Research Article Open Access
Characterization, Properties and Applications of Nonthermal Plasma: A Novel Pulsed-Based Option
James R Ferrell1, Aleksandr S Galov2, Valery A Gostev2, Bruce A Banks3, Steven P Weeks4, Judith A Fulton5 and Christopher J Woolverton6*1Department of Biological Sciences, Kent State University, Kent, OH, 44242, USA
2Department of Physical Engineering, Petrozavodsk State University, Petrozavodsk, Russia
3BAB Technology, LLC, Olmsted Township, OH, 44135, USA
4Sterionics Inc., Cleveland, OH, 44114, USA
5Wound Healing and Limb Preservation Center, Akron General Medical Center, Akron, OH, 44307, USA
6College of Public Health, Kent State University, Kent, OH, 44242, USA
- Corresponding Author:
- Christopher J Woolverton
College of Public Health, Kent State University, Kent
OH, 44242, USA
Tel: (330) 672-4648
Fax: (330) 672-6505
E-mail: cwoolver@kent.edu
Received date: February 28, 2013; Accepted date: April 12, 2013; Published date: April 17, 2013
Citation: Ferrell JR, Galov AS, Gostev VA, Banks BA, Weeks SP, et al. (2013) Characterization, Properties and Applications of Nonthermal Plasma: A Novel Pulsed-Based Option. J Biotechnol Biomater 3:155. doi:10.4172/2155-952X.1000155
Copyright: © 2013 Ferrell JR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The glow discharge from a novel spark-based nonthermal plasma generator is described and characterized using spectrophotometry, radiometry and gas detectors. Spectral information identified ultraviolet radiation within the plasma discharge. High levels of reactive oxygen and nitrogenous species were also detected within the analyzed plasma. The plasma discharge is found to have a significant germicidal effect on Gram-positive bacteria, independent of temperature increase. The ultraviolet radiation and ionized gaseous species are found to contribute to this observed effect, synergistically. Plasma filtration reduced germicidal survivability significantly. The liquid environment in which bacterial cells were exposed to the nonthermal plasma also was important, when determining the efficacy of nonthermal plasma, as a germicidal agent. Staphylococcus aureus cultures exposed to nonthermal plasma for two minutes within low nutrient sources, such as water or phosphate-buffered saline, had very low survival. Cultures exposed to the same plasma interval in nutrient rich liquids tolerated the plasma exposure better, and survived at higher numbers. This plasma discharge is highly bactericidal, due to the high concentrations of known antimicrobial agents, but its effectiveness is subject to a synergistic effect, that depends upon the surrounding environment.
Keywords
Characterization; Composition; Nonthermal plasma; Sterilization
Introduction
Plasma science has been of particular interest to researchers in several disciplines, since the 1990s. This continuously evolving field has spawned investigations into diverse applications, including industrial sterilization, pollution control, polymer science, food safety and biomedicine [1-7]. Additional considerations of plasma science in therapeutic medicine may develop, as more basic science evaluates the impact of plasma on the human host. For example, consideration must be given to the effects of plasma on whole tissue, damaged tissue, including its micro-environment (devitalized tissue, debris, foreign and host bacteria), individual cell types (keratinocytes, fibroblasts, melanocytes, blood, etc.), as well as inter- and intra-cellular biochemical reactions. Data demonstrating positive effects on human cells, such as cytolysis of cancer cells, enhanced wound healing, angiogenesis, etc., in the absence of toxic effects, would certainly promote additional applications [8-12]. Here, we review the present understanding of plasma science, including a newer periodic discharge nonthermal plasma device.
Plasma is generated, when sufficient energy is supplied to a neutral gas, to cause individual gas molecules to become charged. Once an electric field is generated and subsequently maintained, in order to permit the initial electrification of an ambient gas, ionization of particles occurs. The electric field permits acceleration of electrons within the gaseous environment, due to the small masses of gas molecules [13]. Once initial electrification of the gas molecules occurs, the excitatory action of charged electrons and ions intensifies (Townsend mechanism), and results in what is called an “electron avalanche” [14,15]. The very nature of the localized environment can also vary, depending on the type of plasma that is needed. Plasmas can be created from most gaseous mixtures, including noble gases, ambient air or aqueous vapors [16]. The starting medium that is used to generate the plasma can convey different properties to the plasma. For example, using water vapor can dramatically increase the amount of hydroxides that make up the plasma’s composition [17]. “Customization” of a specific sub-type of plasma, in order to perform a specific task, is something that merits additional investigation. The specific plasma generator studied here is capable of using a wide variety of starting materials; therefore, the potential for the creation of a range of plasma subtypes is vast. Several recent studies have referenced the use of water vapor in the generation of nonthermal plasma. These studies have identified discharge spectra profiles, composed of elevated hydroxyl radicals and hydrogen peroxide [18,19]. This current paper does not discuss the use of humidified air or water vapor as a source of plasma starting material. Inclusion of water vapor as a source of starting plasma medium in additional plasmabased experimentation, deserves consideration. Expansion of available gaseous (or vapor-based) media, for use in the creation of nonthermal plasma discharges, would permit the development of a wide-ranged, customizable plasma, capable for use in a variety of applications.
Plasma generators that utilize pulsed periodic discharge, produce an uncontrolled random discharge, sometimes referred to as a “spark” discharge [20,21]. The generated electric field occurs between the electrode in the needle and the electrode in the head nozzle, creating a discharge gap. As the spark occurs, the plasma materials are expelled out through the gap. There is no direct electric field outside of the discharge gap, making it safe to use on biological specimens. Plasma generated using this type of device is lower in temperature and larger in overall volume, compared to plasma discharges from other generators, such as Dielectric Barrier Discharge (DBD), or plasma jets. This current investigation evaluates a variant of the pulse-producing periodic discharge device (Gostev Plasma Pulse or GPP), capable of controlled nonthermal plasma discharges.
The specificity of plasmas are dependent upon gas compositions, pressure, air flow, frequency of the applied voltage, construction of the cathode/anode configuration, and sustainment of the electric field [1,22]. Nonthermal plasmas can be produced by various discharge methods, including dielectric barrier discharge, plasma jet and plasma spark discharge [7,13,23,24]. In general, nonthermal plasmas possess very low temperatures compared to thermal plasmas. This occurs because the majority of the electrical energy supplied to create plasma from starting gases, primarily excites the electrons within the starting medium, instead of heating the surrounding gas. This implies that the electron temperatures are not in thermal equilibrium with the surrounding gas itself. The elevated temperature and excitatory nature of the charged electrons and plasma molecules are responsible for initializing the chain reactions associated with plasma generation. For many types of discharges, the gas kinetic temperature is room temperature. Low gas temperature is also achieved by limiting the time, the gas spends in the electrification process [13].
Given the variety of methods used to create nonthermal plasma, determination of the physical parameters and composition, the generated plasma discharge is essential. Several nonthermal plasma generators display higher levels of hydroxyl radicals, compared with non-water vapor plasma discharges, when supplied with water vapor [18,19,25]. Alteration of the amount of oxygen in the starting plasma, gaseous medium has been shown to affect the amount of nitrogen oxide during association with nitrogen. Zhao et al. [26] described a critical oxygen concentration needed to produce nitrogen dioxide gas, instead of nitric oxide. Other investigations have used specific ratios of nitrogen and oxygen mixtures in their starting gaseous environment, which led to the intentional production of nitric oxide [27]. This concept was expanded upon leading to the proposal of a nitric oxide generator for therapeutic purposes [28]. Characterization of plasma constituents is critical, if mechanisms for observed results are to be postulated with accuracy. Some plasma generators rely on noble gases such as argon or xenon, whereas others function on ambient air, made up primarily of nitrogen and oxygen. The composition of plasmas generated with different starting mediums can be radically different due to electron amplification ,and subsequent creation of downstream molecules and free radicals [16].
The localized micro-environments exposed to nonthermal plasma discharge also must be taken under consideration. Nonthermal plasma has been successfully utilized to kill a variety of microorganisms for many years. This observed germicidal effect is not linked to only one type of plasma generator subtype [3,4,29-32]. However, there are several important aspects, when taking the germicidal efficacy of nonthermal plasma with respect to the localized environments. Different nutrient media used to culture microorganisms contain a variety of macromolecules, such as carbohydrates and sugars, depending on their formulation. Their inclusion may limit the germicidal capabilities due to macromolecular shielding of targeted microorganisms [33]. Likewise, the pH [34], and temperature [16], are susceptible to alteration after nonthermal plasma discharge, and emission due to the activity of reactive secondary messenger molecules and ions [35]. In this investigation, macromolecule concentrations and alterations of pH and temperatures in aqueous bacterial micro-environments were evaluated concurrently with microbial survivability, after nonthermal plasma discharge. Collectively, this information provides a larger understanding of the physical properties and capabilities of the studied plasma generator.
Methods
Physical description of the Plasma Generator (GPP)
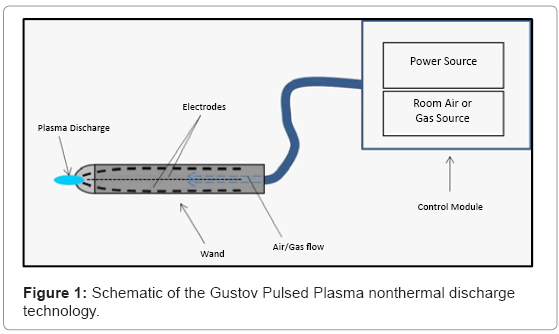
The GPP generator is composed of three main components: a control module, plasma wand and plasma nozzle. The gaseous medium is introduced into the plasma generator through an air flow inlet (~ 1/8 of an inch in diameter). Electrical voltage is supplied to generate an electrical field that resonates throughout the device. The gas is then passed to a connecting wand (Figure 1), composed of additional tubing, and it is transported downstream to the plasma nozzle, which contains two electrodes. The voltage generated by the creation of an electric field upstream in the plasma control module creates a discharge between the electrodes, which subsequently ionizes the gas in this localized environment between the discharge gap. As discharge events continue and amplify inside of the discharge gap, the generated plasma is expelled through the orifice of the nozzle.
Plasma spectra characteristics
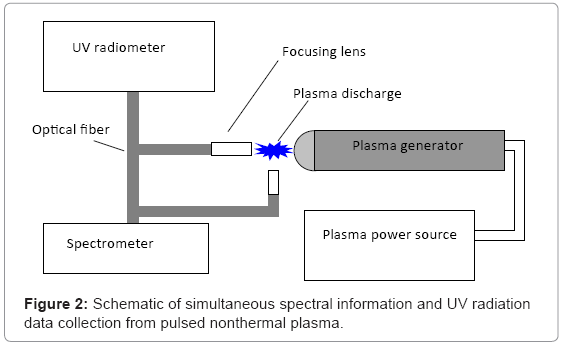
To determine the makeup of ultraviolet (UV) radiation found in the plasma discharge, a TKA-PKM UV-radiometer (TKA, St. Petersburg, Russia) was utilized (Figure 2). The sensor was fixed 1 cm crosswise and lengthwise direction, with respect to the discharge corona. Experimental data were collected continuously over 1 minute, at a frequency discharge of 0.25 Hz. An AvaSpec-2048FT spectrophotometer (Avantes, Broomfield, CO) was used to measure spectral information from the nonthermal plasma discharge arc (Figure 3). The plasma discharge frequency was set to 0.25 Hz, and the spectrophotometer was placed 1 cm from the corona of the plasma discharge. An optical resolution of 0.3 nm was selected during subsequent and continuous analysis.
Plasma composition measurements
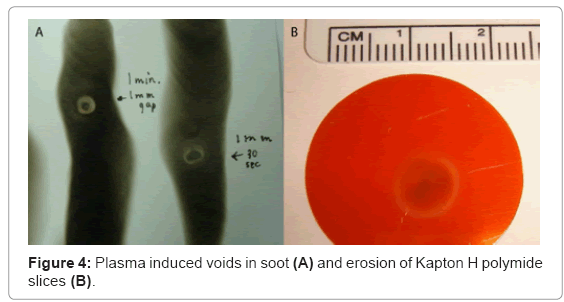
Kapton H polyimide slices (5 mm diameter, 0.127 mm thickness; Dupont, Fayetteville, NC) were used to measure atomic oxygen flux within the plasma. The plasma discharge was fixed at a constant distance of 5 mm above the surface of the polyimide slices, and fired continuously at exposure times that ranged from 15 seconds to 2 minutes. Images and measurements of induced voids were collected for mass analysis.
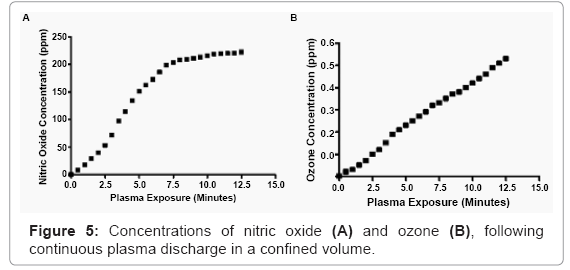
Non-purified, room temperature air was introduced into the control module of the plasma generator. After electrification, the ionized molecules were discharged through the tip of the plasma wand into a sealed container, with the exception of a outflow orfice that went to a bubbler in water, to prevent any backwards transport of room air. A nitric oxide detector (RAE Systems, San Jose, CA) was placed in close proximity (2 mm) to the pulsed plasma. In order to reduce any electrical interference, a grounded copper Faraday cage was constructed, and surrounded the monitor. Results were collected over ten minutes of continuous plasma discharge.
Ozone within the plasma discharge was measured by confining the plasma in a sealed chamber, with the exception of a outflow orfice that went to a bubbler in water, to prevent any backwards transport of room air. An ozone detector (EcoSensors, Newark, CA) was used to obtain readings over time, by positioning the sensor, approximately 5 inches from the plasma nozzle fixed inside the sealed chamber.
To determine if the initiation voltage of the plasma was sufficient to produce soft X-rays, dental film (Kodak, Rochester, NY) covered with a perforated molybdenum sheet, was used to develop X-rays through contrasting images. The film was exposed to the plasma, for durations ranging from 2 minutes to 10 minutes, at a fixed distance of 2 mm from the surface of the film.
pH measurement
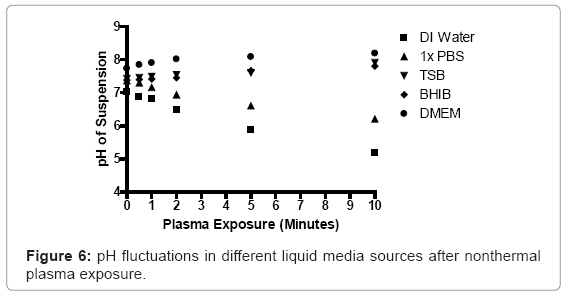
An Accumet model pH meter (Fisher Scientific, Pittsburgh, PA), equipped with a Symphony probe (VWR, Radnor, PA), was used to measure changes in pH in prepared bacterial suspensions, following plasma discharge. Prepared S. aureus suspensions were transferred to Costar 24-well plates (0.8 cm depth and 1.75 cm diameter; Corning Inc., Corning, NY), at a total depth of 5 mm. The tip of the plasma discharge was fixed 3 mm above the surface of the bacterial suspension. The plasma generator was operated for up to ten minutes for each sample. Each sample was analyzed in real time using the pH probe, during and after nonthermal plasma discharge.
Temperature measurement
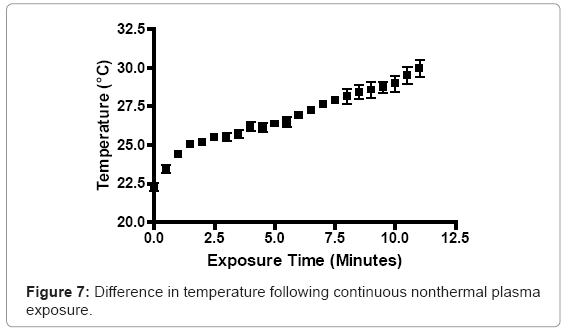
A RMS multimeter (Fluke, Everett, WA) was used to measure temperature increases in prepared bacterial suspensions in real time, during plasma discharge. Prepared S. aureus suspensions were transferred to Costar 24-well plates (0.8 cm depth and 1.75 cm diameter; Corning Inc., Corning, NY), at a total depth of 5 mm. The probe adjusted to room temperature (23°C), prior to experimentation. The tip of the plasma discharge was fixed 3 mm above the surface of the bacterial suspension. The plasma generator was operated for 10 minutes continuously, with temperature values recorded at 30 second intervals.
Nutrient media variation (Macromolecular concentrations)
Overnight S. aureus cultures were obtained and re-suspended in: sterile distilled water, 1 M phosphate buffered saline (PBS; pH 7.4), tryptic soy broth (TSB), Dulbecco’s Modified Eagle Medium (DMEM; pH 7.4; Invitrogen, Carlsbad, CA), enriched with 5% fetal bovine serum (Cleveland Clinic, Cleveland, OH), or Brain Heart Infusion Broth (BHIB), to provide a starting bacterial concentration of 3.0×107 CFU/ mL. Each culture was exposed to nonthermal plasma, for lengths of time ranging from 0.5 minutes to 10 minutes, at a fixed distance of 3 mm above the surface of the liquid. All cultures were then plated on nutrient agar plates and incubated overnight at 37°C. Plate counts were collected the following day, enabling survivability calculations based on cell culture densities.
Bacterial cultures
The following bacterial strains were used in these studies: S. aureus ATCC 12598, P. aeruginosa ATCC 29260, Klebsiella pneumoniae ATCC 8045, Escherichia coli ATCC 25297, B. subtilis ATCC 465, and B. atrophaeus ATCC 6455. One hundred microliters of freezer stock culture for each bacterium was diluted with 14.9 mL of Brain Heart Infusion Broth (6 g Brain, Heart infusion from solids, 6 g Peptic digest of Animal Tissue, 5 g Sodium Chloride, 3 g Dextrose, 14.5 g Pancreatic digest of Gelatin, and 2.5 g Disodium Phosphate per liter of deionized water, pH 7.4), obtained from BD Biosciences, Sparks, MD. Following overnight incubation at 37°C, 1 mL aliquots for each bacterial species were taken from each suspension, placed into UVtransparent quartz cuvettes, and measured using a Genesys 10 uV Thermospectronic spectrophotometer (Thermo Scientific, Pittsburgh, PA), at an optical density of 600 nm (OD600). The reading was in tandem with an established (average of five experiments) standard curve for the microorganism of interest, which provided a starting concentration for each organism. For most experiments, the starting concentration of each respective bacterium was 3.0×106 CFU/mL, unless otherwise noted.
Depth experiments
Bacterial suspensions were prepared and then added into fresh, sterile Tryptic Soy Agar (15 g Pancreatic Digest of Casein, 5 g Digest of Soybean, 5 g Sodium Chloride, and 15 g Agar per liter of deionized water, pH 7.4, 56°C), obtained from BD Biosciences, Franklin Lakes, NJ, USA. The ratio of inoculated bacterial culture to agar per mL was 1:50, unless otherwise noted. Prepared, inoculated agar was mixed thoroughly and poured into plates in 20 mL aliquots. This provided a depth of agar of 0.5 cm. Other amounts of the inoculated agar were poured as indicated, to yield different depths. The nonthermal plasma device was fixed 3 mm from the surface of each plate. Treatment with the cold plasma was marked on the bottom of each plate, indicating an individual exposure focus. Exposure doses ranged from 15 seconds to 3 minutes. Following exposure, all cultures were incubated for approximately 24 hours at 37°C, and then photographed from the top and bottom of each plate, using a BioMic plate reader (Giles Scientific, Santa Barbara, CA). A sterile scalpel was used to core around the perimeter of the treatment zones in the agar. These pieces were placed on a transparent glass surface, and then analyzed for turbidity or cloudiness through the agar cores.
Diameter experiments
Overnight bacterial cultures (prepared as described above) were spread across the entire surface of TSA plates in 150 uL aliquots. Individual exposure zones were marked on the bottom of the plates, to indicate where portions of the plate had been exposed to nonthermal plasma. Following exposure, the plates were placed in an incubator for 24 hours at 37°C. The plates were then removed and imaged. Following image acquisition, zones of inhibition were observed and then measured.
Colony counts and viability
Sterile, deionized water was used to dilute bacterial stock cultures to experimental starting concentrations. One mL aliquots of a prepared bacterial suspension were placed in the wells of 24-well plastic plate,s with depths and a diameter of 0.8 cm and 1.75 cm, respectively (Corning Inc., Corning, NY, USA). The probe of the nonthermal plasma device was fixed 3 mm from the direct surface of the air-liquid interface. Exposure ranged from 15 seconds to 5 minutes. After plasma exposure, each individual sample well was then diluted sequentially at concentrations ranging from 10-4 to 10-8 of exposed cultures. One hundred uL aliquots from each dilution group was then plated evenly across freshly prepared TSA plates. All plates incubated for 24 hours at 37°C. Original cell density (OCD) values were calculated for each sample using counted
Magnesium fluoride windows with a 0.2 mm thickness (Edmund Optics, Barrington, NJ, USA), were selected to transmit UV radiation, and simultaneously block reactive oxygen and nitrogenous species. MgFl2 windows were placed on top of Costar 24-well plates (0.8 cm depth and 1.75 cm diameter; Corning Inc., Corning, NY, USA). The plasma discharge probe was fixed 2 mm above the window, resulting in a total exposure gap of 5 mm from the surface of the liquid. Bacterial suspensions were then exposed to the nonthermal plasma at l intervals that ranged from 30 seconds to 4 minutes. Following exposure, resulting cultures were serially diluted and plated on nutrient agar. Bacterial colonies were counted after 18 hour incubations at 37°C.
Ultraviolet radiation filtration
Nonthermal plasma filtration was repeated using sterile, “Breathe Easy film,” a 1 mm-thick non-toxic, polyurethane material (USA Scientific, Ocala, FL), painted black, to absorb UV-radiation from the plasma discharge. The material was designed to remain porous, allowing oxygen and its reactive derivatives penetration through to cultures. S. aureus cultures were sealed with 24-well Costar plates, using the film material. The plasma discharge probe was fixed 3 mm above the surface, yielding a total discharge gap of 5 mm. Bacterial suspensions were then exposed to the nonthermal plasma, at intervals that ranged from 30 seconds to 4 minutes. Following exposure, resulting cultures were serially diluted and plated on nutrient agar. Bacterial colonies were counted after 18 hour incubations at 37°C. Viability was determined using original cell density calculations of remaining survivors that grew on collected plates.
Results
The physical dimensions and construction of the device are described as the following. The air flow rate through the nozzle was measured to be 10.6 cm3 per sec. The input voltage required to generate the plasma was 220 volts, which corresponded to a plasma pulse rate of approximately 4 pulses per second. The diameter of the plasma discharge ranged from 3 to 5 mm from the central exposure point, after continuous discharge ranged from 1 minute to 5 minutes. The plasma discharge extended over 1 cm out from the orifice of the nozzle, as determined visually, and confirmed utilizing photography and spectroscopy. The interaction between the positive nickel-chromium alloy center electrode and negative outer electrode formed a warm cathode spot on the brass outer electrode, at continually varying places. These spots produce vaporized atoms and colloidal electrode particles that are deposited on surfaces downstream of the plasma discharge arc. Manipulation or optimization of the electrode materials could be a beneficial niche to exploit further.
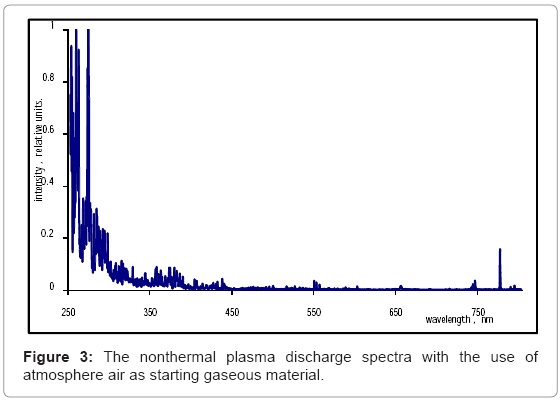
When air is used as generation matter, the discharge spectra are characterized by intense lines of oxygen and associated ionized derivatives. A peak at 257 nm corresponded to O+ ions, and had a relative concentration of 0.38 units. Another peak at 281 nm corresponded to a high concentration of OH-, with a relative concentration of 0.65 units. The last significant peak below 300 nm corresponded to O2+, and displayed a relative concentration of 0.77 units. The system of bands in the spectral range from 310 to 437 nm can be assigned to molecular oxygen. Low intensity levels of ozone were detected in the 310-340 nm spectral range. The light emission spectroscopy data displayed lines around 460 and 700 nm, and can be identified as ionized copperderived electrode materials (Figure 3).
The relative power densities of the three types of UV radiation were also determined using air as the plasma generation matter. Power densities of UV-A (long wave) and UV-B (medium wave), each equaled 180 mW/cm2, during the 10 minute continuous discharge, while UV-C equaled 330 mW/cm2 for the same time interval. The power densities associated with the UV in this plasma generator contained almost double the amount of UV-C, compared to UV-A and UV-B. The American Conference of Governmental Industrial Hygienists recommends a maximum energy deposition of 1 J/cm2 per 1000 seconds of exposure (approximately 16.5 minutes). The values associated here would safely fall under this recommendation, even though there is much more UV-C in the emission profile, compared to lesser energy ultraviolet radiation sub-types.
Oxygen-mediated mass reduction in Kapton H polyimide slices was used to measure atomic oxygen content of the plasma discharge [36]. Erosion rings measured approximately 8 mm in diameter, after 10 minutes of continuous plasma exposure. In addition, the mass lost corresponded to an atomic flux of at least 1.15×1017 atoms per cm2 per second (Figure 4).
The level of detected nitric oxide (NO) was 18 parts per million (ppm), after one minute of continuous plasma discharge. Doubling the exposure time to two minutes accumulated 40 ppm. Four minutes The level of detected nitric oxide (NO) was 18 parts per million (ppm), after one minute of continuous plasma discharge. Doubling the exposure time to two minutes accumulated 40 ppm. Four minutes
The pH of low nutrient aqueous media, such as deionized water and 1X phosphate buffered saline (PBS) decreased, whereas higher nutrient media sources increased slightly. The pH of deionized water decreased from 7.04 to 6.83, after one minute of plasma exposure, and decreased further to values of 6.489, 5.88, and 5.18 at exposure intervals of two, five, and ten minutes respectively. The pH of brain heart infusion broth and Dulbecco’s Modified Eagle Medium rose from 7.38 and 7.73 to 7.43 and 7.9, following one minute of exposure. The pH of these liquids increased further to 7.81 and 8.19, after ten minutes of continuous nonthermal plasma exposure, respectively (Figure 6).
A localized area exposed to one minute of continuous nonthermal plasma discharge increased in temperature by 1.7°C. Two minutes increased the temperature of the localized area by 2.9°C. At subsequent intervals of four, six and eight minutes, the temperature rose 3.9°C, 4.6°C, and 5.83°C, respectively. Ten minutes of continuous plasma generation increased the local environment’s temperature from 23°C to approximately 31°C (Figure 7).
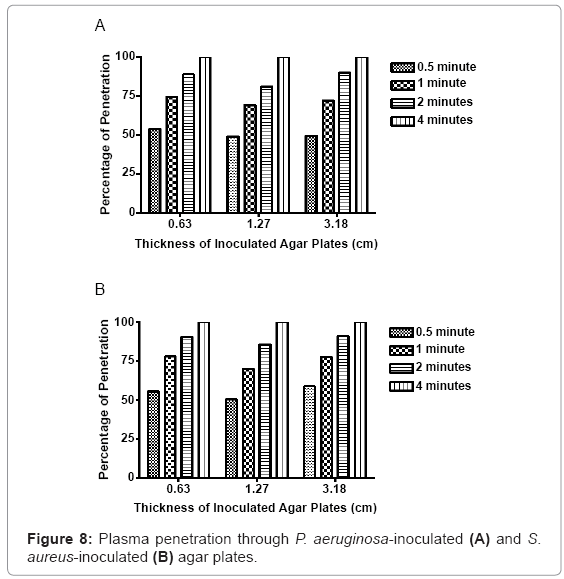
Thirty seconds of nonthermal plasma exposure penetrated through 0.34 centimeters of P. aeruginosa-embedded agar, equivalent to over 50% penetration of the total depth. When the exposure was increased to 1 minute, penetration through the first group increased to 0.47 centimeters, or approximately 74% total penetration. Two minutes of exposure increased the total penetration to 0.56 centimeters, or 88%. Four minutes of continuous exposure was sufficient to penetrate completely through 0.63 centimeters of inoculated agar. Nonthermal plasma exposure at 30 seconds, 1 minute, 2 minutes, and 4 minutes in the 1.3 centimeter agar model group generated penetration depths of 0.62, 0.88, 1.03, and 1.3 centimeters, respectively. Likewise, in 3.2 centimeter group, exposure doses of 30 seconds, 1 minute, 2 minutes, and 4 minutes generated penetration depths of 1.58, 2.29, 2.86, and 3.2 centimeters, respectively (Figure 8).
Similar results were observed in the embedded S. aureus agar plates. In the 0.63 centimeter agar model group, 30 seconds of plasma exposure generated penetration of 0.35 centimeters, or 55.5% of the total depth. When exposure was increased to 1 minute, 0.49 centimeters, or 76% of the total depth was penetrated. Two minutes increased penetration to 0.57 centimeters, equivalent to approximately 89% of the total depth. In the 1.3 centimeter agar model experimental group, nonthermal plasma exposure of 30 seconds, 1 minute, 2 minutes and 4 minutes generated penetration depths of 0.64, 0.89, 1.08 and 1.3 centimeters, respectively. In the 3.2 centimeter experimental agar model group, nonthermal plasma exposure at 30 seconds, 1 minute, 2 minutes and 4 minutes generated penetration depths of 1.86, 2.47, 2.89 and 3.2 centimeters, respectively. Complete penetration was observed with 4 minutes of continuous exposure. A positive correlation between plasma exposure and total agar penetration was observed in all depth groups, in both bacterial species. Overall nonthermal plasma-mediated penetration was reduced, as the depth and overall amount of inoculated agar increased. However, this effect was not deemed significantly detrimental to penetration or lethality of the plasma discharge in most samples.
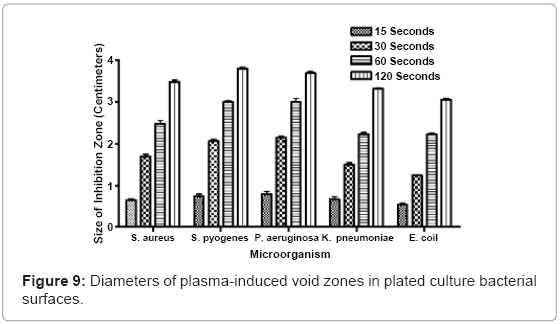
Nonthermal plasma discharge-induced damage voids in S. aureus, P. aeruginosa, S. pyogenes, K. pneumonia, and E. coli, were measured after exposure. Fifteen seconds of exposure generated average void sizes of 0.642 centimeters, 0.736 centimeters, 0.788 centimeters, 0.669 centimeters, and 0.531 centimeters in S. aureus, P. aeruginosa, S. pyogenes, K. pneumonia, and E. coli cultures, respectively. Thirty seconds of exposure increased the average void sizes to 1.691 centimeters, 2.054 centimeters, 2.144 centimeters, 1.498 centimeters, 1.498 centimeters and 1.242 centimeters. One minute of plasma exposure generated zones, with diameters ranging in size from 2.471 centimeters, observed in S. aureus plates to 2.218 centimeters in E. coli plates. As exposure increased to two minutes, inhibition zones also increased with measured values of 3.475 centimeters, 3.792 centimeters, 3.681 centimeters, 3.331 centimeters, and 3.055 centimeters in S. aureus, P. aeruginosa, S. pyogenes, K. pneumonia, and E. coli cultures, respectively. Some of the measured zones surpassed 4 centimeters in total diameter. Although damage void sizes varied amongst the different bacterial species, a direct positive correlation between increased plasma exposure and increased void size diameter was observed (Figure 9).
Fifteen seconds of continuous nonthermal plasma exposure reduced the logarithmic count of viable P. aeruginosa cultures by 0.24 logs. Log reduction increased to 1.24, once the exposure length was increased to 30 seconds of continuous exposure. A 1 minute exposure increased log reduction of viable bacteria by an additional 2.45 logs. Two minutes of exposure, reduced viable culture by 6.33 logs. Complete sterilization of cultures, with an average starting logarithmic concentration of 7.42, occurred after 4 minutes of continuous plasma exposure in P. aeruginosa cultures. Similar log reductions in viability were noted in the S. aureus cultures. Fifteen seconds of plasma exposure reduced S. aureus cultures by 0.26 logs. When the exposure interval was increased to 30 seconds and 1 minute, the log reduction of viable organisms increased to 0.796 and 2.26 logs, respectively. Nonthermal plasma exposure intervals of 2 minutes and 4 minutes generated log reductions of 6.08 logs and 7.56 logs, respectively. Four minutes of exposure completely sterilized all S. aureus cultures (Figure 10).
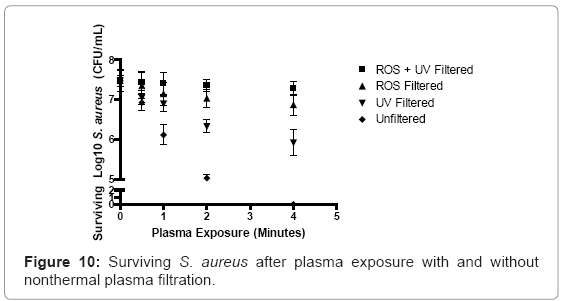
Unfiltered nonthermal plasma discharge reduced S. aureus cultures by 0.55 logs, or a 7.4% overall reduction, after 30 seconds of exposure. As plasma exposure increased to 1 and 2 minutes, bacterial logarithmic values decreased by 1.37 and 2.45 logs, equivalent to 18.3% and 32.8% overall reduction compared to negative control values. Four minutes of nonthermal plasma discharge was found to sterilize all cultures. UV-filtration reduced logarithmic survivability by only 5.7%, 7.8%, 15.4% and 27.5% after 30 seconds, 1 minute, 2 minutes and 4 minutes of plasma exposure. Reactive oxygen species (ROS) filtration reduced overall viability by only 1.62%, 4.41%, 5.9%, and 9.1% after 30 seconds, 1 minute, 2 minutes and 4 minutes of plasma exposure. Plasma discharges that were selectively filtered for both ROS and UV radiation displayed almost no germicidal capability, when compared to other experimental groups. Logarithmic reduction percentages equal to 0.61%, 1.02%, 1.5%, and 2.6% were observed after 30 seconds, 1 minute, 2 minutes, and 4 minutes of plasma exposure (Figure 10).
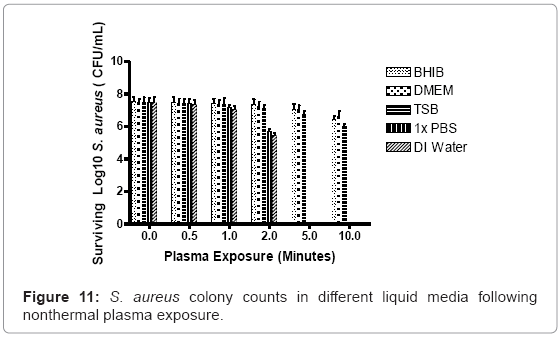
Surviving S. aureus culture log scale values after 2 minutes of continuous nonthermal plasma discharge were normalized against negative control, non-exposed culture values: 98% (DMEM), 97% (BHIB), 96% (TSB), 81% (PBS), and 76% (water). As plasma exposure gradually increased to 5 minutes, logarithmic values changed to: 97% (DMEM), 95% (BHIB), and 94% (TSB).The PBS and water groups were completely sterilized at 5 minutes. At 10 minutes of exposure, the surviving percentages decreased further in the heavier nutrient groups to: 94% (DMEM), 91% (BHIB), and 88% (TSB) (Figure 11).
Discussion
A reasonably high atomic oxygen flux was observed during characterization and measurement of the GPP plasma’s main constituents. Atomic oxygen has been found to be an important component associated with plasma sterilization [37-39]. Its presence in the plasma discharge of the studied device is encouraging for future sterilization applications. Spectrophotometric analyses indicated the plasma discharge associated with this generator produces elevated amounts of O+, OH-, and O2 +. Nitric oxide (NO), another main component found within this plasma discharge, is known to possess antimicrobial properties, and also act as an important signaling molecule in a variety of systems, including wound healing [40]. We report that continuous nonthermal plasma discharge generated a significant amount of NO. The significance of the concentration of NO observed in this study is apparent, when compared to the OSHA Time Weighted Average of 25 parts per million (ppm), associated with eight hours of transient exposure [41]. High concentrations of this reactive species are the result of elevated nitrogen species present in the starting gaseous medium fed into the plasma electrode. The production of nitric oxide is due to reactive ionization events caused during gaseous electrification, prior to expulsion from the plasma generator. The relatively high values of NO, derived from nonthermal plasma exposure, were important to note due to how quickly the molecule reacts in its environment. Nitric oxide’s impact on cellular activity is significant, and is likely a primary vehicle for physiological changes observed in prokaryotic and eukaryotic cell responses to nonthermal plasma discharge. However, bacterial exposures were performed in an open system.
All three types of ultraviolet radiation were detected and measured using radiometry. Although UV-A and UV-B have been linked to ultraviolet-radiation-induced damage in sporulating bacteria [42], these wavelengths were detected at lesser intensity than short wave UV-C radiation, during spectrophotometric analyses of studied plasma discharges. High energy UV-C is likely more directly associated with germicide, and DNA damage observed in exposed bacterial cultures [43,44]. While the UV components detected within the plasma discharge spectral profile are independently antimicrobial, they may function collectively in tandem.
The amount of ozone detected, the exposure interval, and the starting concentrations of bacteria, are all important factors to consider, when evaluating the germicidal capabilities of the ozone found within the plasma generator presented here. Ozone was detected spectrophotometrically in the discharge associated with this plasma discharge. Thanomsub et al. [45] reported that levels of ozone less than 1 ppm can have bactericidal properties. While the results from that study indicate ozone at low concentrations can have antimicrobial properties, we believe that it does not contribute significantly to the overall germicidal capability of the nonthermal plasma discharge independently. It likely plays a role, but given the large concentration of other known potent oxidizing radicals and ultraviolet radiation, play a larger collective role. Additionally, the measured concentration of ozone, following extensive continuous nonthermal plasma discharge (>10 minutes), is less than half of the ozone threshold limit value concentration of 1 ppm per eight hours, established by the American Conference of Governmental Industrial Hygienists of exposure and the Occupational Safety and Health Administration [46]. This suggests that nonthermal plasma could be used safely at exposure intervals on eukaryotic cells and tissues.
Nonthermal plasma-mediated temperature increases were not found to be significant. An 8°C increase from the starting, room temperature value was observed after ten minutes of continuous discharge. Since S. aureus and P. aeruginosa are mesophilic bacteria capable of tolerating temperatures up to 44°C, plasma-mediated temperature increases to 31°C will have no effect on their bacterial physiology. The plasma discharge slightly altered the pH of various high nutrient media. Increases in the high nutrient media pH values were less than 1 log difference. Likewise, the pH decrease associated with low nutrient media, deionized water and 1X PBS, while greater than the change in the higher nutrient media, did not vary significantly. The changes in pH are not outside the threshold for normal pH tolerance for S. aureus, which is typically found between pH 4 and 9 [47,48]. The heightened change in aqueous environments devoid of nutrient sources, such as proteins and sugars, may support the notion that the reactive species and ultraviolet radiation within nonthermal plasma discharges react with macromolecules in micro-environments, alongside the intended, targets cells. Other studies have found that extended usage of nonthermal plasma can significant affect and alter the pH of solutions. The alteration of the pH can have a very prolific effect on the viability of bacterial cells, within these liquid environments [49]. A specific pH critical value of 4.7 is presented as a threshold, with respect to plasmamediated germicide. The authors believed that superoxide was a chief effector in not only pH flux, but also bacterial germicide. Oehmingen et al. [50] found that acidification occured, when using 5-15 minutes of a DBD-generating plasma. This acidification effect was linked to elevated release of NO from the discharge resulting in logarithmic reduction of viable bacteria over time. Diferences in pH fluxuations, based on the macromolecular content of the liquid medium environment, is likely due to a shielding effect due to plasma interaction with complex macromolecules, prior to affecting target bacterial cells. Stoffels et al. [51] observed a similar phenomenon in an earlier work. The density of the bacterial cells combined with macromolecular buffering of plasma can affect how the plasma is able to interact with downstream cells.
Extensive testing with the penetrative properties of this plasma was performed in part, to determine the effectiveness of using nonthermal plasma, as a tool used to treat bacterial infections complicated by cryptic planktonic bacteria or biofilms. Agar was chosen to serve as a semi-solid matrix to embed bacteria uniformly throughout a fixed area. Since agar is also a polysaccharide polymer, it also provides a three-dimension barrier for the microorganisms embedded within. The agar medium served as a simple way to assess nonthermal plasma penetrative efficacy, through biological polymers. Since the surrounding medium plays a role in how the plasma is able to interact with target cells, penetrative efficacy must be considered. Three different volumes of agar were chosen to evaluate plasma response to varied depth and total volume of biopolymer-bacteria models. Although the plasma discharge was able to penetrate through all three agar model groups, the total penetration was reduced, as the depth of starting agar concentrations increased. The penetrative nature of the plasma discharge is encouraging, and allows for more advanced model development to occur. The limits of plasma penetration provided additional capability information, with respect to this plasma generator subtype.
Agar penetration studies show that thirty seconds of nonthermal plasma exposure is able to penetrate through 0.133 inches of P. aeruginosa-embedded agar, and 0.139 inches of S. aureus-embedded agar. Increased exposure lengths directly correspond to greater penetration through bacterial embedded agar hydrogel models. Four minutes of continuous plasma exposure penetrates completely through the largest volume of agar, while simultaneously sterilizing uniform columns through these inoculated matrices. The dispersal of reactive oxygen species, charged ions, molecules and secondary plasmaassociated molecules, was observed using bacteria spread evenly on plates. As little as 15 seconds of exposure generated inhibition zones where bacteria had been killed and etched away from the surface. Fifteen seconds of exposure generated average void sizes of 0.642 centimeters, 0.736 centimeters, 0.788 centimeters, 0.669 centimeters, and 0.531 centimeters, in each of the respective bacterial species plated. When the exposure increased to 2 minutes, the zone sizes increased to values of 3.475 centimeters, 3.792 centimeters, 3.681 centimeters, 3.331 centimeters, and 3.055 centimeters, in each of the respective bacterial species.
The other main physical parameter of experimental interest was the diameter of plasma-induced exposure zones. These voids served as indicators of the distance that plasma discharges radiate from a central fixed point. Void zones were observed in a variety of pathogenic bacteria, including P. aeruginosa and S. aureus (Figure 9). In many of the experiments, a central void was accompanied by a surrounding corona that displayed a fringe germicidal effect. These coronas served as the boundary limits for all void measurements. Evidence of a biphasic, coronal effect suggests that the plasma has a direct, immediate sterilization effect on the localized area where the discharge is fixed, and also a secondary lesser germicidal effect at the periphery of its displayed range. There is a direct positive correlation between larger void zones and elevated nonthermal plasma usage. This expansion is due to an increase in the germicidal effect at the periphery of the growing zones, due to continued release of germicidal free radicals and other plasma-associated ions. Because the chief components of the plasma discharge disperse rapidly into the atmosphere, or interact with its local environment, cessation of the plasma discharge after shorter exposure lengths could explain reduced size of killing zone voids.
Log reduction of viable bacteria, following plasma exposure, is a core confirmation of previous plasma germicidal capability in other plasma subtypes. It is also a fundamental observation within this study as a whole. Ultraviolet radiation, atomic oxygen and reactive oxygen species, all possess antimicrobial properties [43,46,52]. The nonthermal plasma discharge profile was found to possess each of these components, suggesting significant germicidal potential in this generator. Averages of germicidal values were obtained following multiple independent experiments, and these results reveal increased nonthermal plasma exposure results in a direct negative correlation in planktonic bacteria assessed in vitro.
As little as fifteen seconds of nonthermal plasma discharge was sufficient to reduce bacterial viability in both Gram-positive and Gram-negative bacteria. Exposure up to two minutes of continuous nonthermal plasma exposure reduced bacterial viability by over six logs, in cultures with starting bacterial concentrations of 3.0×107 CFU/mL. Complete sterilization was observed between three and four minutes of plasma exposure. Significant sporicidal results were also collected from experiments using continuous plasma exposure. The length of time needed to reduce spore germination was much greater than the planktonic bacteria. Five minutes began to reduce viability, and it was not until after ten minutes of exposure, until more dramatic results were achieved. Complete sterilization of spores occurred around fifteen minutes of nonthermal plasma exposure. Collectively, these data provide a more complete profile, for not only the physical parameters of the plasma discharge, but also the potential germicidal capabilities of this novel plasma generator.
Reactive oxygen and nitrogen species have been implicated in the attack and destabilization of membranes in bacteria [35,52]. A membrane attack event can be viewed as a short term germicidal effect, because the induced damage would be a highly dynamic and amplifying sequence of events. The UV radiation profile of this nonthermal plasma is predominantly UV-C. Short wave ultraviolet radiation has been linked to DNA damage for decades. It is certainly probable that the UV radiation in the nonthermal plasma discharge is able to penetrate into the bacteria, especially as integrity of cellular membranes is weakened over time, and then lost entirely. The accumulation of DNA damage ultimately results in genomic instability, which ultimately leads to necrotic lysis of the cell. Genomic damage in bacterial cells can be thought of as a long term killing event that contrasts the immediate membrane-damaging attack on bacterial cell membranes. The data suggest that when reactive oxygen and nitrogen species are removed from the plasma discharge profiles, the time required to damage bacterial cells increases dramatically. This delay increases further in cultures exposed to ROS-filtered nonthermal plasma. Removal of the reactive species from the nonthermal plasma discharge profile eliminates the rapid membrane destabilizing mechanism of action associated with high ROS/RNS-associated membrane catastrophe. The drop off between ROS/RNS-filtered plasma suggests that a significant portion of the germicidal effect likely occurs very rapidly, and could support this proposed “short-term” mechanism.
The UV component within the nonthermal plasma discharge is still significant, even though it independently takes longer to impact significant germicidal capability. Ultraviolet radiation is able to damage the DNA within the bacterial cell. The penetrative nature associated with UV radiation could be advantageous against higher starting concentrations of bacteria, since the ROS, RNS and other plasma ions could dissipate and react rapidly on the initial surface of an exposed area. UV-mediated germicide is a longer term effect, that is effective at aiding removal of high density populations of cells. Bacteria possess a class of enzymes called superoxide dismutases, to eliminate reactive oxygen species and their derivatives from localized environments. Should ROS attack bacterial membranes, bacteria can use these enzymes to neutralize offending ROS. However, if over time, the UV radiation damages the enzymes that are used to code for this class of enzymes, the bacteria ultimately would become more susceptible to ROS damage. A two-pronged effect, linking the two mechanisms together, could be postulated, in order to explain the synergistic effect of both constituents and their activity within nonthermal plasma discharge.
Staphylococcal cultures exposed to the plasma in a variety of liquid media, ranging from the highly fortified, nutrient-rich Brain Heart Infusion Broth (BHIB) to nutrient-devoid sterile distilled water, indicated that a local environment does play a role in plasma penetration, and its ability to affect targeted cells. Because each medium contains different types and ratios of proteins, sugars and vitamins, this study simulated different environmental conditions, with respect to bacterial growth macromolecular content. The survivability of S. aureus observed in high nutrient media exposed to unfiltered plasma, and lownutrient media exposed to plasma with reactive species and charged molecules, is similar. It remains possible that environment composed of higher amounts of macromolecules inhibit the reactivity of plasma molecules. Reactive species and free radicals are unstable, and will react readily with a wide variety of compounds both in vitro and in vivo [53]. Environments with elevated sugar or protein content may shield cells in localized areas from reactive species, as the macromolecules could react with plasma-associated molecules and ions, before reaching intended target cells. If the reactive and charged molecules are unable to reach the target cells, then some other component must be responsible for the reduction of bacteria.
The reduction of viability in liquid cultures, when both ROS and UV radiation was removed, suggests that there are other components within the plasma discharge that contributes to germicidal efficacy. A possible target could be the ionized colloidal nozzle materials produced during extended, continuous nonthermal plasma discharges. The ionized materials were detected spectrophotometrically, and traces were visibly seen on glass slides and in sample solutions. These data suggest that the environment in which cells are placed has a role in the effectiveness of nonthermal plasma exposure. This further supports that the observed germicide in the S. aureus cultures are the result of more than one chief effector, and possibly lesser secondary elements.
Comprehensive understanding of the micro-environment where cells are exposed to nonthermal plasma is important, as one considers how the environment can change was exposed to reactive species at high densities from nonthermal plasma discharge. Knowledge of how the environment plays a role in the efficacy of nonthermal plasmamediated effects, are critical as plasma generator designs are updated and developed. Optimization of starting gaseous materials fed into nonthermal plasmas could drastically altered the subsequent, produced plasmas. Customizable plasma discharges could greatly benefit the user in a wide variety of applications. The foundation upon how one chooses their gaseous materials will be dependent upon how the discharge interacts with the environment interface. This plasma-environment interaction is one that merits additional investigation and development as plasma development, and therapies continue to evolve.
References
- Fridman G, Friedman G, Gutsol A, Gutsol AB, Vasilets VN, et al. (2008) Applied plasma medicine. Plasma Process Polym 5: 503-533.
- Vleugels M, Shama G, Deng XT, Greenacre E, Brocklehurst T, et al. (2005) Atmospheric plasma inactivation of biofilm-forming bacteria for food safety control. IEEE Trans Plasma Sci 33: 824-828.
- Birmingham JG, Hammerstrom DJ (2000) Bacterial decontamination using ambient pressure nonthermal discharges. IEEE Trans Plasma Sci 28: 51-55.
- Gaunt LF, Beggs CB, Georghiou GE (2006) Bactericidal action of the reactive species produced by gas-discharge nonthermal plasma at atmospheric pressure: A review. IEEE Trans Plasma Sci 34: 1257-1269.
- Kong MG, Kroesen G, Morfill G, Nosenko T, Shimizu T, et al. (2009) Plasma medicine: an introductory review. New J Phys 11: 115012.
- Lerouge S, Wertheimer MR, Yahia LH (2001) Plasma sterilization: A review of parameters, mechanisms, and limitations. Plasmas Polym 6: 175-188.
- Kim HH (2004) Nonthermal plasma processing for air pollution control: A historical review, current issues, and future prospects. Plasma Process Polym 1: 91-110.
- Fridman G, Peddinghaus M, Balasubramanian M, Ayan H, Fridman H, et al. (2006) Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chemistry And Plasma Processing 26: 425-442.
- Fridman G, Shereshevsky A, Jost MM, Brooks AD, Fridman A, et al. (2007) Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chemistry And Plasma Processing 27: 163-176.
- Heinlin J, Isbary G, Stolz W, Morfill G, Landthaler M, et al. (2011) Plasma applications in medicine with a special focus on dermatology. J Eur Acad Dermatol Venereol 25: 1-11.
- Liebmann J, Scherer J, Bibinov N, Rajasekaran P, Kovacs R, et al. (2011) Biological effects of nitric oxide generated by an atmospheric pressure gas-plasma on human skin cells. Nitric Oxide 24: 8-16.
- Kalghatgi SU, Fridman G, Cooper M, Nagaraj G, Peddinghaus M, et al. (2007) Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans Plasma Sci 35: 1559-1566.
- Napartovich A (2001) Overview of atmospheric pressure discharges producing nonthermal plasma. Plasmas Polym 6: 1-14.
- Massines F, Gherardi N, Naude N, Segur P (2005) Glow and Townsend dielectric barrier discharge in various atmosphere. Plasma Phys Control Fusion 47: B577.
- Yokoyama T, Kogoma M, Moriwaki T, Okazaki S (1990) The mechanism of the stabilisation of glow plasma at atmospheric pressure. J Phys D: Appl Phys 23: 1125.
- Ogata A, Shintani N, Yamanouchi K, Mizuno K, Kushiyama S, et al. (2000) Effect of water vapor on benzene decomposition using a nonthermal-discharge plasma reactor. Plasma Chemistry and Plasma Processing 20: 453-467.
- Lukes P, Appleton AT, Locke BR (2004) Hydrogen peroxide and ozone formation in hybrid gas-liquid electrical discharge reactors. IEEE Trans Ind Appl 40: 60-67.
- Plimpton SR, Gołkowski M, Mitchell DG, Austin C, Eaton SS, et al. (2013) Remote delivery of hydroxyl radicals via secondary chemistry of a nonthermal plasma effluent. Biotechnol Bioeng.
- Golkowski M, Golkowski C, Leszczynski J, Plimpton SR, Maslowski P, et al. (2012) Hydrogen-peroxide-enhanced nonthermal plasma effluent for biomedical applications. IEEE Trans Plasma Sci 40: 1984-1991.
- Chang JS (2001) Recent development of plasma pollution control technology: a critical review. Sci Technol Adv Mater 2: 571-576.
- Fridman A, Chirokov A, Gutsol A (2005) Non-thermal atmospheric pressure discharges. J Phys D: Appl Phys 38.
- Conrads H, Schmidt M (2000) Plasma generation and plasma sources. Plasma Sources Sci Technol 9: 441.
- Akishev Y, Grushin M, Napartovich A, Trushkin N (2007) Novel AC and DC non-thermal plasma sources for cold surface treatment of polymer films and fabrics at atmospheric pressure. Plasmas Polym 7: 261-289.
- Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, et al. (2001) Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm 226: 1-21.
- Burlica R, Kirkpatrick MJ, Locke BR (2006) Formation of reactive species in gliding arc discharges with liquid water. J Electrostat 64: 35-43.
- Zhao GB, Janardhan Garikipati SVB, Hu X, Argyle MD, Radosz M (2005) Effect of oxygen on nonthermal plasma reactions of nitrogen oxides in nitrogen. AIChE J 51: 1800-1812.
- Namihira T, Katsuki S, Reuben H, Akiyama H, Okamoto K (2002) Production of nitric oxide using a pulsed arc discharge. IEEE Trans Plasma Sci 30: 1993-1998.
- Sakai S (2008) Nitric oxide generator based on pulsed arc discharge.
- Burts ML, Alexeff I, Meek ET, McCullers JA (2009) Use of atmospheric non-thermal plasma as a disinfectant for objects contaminated with methicillin-resistant Staphylococcus aureus. Am J Infect Control 37: 729-733.
- Laroussi M (1996) Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans Plasma Sci 24: 1188-1191.
- Laroussi M, Leipold F (2004) Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int J Mass Spectrom 233: 81-86.
- Laroussi M, Mendis DA, Rosenberg M (2003) Plasma interaction with microbes. New J Phys 5: 41.
- Laroussi M (2002) Nonthermal decontamination of biological media by atmospheric-pressure plasmas: Review, analysis, and prospects. IEEE Trans Plasma Sci 30: 1409-1415.
- Locke BR, Sato M, Sunka P, Hoffmann MR, Chang JS (2006) Electrohydraulic discharge and nonthermal plasma for water treatment. Ind Eng Chem Res 45: 882-905.
- Liu C, Cui N, Brown NMD, Meenan BJ (2004) Effects of DBD plasma operating parameters on the polymer surface modification. Surf Coat Technol 185: 311-320.
- ASTM E 2089-00 (2000) Standard Practices for Ground Laboratory Atomic Oxygen Interaction Evaluation of Materials for Space Applications.
- Choi JH, Hana I, Baika KH, Leeb MI, Hanb DW, et al. (2006) Analysis of sterilization effect by pulsed dielectric barrier discharge. J Electrostat 64: 17-22.
- Hayashi N, Guan W, Tsutsui S, Tomari T, Hanada Y (2006) Sterilization of medical equipment using radicals produced by oxygen/water vapor RF plasma. Jpn J Appl Phys 45: 8358.
- Moreira AJ (2004) Sterilization by oxygen plasma. Appl Surf Sci 235: 151-155.
- Nathan CF, Hibbs JB Jr (1991) Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol 3: 65-70.
- Phillips ML, Hall TA, Sekar K, Tomey JL (1999) Assessment of medical personnel exposure to nitrogen oxides during inhaled nitric oxide treatment of neonatal and pediatric patients. Pediatrics 104: 1095-1100.
- Slieman TA, Nicholson WL (2000) Artificial and solar UV radiation induces strand breaks and cyclobutane pyrimidine dimers in Bacillus subtilis spore DNA. Appl Environ Microbiol 66: 199-205.
- Friedberg EC (2003) DNA damage and repair. Nature 421: 436-440.
- Kielbassa C, Roza L, Epe B (1997) Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis 18: 811-816.
- Thanomsub B, Anupunpisit V, Chanphetch S, Watcharachaipong T, Poonkhum R, et al. (2002) Effects of ozone treatment on cell growth and ultrastructural changes in bacteria. J Gen Appl Microbiol 48: 193-199.
- Bergamini CM, Gambetti S, Dondi A, Cervellati C (2004) Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des 10: 1611-1626.
- Booth IR (1999) The regulation of intracellular pH in bacteria. Novartis Found Symp 221: 19-28.
- Sutherland JP, Bayliss AJ, Roberts TA (1994) Predictive modelling of growth of Staphylococcus aureus: the effects of temperature, pH and sodium chloride. Int J Food Microbiol 21: 217-236.
- Ikawa S, Kitano K, Hamaguchi S (2010) Effects of pH on bacterial inactivation in aqueous solutions due to low-temperature atmospheric pressure plasma application. Plasma Process Polym 7: 33-42.
- Oehmigen K, Hähnel M, Brandenburg R, Wilke Ch, Weltmann KD, et al. (2010) The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process Polym 7: 250-257.
- Stoffels E, Sakiyama Y, Graves DB (2008) Cold atmospheric plasma: charged species and their interactions with cells and tissues. IEEE Trans Plasma Sci 36: 1441-1457.
- Bandyopadhyay U, Das D, Banerjee RK (1999) Reactive oxygen species: Oxidative damage and pathogenesis. Curr Sci 77: 658-666.
- Thorpe SR, Baynes JW (2003) Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids 25: 275-281.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16186
- [From(publication date):
June-2013 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 11443
- PDF downloads : 4743