
- Miwa N, Furuse M, Tsukita S, Niikawa N, Nakamura Y, et al. (2001) Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res 12: 469-476.
- Sakaguchi T, Gu X, Golden HM, Suh E, Rhooads DB, et al. (2002) Cloning of the human claudin-1 5¨-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1alpha. J Biol Chem 277: 21361-21370.
- Katoh M (2005) Epithelial-mesenchymal transition in gastric cancer (Review). Int J Oncol 27: 1677-1683.
- Yamamoto T, Harada N, Kawano Y, Taya S, Kaibuchi K (1999) In vivo interaction of AF-6 with activated Ras and ZO-1. Biochem Biophys Res Commun 259: 103-107.
- Denker BM, Saha C, Khawaja S, Nigam SK (1996) Involvement of a heterotrimeric G protein alpha subunit in tight junction biogenesis. J Biol Chem 271: 25750-25753.
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, et al. (1998) Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 273: 12725-12731.
- Parisi F, Vidal M (2011) Epithelial delamination and migration: lessons from Drosophila. Cell Adh Migr 5: 366-372.
- Baud J, Varon C, Chabas S, Chambonnier L, Darfeuille F, et al. (2013) Helicobacter pylori initiates a mesenchymal transition through ZEB1 in gastric epithelial cells. PLoS One 8: e60315.
- Bessède E, Staedel C, Acuña Amador LA, Nguyen PH, Chambonnier L, et al. (2013) Helicobacter pylori generates cells with cancer stem cell properties via epithelial-mesenchymal transition-like changes. Oncogene.
- Severson EA, Kwon M, Hilgarth RS, Parkos CA, Nusrat A (2010) Glycogen Synthase Kinase 3 (GSK-3) influences epithelial barrier function by regulating occludin, claudin-1 and E-cadherin expression. Biochem Biophys Res Commun 397: 592-597.
- Gupta IR, Ryan AK (2010) Claudins: unlocking the code to tight junction function during embryogenesis and in disease. Clin Genet 77: 314-325.
- Kominsky SL, Vali M, Korz D, Gabig TG, Weitzman SA, et al. (2004) Clostridium perfringens enterotoxin elicits rapid and specific cytolysis of breast carcinoma cells mediated through tight junction proteins claudin 3 and 4. Am J Pathol 164: 1627-1633.
- Michl P, Barth C, Buchholz M, Lerch MM, Rolke M, et al. (2003) Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res 63: 6265-6271.
- Zavala-Zendejas VE, Torres-Martinez AC, Salas-Morales B, Fortoul TI, Montaño LF, et al. (2011) Claudin-6, 7, or 9 overexpression in the human gastric adenocarcinoma cell line AGS increases its invasiveness, migration, and proliferation rate. Cancer Invest 29: 1-11.
- Singh AB, Sharma A, Dhawan P (2010) Claudin family of proteins and cancer: an overview. J Oncol 2010: 541957.
- del Castillo G, Alvarez-Barrientos A, Carmona-Cuenca I, Fernández M, Sánchez A, et al. (2008) Isolation and characterization of a putative liver progenitor population after treatment of fetal rat hepatocytes with TGF-beta. J Cell Physiol 215: 846-855.
- Satohisa S, Chiba H, Osanai M, Ohno S, Kojima T, et al. (2005) Behavior of tight-junction, adherens-junction and cell polarity proteins during HNF-4alpha-induced epithelial polarization. Exp Cell Res 310: 66-78.
- Miyoshi J, Takai Y (2005) Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev 57: 815-855.
- Chiba H, Itoh T, Satohisa S, Sakai N, Noguchi H, et al. (2005) Activation of p21CIP1/WAF1 gene expression and inhibition of cell proliferation by overexpression of hepatocyte nuclear factor-4alpha. Exp Cell Res 302: 11-21.
- Calvisi DF, Frau M, Tomasi ML, Feo F, Pascale RM (2012) Deregulation of signalling pathways in prognostic subtypes of hepatocellular carcinoma: novel insights from interspecies comparison. Biochim Biophys Acta 1826: 215-237.
- Xu X, Jin H, Liu Y, Liu L, Wu Q, et al. (2012) The expression patterns and correlations of claudin-6, methy-CpG binding protein 2, DNA methyltransferase 1, histone deacetylase 1, acetyl-histone H3 and acetyl-histone H4 and their clinicopathological significance in breast invasive ductal carcinomas. Diagn Pathol 7: 33.
- Swisshelm K, Macek R, Kubbies M (2005) Role of claudins in tumorigenesis. Adv Drug Deliv Rev 57: 919-928.
- Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH (2007) The first extracellular domain of claudin-7 affects paracellular Cl- permeability. Biochem Biophys Res Commun 357: 87-91.
- Nübel T, Preobraschenski J, Tuncay H, Weiss T, Kuhn S, et al. (2009) Claudin-7 regulates EpCAM-mediated functions in tumor progression. Mol Cancer Res 7: 285-299.
- Kitajiri SI, Furuse M, Morita K, Saishin-Kiuchi Y, Kido H, et al. (2004) Expression patterns of claudins, tight junction adhesion molecules, in the inner ear. Hear Res 187: 25-34.
- Niimi T, Nagashima K, Ward JM, Minoo P, Zimonjic DB, et al. (2001) Claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol 21: 7380-7390.
- Jovov B, Van Itallie CM, Shaheen NJ, Carson JL, Gambling TM, et al. (2007) Claudin-18: a dominant tight junction protein in Barrett's esophagus and likely contributor to its acid resistance. Am J Physiol Gastrointest Liver Physiol 293: G1106-1113.
- Katoh M, Katoh M (2003) CLDN23 gene, frequently down-regulated in intestinal-type gastric cancer, is a novel member of CLAUDIN gene family. Int J Mol Med 11: 683-689.
- Brenner H, Rothenbacher D, Arndt V (2009) Epidemiology of stomach cancer. Methods Mol Biol 472: 467-477.
- Goldenring JR, Nam KT, Mills JC (2011) The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res 317: 2759-2764.
- Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, et al. (2007) The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134: 211-222.
- Hanby AM, Poulsom R, Playford RJ, Wright NA (1999) The mucous neck cell in the human gastric corpus: a distinctive, functional cell lineage. J Pathol 187: 331-337.
- El-Zimaity HMT, Ramchatesingh J, Saeed MA, Graham DY (2001) Gastric intestinal metaplasia: subtypes and natural history. J Clin Pathol 54: 679-683.
- Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, et al. (2000) Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 118: 36-47.
- Fox JG, Li X, Cahill RJ, Andrutis K, Rustgi AK, et al. (1996) Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology 110: 155-166.
- Hoffmann W (2012) Stem cells, self-renewal and cancer of the gastric epithelium. Curr Med Chem 19: 5975-5983.
- Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, et al. (1999) Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest 79: 639-646.
- Lennerz JK, Kim SH, Oates EL, Huh WJ, Doherty JM, et al. (2010) The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol 177: 1514-1533.
- Resnick MB, Gavilanez M, Newton E, Konkin T, Bhattacharya B, et al. (2005) Claudin expression in gastric adenocarcinomas: a tissue microarray study with prognostic correlation. Hum Pathol 36: 886-892.
- Kimura Y, Shiozaki H, Hirao M, Maeno Y, Doki Y, et al. (1997) Expression of occludin, tight-junction-associated protein, in human digestive tract. Am J Pathol 151: 45-54.
- Katoh M, Katoh M (2003) MGC29506 gene, frequently down-regulated in intestinal-type gastric cancer, encodes secreted-type protein with conserved cysteine residues. Int J Oncol 23: 235-241.
- Soini Y, Tommola S, Helin H, Martikainen P (2006) Claudins 1, 3, 4 and 5 in gastric carcinoma, loss of claudin expression associates with the diffuse subtype. Virchows Arch 448: 52-58.
- Rendón-Huerta E, Teresa F, Teresa GM, Xochitl GS, Georgina AF, et al. (2010) Distribution and expression pattern of claudins 6, 7, and 9 in diffuse- and intestinal-type gastric adenocarcinomas. J Gastrointest Cancer 41: 52-59.
- Park JY, Park KH, Oh TY, Hong SP, Jeon TJ, et al. (2007) Up-regulated claudin 7 expression in intestinal-type gastric carcinoma. Oncol Rep 18: 377-382.
- Chen GY, Núñez G (2011) Inflammasomes in intestinal inflammation and cancer. Gastroenterology 141: 1986-1999.
- Zitvogel L, Kepp O, Galluzzi L, Kroemer G (2012) Inflammasomes in carcinogenesis and anticancer immune responses. Nat Immunol 13: 343-351.
- Di Virgilio F (2013) The therapeutic potential of modifying inflammasomes and NOD-like receptors. Pharmacol Rev 65: 872-905.
- Ferrone C, Dranoff G (2010) Dual roles for immunity in gastrointestinal cancers. J Clin Oncol 28: 4045-4051.
- Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, et al. (2008) Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14: 408-419.
- Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J (2012) IL-1ß promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer 11: 87.
- Yang J, Weinberg RA (2008) Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14: 818-829.
- Rakoff-Nahoum S, Medzhitov R (2007) Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 317: 124-127.
- Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS (2005) Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A 102: 99-104.
- Jiang D, Liang J, Fan J, Yu S, Chen S, et al. (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173-1179.
- Beachy PA, Karhadkar SS, Berman DM (2004) Tissue repair and stem cell renewal in carcinogenesis. Nature 432: 324-331.
- Brawley C, Matunis E (2004) Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 304: 1331-1334.
- Taipale J, Beachy PA (2001) The Hedgehog and Wnt signaling pathways in cancer. Nature 411: 349-354.
- Mankertz J, Hillenbrand B, Tavalali S, Huber O, Fromm M, et al. (2004) Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem Biophys Res Commun 314: 1001-1007.
- Neal JT, Peterson TS, Kent ML, Guillemin K (2013) H. pylori virulence factor CagA increases intestinal cell proliferation by Wnt pathway activation in a transgenic zebrafish model. Dis Model Mech 6: 802-810.
- Oguma K, Oshima H, Oshima M (2010) Inflammation, tumor necrosis factor and Wnt promotion in gastric cancer development. Future Oncol 6: 515-526.
- Lim S, Becker A, Zimmer A, Lu J, Buettner R, et al. (2013) SNAI1-mediated epithelial-mesenchymal transition confers chemoresistance and cellular plasticity by regulating genes involved in cell death and stem cell maintenance. PLoS One 8: e66558.
- Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, et al. (2013) Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol 10: 643-655.
- Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, et al. (2002) SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295: 683-686.
- Hatakeyama M (2004) Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer 4: 688-694.
- Segal ED, Cha J, Lo J, Falkow S, Tompkins LS (1999) Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci U S A 96: 14559-14564.
- Bagnoli F, Buti L, Tompkins L, Covacci A, Amieva MR (2005) Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc Natl Acad Sci U S A 102: 16339-16344.
- Buti L, Spooner E, Van der Veen AG, Rappuoli R, Covacci A, et al. (2011) Helicobacter pylori cytotoxin-associated gene A (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proc Natl Acad Sci U S A 108: 9238-9243.
- Lee IO, Kim JH, Choi YJ, Pillinger MH, Kim SY, et al. (2010) Helicobacter pylori CagA phosphorylation status determines the gp130-activated SHP2/ERK and JAK/STAT signal transduction pathways in gastric epithelial cells. J Biol Chem 285: 16042-16050.
- Sharma SA, Tummuru MK, Blaser MJ, Kerr LD (1998) Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J Immunol 160: 2401-2407.
- Sasaki A, Kitadai Y, Ito M, Sumii M, Tanaka S, et al. (2003) Helicobacter pylori infection influences tumor growth of human gastric carcinomas. Scand J Gastroenterol 38: 153-158.
- Kitadai Y, Sasaki A, Ito M, Tanaka S, Oue N, et al. (2003) Helicobacter pylori infection influences expression of genes related to angiogenesis and invasion in human gastric carcinoma cells. Biochem Biophys Res Commun 311: 809-814.
- Ding SZ, Zheng PY (2012) Helicobacter pylori infection induced gastric cancer; advance in gastric stem cell research and the remaining challenges. Gut Pathog 4: 18.
- Müller A, Solnick JV (2011) Inflammation, immunity, and vaccine development for Helicobacter pylori. Helicobacter 16 Suppl 1: 26-32.
- Lagunes-Servin H, Torres J, Maldonado-Bernal C, Pérez-Rodríguez M, Huerta-Yépez S, et al. (2013) Toll-Like Receptors and Cytokines are Upregulated during Helicobacter pylori Infection in Children. Helicobacter 18: 423-432.
- Kolář M, Szabo P, Dvořánková B, Lacina L, Gabius HJ, et al. (2012) Upregulation of IL-6, IL-8 and CXCL-1 production in dermal fibroblasts by normal/malignant epithelial cells in vitro: Immunohistochemical and transcriptomic analyses. Biol Cell 104: 738-751.
- Aceto N, Duss S, Macdonald G, Meyer DS, Roloff TC, et al. (2012) Co-expression of HER2 and HER3 receptor tyrosine kinases enhances invasion of breast cells via stimulation of interleukin-8 autocrine secretion. Breast Cancer Res 14: R131.
- Oshima T, Shan J, Okugawa T, Chen X, Hori K, et al. (2013) Down-regulation of claudin-18 is associated with the proliferative and invasive potential of gastric cancer at the invasive front. PLoS One 8: e74757.
- Kwon MJ (2013) Emerging roles of claudins in human cancer. Int J Mol Sci 14: 18148-18180.
- Li X, Li Y, Qiu H, Wang Y (2013) Downregulation of claudin-7 potentiates cellular proliferation and invasion in endometrial cancer. Oncol Lett 6: 101-105.
- Farkas AE, Capaldo CT, Nusrat A (2012) Regulation of epithelial proliferation by tight junction proteins. Ann N Y Acad Sci 1258: 115-124.
- Waugh DJ, Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14: 6735-6741.
- de Vries AC, Kuipers EJ, Rauws EA (2009) Helicobacter pylori eradication and gastric cancer: when is the horse out of the barn? Am J Gastroenterol 104: 1342-1345.
- Stein M, Ruggiero P, Rappuoli R, Bagnoli F (2013) Helicobacter pylori CagA: From Pathogenic Mechanisms to Its Use as an Anti-Cancer Vaccine. Front Immunol 4: 328.
- Arnold IC, Hitzler I, Muller A (2012) The immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Front Cell Infect Microbiol 2: 10.
- Rendón-Huerta EP, Torres-Martínez A, Charles-Niño C, Rivas-Estilla AM, Paez A, et al. (2013) Pegylated interferon-a2b and ribavirin decrease claudin-1 and E-cadherin expression in HepG2 and Huh-7.5 cells. Ann Hepatol 12: 616-625.
- Krishnan M, Singh AB, Smith JJ, Sharma A, Chen X, et al. (2010) HDAC inhibitors regulate claudin-1 expression in colon cancer cells through modulation of mRNA stability. Oncogene 29: 305-312.
- Wang H, Zhang H, Tang L, Chen H, Wu C, et al. (2013) Resveratrol inhibits TGF-β1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology 303: 139-146.
- Kang H, Lee M, Jang SW (2013) Celastrol inhibits TGF-ß1-induced epithelial-mesenchymal transition by inhibiting Snail and regulating E-cadherin expression. Biochem Biophys Res Commun 437: 550-556.
- Rippa E, La Monica G, Allocca R, Romano MF, De Palma M, et al. (2011) Overexpression of gastrokine 1 in gastric cancer cells induces Fas-mediated apoptosis. J Cell Physiol 226: 2571-2578.
- Bang YJ (2012) Advances in the management of HER2-positive advanced gastric and gastroesophageal junction cancer. J Clin Gastroenterol 46: 637-648.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15435
- [From(publication date):
November-2013 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10791
- PDF downloads : 4644