Carbaryl, A Pesticide Causes “Reproductive Toxicity” in Albino Rats
Received: 08-Aug-2012 / Accepted Date: 31-Aug-2012 / Published Date: 03-Sep-2012 DOI: 10.4172/2161-0681.1000126
Abstract
The key to man’s health lies largely in his environment & often man is responsible for the pollution of his environment through urbanization, industrialization and other human activities for example commercial agriculture and garden pest control etc. by using pesticides. Pesticides, though present in the environment in small quantities as compared to other contaminants such as industrial wastes and fertilizers, account for public and scientific concern due to their high biological activity. In the recent years, use of carbamate insecticides has gained importance due to ban of the insecticides belonging to Organochlorine groups that is D.D.T., Aldrin, Lindane and Endosulfan. These pesticides have a tendency to persist and have potential to bioaccumulate in the body Kamrin [1]. Concern about the susceptibility of the male reproductive system to drugs or environmental agents has assumed an increasing extent. The outcome of such exposures have included not only reduced fertility but also embryo/fetal loss, birth defects, childhood cancer, and other postnatal or functional deficits. Carbaryl is one of the most important insecticides as it is widely produced and used which has prompted us to initiate this study . The present study was conducted on 40 male Wistar albino rats as experimental animals. The rats were procured from the Animals House of the Department of Pharmacology, Government Medical College, Jammu. The rats were divided in four groups as normal control group I, group II, group III and group IV. All the rats were group housed and were fed with standard pellet diet and water for two weeks. After two weeks, the rats of group I were left as such and rats of groups II, III and IV were given 50 mg, 100 mg and 200 mg/kg body weight/day of carbaryl drug in 0.2 ml of groundnut oil orally, 6 days/week for 60 days, respectively. After 60 days, all the rats were anaesthetized by keeping them in an inverted glass jar containing large piece of cotton soaked in anaesthetic ether. The testis were dissected out from each rat and were cut into smaller pieces. These pieces were immediately fixed in 10% formalin. The blocks were prepared for section cutting with a microtome by paraffin wax embedding method. The sections of 7 μ thickness were mounted on glass slides and were stained by H&E and Masson’s trichome stain. The following findings are drawn from the study:- (1) There is variation in the shape of seminiferous tubules of testis. (2) There is sloughing of the germinal cells from the basement membrane. (3) There is depressed spermatogenesis and loss of sperms. (4) Some tubules are showing accumulation of cellular masses in the lumen of seminiferous tubules of testis. (5) Interstitial spaces are showing the oedema. (6) Degenerated Leydig cells are also seen. These findings are highly conclusive of reproductive toxicity produced by an insecticide, Carbaryl. It is concluded that the toxic effects are more pronounced in the peripheral parts of the sections of testis. Moreover, intensity of toxic effects both in peripheral and central parts increases with increase in dosage of the carbaryl drug.
Keywords: Albino rats; Testis; Carbaryl; Depressed spermatogenesis
308202Introduction
The key to man’s health lies largely in his environment. Infact, much of man’s ill health can be traced to adverse environmental factors, such as water pollution, soil pollution, air pollution etc. which pose a constant threat to man’s health. Often man is responsible for the pollution of his environment through urbanization, industrialization and other human activities for example commercial agriculture and garden pest control etc by using pesticides.
Pesticides, though present in the environment in small quantities as compared to other contaminants such as industrial wastes and fertilizers, account for public and scientific concern due to their high biological activity. According to the Stockholm Convention on Persistent Organic Pollutants, 9 of the 12 most dangerous and persistent organic chemicals are pesticides [2]. It is classified as a likely human carcinogen by the United States Environmental Protection Agency (EPA.) [3].
Pesticides is the general term for insecticides, acaricides, rodenticides, herbicides, fungicides, etc. They are widely used in industry, agriculture and for public health purposes. Unfortunately, pesticides are toxic to a greater or lesser extent towards non-target organisms, including humans Ernest and Patricia [4].
The synthetic chemical pesticides can structurally be classified into the following groups:
• Organochlorine pesticides
• Organophosphate pesticides
• Carbamates
• Synthetic pyrethroids
• Miscellaneous.
Benefits from Pesticides
The important contributions of pesticides to our heath and economy guarantee their continued use as a class, so require the most complete knowledge of their toxicology that we can achieve in order to avoid hazards.
They contribute directly to our health through control of certain vector-borne diseases and to the economy through increased production of food. Nearly two-third of world population does not have sufficient food when more than 30% of our food crops continue to be destroyed by pests.
Roughly 55,000 tons of pesticides are annually applied in field crops. Of this 40,000 tons are insecticide, 8000 tons are fungicides and the rest herbicides. On an average the pesticide used in India is one tenth of what is applied per unit area of cropped field in the advanced countries.
Pesticides as Environmental Pollutants
Pesticides hold a unique position among environmental contaminants, being present in the environment in such small quantities as compared to other contaminants such as industrial wastes and fertilizers. The major factors which account for public and scientific concern is their biological activity.
Role of Pesticides on Testis
Of the potential health risks associated with exposure to chemical or physical agents, a prominent concern is that these agents may interfere with the ability of individuals to produce normal, healthy children. A large number of chemicals that have been released into the environment are known to interfere with the endocrine system. Sexual development during the prenatal and neonatal period is under hormonal control and is, therefore, sensitive to exogenous substances with an endocrine effect. Keeping in mind the pivotal role of testis in reproduction this experimental work was done.
Attention in this thesis primarily is focused on toxic effects that involve testicular and postspermatogenic processes that are essential for reproductive success.
Choice of Pesticides for the Present Study
Carbaryl was taken to see the effect on testis of albino rats. Carbaryl which is 1-naphthyl-N-methyl carbamate is a broad-spectrum insecticide used to protect vegetables, cotton, fruits, cereals and other crops against a variety of insects and pests. Carbaryl is 1-naphthyl-N-methyl carbamate and was first synthesized in 1953 and introduced in 1958. It controls over 100 species of insects on citrus fruits, cotton, forests, lawns, ornamentals, shade trees and other crops as well as poultry livestock and pets. It is also used as molluscicide and acaricide Rani et al. [5].
Carbaryl can produce adverse effects in humans by skin contact, inhalation or ingestion. Direct contact of skin or eyes with moderate levels can cause burns. Inhalation or ingestion of very large amounts can be toxic to nervous and respiratory system.
Pesticides clearly have the potential to cause reproductive toxicity in animals and several compounds are known to affect human reproduction [6,7].
Epidemiological studies postulated that in the past 50 years the sperm number and sperm quality in human had been decreased [8,9].
Pathological effects of pesticides on the reproductive system of experimental animals were recorded by many authors [10-13].
Different studies have been done to see the effects of carbamate exposure on the testis of experimental animals.
Degraeve et al. [14] found that in mice injected intraperitoneally with 0.4 mg of carbaryl daily for one week, the incidence of sperm abnormalities were reportedly increased, but no degenerative changes in the testis were seen.
Kitagawa et al. [15] reported reduced numbers of spermatogonia and spermatozoa in rats given 3 mg of carbaryl per week orally for one year.
Rani et al. [5] found marked histopathological changes in testis of albino rats following oral administration of carbaryl in dose of 100 and 200 mg/kg body weight in 0.2 ml of groundnut oil orally, 6 days/week for 60 days.
The light microscopic observation demonstrated distorted shape of seminiferous tubules, disturbed spermatogenesis, accumulation of cellular mass in the lumen of tubules, oedema of interstitial spaces and loss of sperms of varying degrees and detachment of germ cells from the basement membrane. Same findings were also reported in [16-19].
Nonetheless, carbamate use may pose a significant risk of poisoning if handled carelessly. Health professionals may need to assess the consequences of prior exposure and should understand the fate of these compounds after absorption by humans.
The deleterious effects of carbaryl on reproductive system have prompted us to perform this study. The present study is aimed to evaluate the effect of carbaryl on the histomorphological characteristics of the testis in a mammal, the albino rat.
Vashakidze [20] reported teratogenecity and decreased reproduction at subchronic intubated dosages of 100 mg kg/day and higher but not at 50 mg/kg/day. However, a single 50 mg/kg/day in intubated dose on gestation day nine or ten was reported to cause teratogenecity.
Robens [21] observed that carbaryl administered to guinea pigs by gavage at 300 mg/kg/day (a toxic dosage) during organogenesis resulted in fetal skeletal anomalies; fetal mortality resulted from dosing on gestation days 11-21, but not from single doses during gestation.
Dikshith et al. [22] demonstrated that oral administration of carbaryl (200 mg/kg for 3 days a week) for a period of 90 days did not produce any overt toxicological signs in male rats. There were no significant histological changes in testis, epididymis, liver and kidney. Similarly, no marked biochemical changes were seen in testis, liver and brain. The activity of acetycholine esterase in blood of carbaryl treated rats was however, found to be decreased. Carbaryl did not affect the fertility of male rats at 200 mg/kg up to 90 days.
Murray et al. [23] showed that when carbaryl administered to mice by gavage at 100 mg/kg/day on gestation days 6-15 had no fetotoxic effects, dietary exposure at 5660 ppm (1166 mg/kg/day) resulted in decreased fetal size but no teratogenecity.
Martin [24] showed that when carbaryl administered to mice for 5 days upto 800 mg/kg/day or by gavage at 150 mg/kg/every 2 days for upto 68 days, did not affect testis weight, histology, sperm count and frequency of sperm abnormalities.
Osterloh et al. [25] found that when carbaryl administered to mice up for 5 days at upto 800 mg/kg/day did not affect testis weight, histology sperm count and frequency of sperm abnormalities.
Sever and Hessol [26] stated that Carbaryl given to mice at upto 34 mg/kg/day for 5 days did not affect either the weight of the testis and sex glands or the ability of the prostate to assimilate and metabolize testosterone.
Material and Methodology
The present study is based on the findings carried out on 40 male Wistar albino rats as experimental animals.
Collection of material
Healthy male Wistar albino rats weighing between 50-80 grams were obtained on December 13, 2010 from the Animals House of the Department of Pharmacology, Medical College Jammu. Forty rats were included in this study.
Grouping of animals
The rats were divided into the following four groups and identification number was given to the rats of each group.
Group I: Normal control - 10 rats
Group II: Experimental group - 10 rats
Group III: Experimental group - 10 rats
Group IV: Experimental group - 10 rats
All the rats were group housed in small iron cages in a room, where temperature was maintained at 23° ± 1°C. The rats were fed with standard pellet diet and water for two weeks.
After two weeks the rats of normal control group I were left as such. Whereas, the drugs were administered to other groups as shown in (Table 1).
| Experimental groups | Date of administration of 1st dose of carbaryl drug | Route of administration of carbaryl drug | Duration of carbaryl drug given | Dose of carbaryl drug administered to each group |
|---|---|---|---|---|
| Group II | 28/12/2010 | Oral | 6 days/ week for 60 days | 50 mg/kg body weight/ day in 0.2 ml of groundnut oil |
| Group III | 28/12/2010 | Oral | 6 days/ week for 60 days | 100 mg/kg body weight/ day in 0.2 ml of groundnut oil |
| Group IV | 28/12/2010 | Oral | 6 days/ week for 60 days | 200 mg/kg body weight/ day in 0.2 ml of groundnut oil |
Table 1: Administration of Carbaryl Drug To Experimental Groups.
Dissection of experimental animals
After 60 days all the rats were sacrificed after anaesthetizing them in an inverted glass jar containing large piece of cotton soaked in anaesthetic ether and dissection of testis of albino rats was done on the same day.
The scrotum was skinned and a midline incision was given with the help of scalpel and forceps. The testis were dissected out from the scrotum of each rat. The naked eye examination was done to see any external changes. The dissected out testis were cut into smaller pieces (5 mm) and were kept in tissue capsules along with a label indicating the serial groups I, II, III and IV.
Methodology
Manual processing of tissues
The casting and embedding was done with the help of moulds. Two L-shaped blocks were placed on a metallic plate, which acts as a base of the mould and molten wax was poured into it. The tissues were placed in the mould filled with wax and left to solidify. After solidification the blocks of the wax were removed and properly labeled for microtomy. The slides were subsequently stained by a haematoxylin and eosin & Masson’s trichrome. The slides were cleaned beyond the area of tissue implantation, dried and mounted in DPX and examined first under low power and then high power (Table 2).
| S. No | Step | Medium | Type |
|---|---|---|---|
| 1 | Fixation | 10% formal saline | 12 hours |
| 2 | Dehydration | Acetone | 3 changes at intervals of 2 hours |
| 3 | Clearing | Benzene | 3 changes at intervals of 2 hours |
| 4 | Wax embedding | Paraffin wax at 56oC | Two changes in molten wax at its melting point were given at time intervals of 1½ hrs. & 1 hr respectively |
Table 2: Manual processing of tissues.
Results
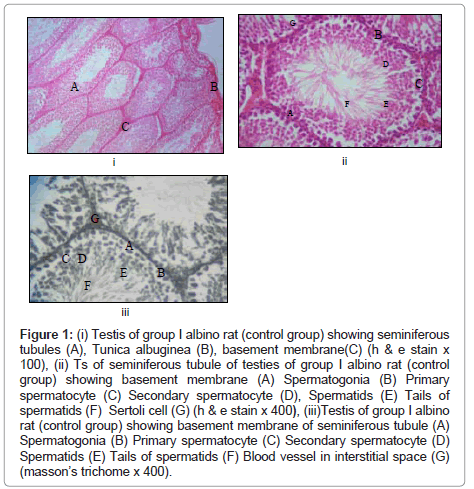
Observations in normal control group I (Figure 1)
Figure 1: (i) Testis of group I albino rat (control group) showing seminiferous tubules (A), Tunica albuginea (B), basement membrane(C) (h & e stain x 100), (ii) Ts of seminiferous tubule of testies of group I albino rat (control group) showing basement membrane (A) Spermatogonia (B) Primary spermatocyte (C) Secondary spermatocyte (D), Spermatids (E) Tails of spermatids (F) Sertoli cell (G) (h & e stain x 400), (iii)Testis of group I albino rat (control group) showing basement membrane of seminiferous tubule (A) Spermatogonia (B) Primary spermatocyte (C) Secondary spermatocyte (D) Spermatids (E) Tails of spermatids (F) Blood vessel in interstitial space (G) (masson’s trichome x 400).
Macroscopic changes: No gross changes seen.
Microscopic changes: Cut sections of testis in various planes show that the testis contain two components as:
(A) Seminiferous tubules with many cells thickened walls.
(B) Interstitial cells disposed in small groups in connective tissue stroma between the tubules.
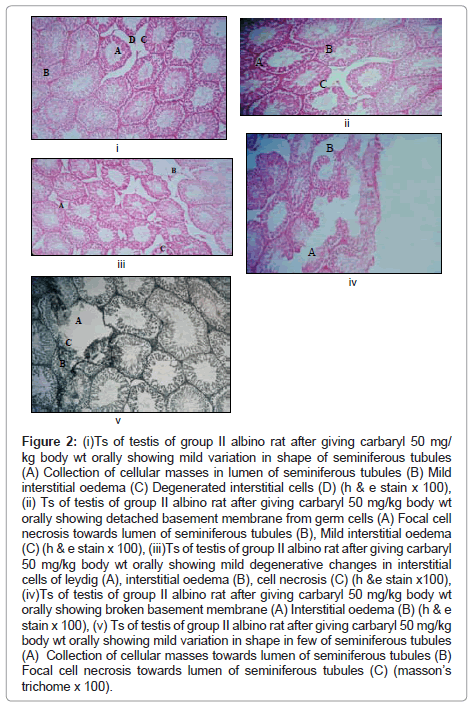
Observations in carbaryl (50 mg/kg/day) treated rats group II (Figure 2)
Figure 2: (i)Ts of testis of group II albino rat after giving carbaryl 50 mg/ kg body wt orally showing mild variation in shape of seminiferous tubules (A) Collection of cellular masses in lumen of seminiferous tubules (B) Mild interstitial oedema (C) Degenerated interstitial cells (D) (h & e stain x 100), (ii) Ts of testis of group II albino rat after giving carbaryl 50 mg/kg body wt orally showing detached basement membrane from germ cells (A) Focal cell necrosis towards lumen of seminiferous tubules (B), Mild interstitial oedema (C) (h & e stain x 100), (iii)Ts of testis of group II albino rat after giving carbaryl 50 mg/kg body wt orally showing mild degenerative changes in interstitial cells of leydig (A), interstitial oedema (B), cell necrosis (C) (h &e stain x100), (iv)Ts of testis of group II albino rat after giving carbaryl 50 mg/kg body wt orally showing broken basement membrane (A) Interstitial oedema (B) (h & e stain x 100), (v) Ts of testis of group II albino rat after giving carbaryl 50 mg/kg body wt orally showing mild variation in shape in few of seminiferous tubules (A) Collection of cellular masses towards lumen of seminiferous tubules (B) Focal cell necrosis towards lumen of seminiferous tubules (C) (masson’s trichome x 100).
The rats fed with 50 mg/kg/day for 60 days show following features in the testis:-
Macroscopic changes: No gross changes seen.
Microscopic changes: (A) Changes in seminiferous tubules
• Seminiferous tubules show mild variation in their size with focal distortion of normal shape which is mostly observed in peripheral seminiferous tubules (only some of the seminiferous tubules).
• The other changes seen are: collection of cellular masses in their lumen (few sections).
• Detachment of the basement membrance from the seminiferous epithelium (in few of the seminiferous tubules).
• Mild to moderate degenerative changes in spermatogenic cells.
• In addition focal individual cell necrosis is also seen especially towards the lumen of tubules.
• In few of tubules, spermatids and spermatozoa are identified.
(B) Changes in the interstitium
• Interstitial cells of Leydig show mild to moderate degenerative changes in the interstitial spaces in between the seminiferous tubules.
• Interstitial oedema is observed in the interstitial spaces which is more prominent in the peripheral region of testis.
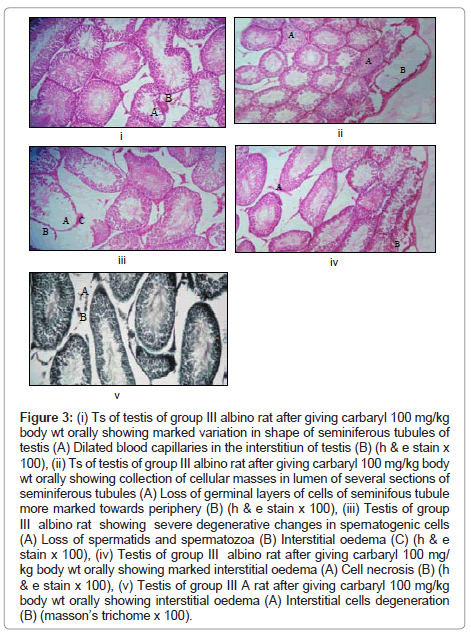
Observations in carbaryl (100 mg/kg/day) treated rats group III (Figure 3)
Figure 3: (i) Ts of testis of group III albino rat after giving carbaryl 100 mg/kg body wt orally showing marked variation in shape of seminiferous tubules of testis (A) Dilated blood capillaries in the interstitiun of testis (B) (h & e stain x 100), (ii) Ts of testis of group III albino rat after giving carbaryl 100 mg/kg body wt orally showing collection of cellular masses in lumen of several sections of seminiferous tubules (A) Loss of germinal layers of cells of seminifous tubule more marked towards periphery (B) (h & e stain x 100), (iii) Testis of group III albino rat showing severe degenerative changes in spernatogenic cells (A) Loss of spermatids and spermatozoa (B) Interstitial oedema (C) (h & e stain x 100), (iv) Testis of group III albino rat after giving carbaryl 100 mg/ kg body wt orally showing marked interstitial oedema (A) Cell necrosis (B) (h & e stain x 100), (v) Testis of group III A rat after giving carbaryl 100 mg/kg body wt orally showing interstitial oedema (A) Interstitial cells degeneration (B) (masson’s trichome x 100).
The group of rats fed with 100 mg/kg/day for 60 days show following observations:-
Macroscopic changes: No gross changes seen.
Microscopic changes: Changes in seminiferous tubules
• Seminiferous tubules show distortion in the normal size. Variation in size is more marked than the previous group and is mostly observed in peripheral tubules, (in many of the seminiferous tubules).
• Collection of cellular masses in the lumen of seminiferous tubules (several sections).
• Separation of the basement membrane from the spermatogenic series of cells, which is more marked than the findings seen in the rats given with low doses of carbaryl ( in several of seminiferous tubules).
• Moderate to severe degenerative changes in spermatogenic cells (in several sections of seminiferous tubules).
• Marked cell necrosis in seminiferous tubules towards the lumen.
• Many of tubular sections show loss of spermatids and spermatozoa.
(B) Changes in interstitium
• Moderate degenerative changes in the interstitial cells of Leydig.
• Marked interstitial oedema especially in the peripheral region of the testis. The interstitial oedema is more marked as compared to the findings seen in rats given with (50 mg/kg/day).
• Dilated blood capillaries are observed in the focal areas in the interstitium of the seminiferous tubules.
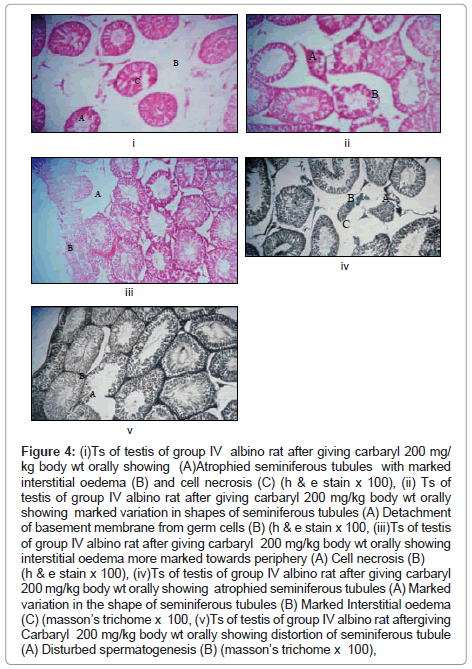
Observations in carbaryl (200 mg/kg/day) treated rats group IV (Figure 4)
Figure 4: (i)Ts of testis of group IV albino rat after giving carbaryl 200 mg/ kg body wt orally showing (A)Atrophied seminiferous tubules with marked interstitial oedema (B) and cell necrosis (C) (h & e stain x 100), (ii) Ts of testis of group IV albino rat after giving carbaryl 200 mg/kg body wt orally showing marked variation in shapes of seminiferous tubules (A) Detachment of basement membrane from germ cells (B) (h & e stain x 100, (iii)Ts of testis of group IV albino rat after giving carbaryl 200 mg/kg body wt orally showing interstitial oedema more marked towards periphery (A) Cell necrosis (B) (h & e stain x 100), (iv)Ts of testis of group IV albino rat after giving carbaryl 200 mg/kg body wt orally showing atrophied seminiferous tubules (A) Marked variation in the shape of seminiferous tubules (B) Marked Interstitial oedema (C) (masson’s trichome x 100, (v)Ts of testis of group IV albino rat aftergiving Carbaryl 200 mg/kg body wt orally showing distortion of seminiferous tubule (A) Disturbed spermatogenesis (B) (masson’s trichome x 100),
The following findings are seen in the testis of rats fed with 200 mg/kg/day for 60 days:-
Macroscopic changes: No gross changes seen.
Microscopic changes: (A) Changes in the seminiferous tubules
• Seminiferous tubules show small atrophied seminiferous tubules with marked distortion in their shape and size(multiple sections). Variations seen in their shape are more pronounced than the previous groups with low doses of carbaryl.
• The seminiferous tubules show cell necrosis towards the lumen of tubules which is more marked than the previous groups. These changes are more prominent in the peripheral tubules of testis.
• Sloughing of the germinal cells from the basement membrane (in several seminiferous tubules).
• The spermatogenesis is much disturbed as compared with the previous groups with low doses of carbaryl.
• Loss of spermatids and spermatozoa (in many seminiferous tubules).
• Accumulation of cellular masses in their lumen (in several seminiferous tubules).
• Detachment of germ cells from the basement membrane. This detachment of basement membrane is more marked as compared with groups with low doses of carbaryl.
(B) Changes in the interstitium:
• Oedema of the interstitial spaces (more marked in the peripheral region). Interstitial oedema is also much significant in this group compared with other groups.
• Prominent degenerative changes in the Leydig cells compared to other groups with low doses of carbaryl.
Discussion
An extremely complex mechanism underlies the effects of various substances on reproductive components and functions. Various chemicals may interfere in different ways with components of reproductive system. They may affect directly by interference of the substance with reproductive components or indirectly by altering hormonal regulations.
The carbamate insecticides, one of which is Carbaryl, exert their insecticidal action by inhibiting cholinesterase enzymes. This inhibition is the primary mechanism by which these insecticides cause toxicity in mammals. The cholinesterase enzymes hydrolyze acetycholine and other choline esters; consequently, their inhibition leads to the accumulation of endogenous acetycholine and other choline esters. Probably most of the biologic effects of anticholinesterase agents, including carbaryl, are due to the inhibition of acetylcholinesterase which leads to the accumulation of endogenous acetycholine, the principal choline ester that has demonstrated physiologic significance in humans.
The present study shows distorted shape of seminiferous tubules, disturbed spermatogenesis, accumulation of cellular mass in the lumen of seminiferous tubules, oedema of interstitial spaces, loss of sperms of varying degrees and detachment of germ cells from the basement membrane of seminiferous tubles of testis. Same findings were also reported by Rani et al. [5]. In the testis of albino rats following administration of carbaryl in dose of 100 and 200 mg/kg body weight in 0.2 ml of groundnut oil orally/6 days week for 60 days. Similar findings were also reported in [16-19], who have reported spermatotoxic effect of carbaryl in adult and young male rats given with 50 and 100 mg/kg body weight. Male fed 5 days/week for 60 days, caused dose and age-dependent decline in epidydmal sperm count and sperm motility and an increase in number of sperms with abnormal morphology. Young animals in comparison to adults exhibited pronounced spermatotoxic effects. Some of these findings are in accordance with the present study in which the dose-related decreased spermatogenesis and loss of sperms of varying degrees have been found.
The present study reveals marked histomorphological and degenerative changes of the lining cells of seminiferous tubules. These findings are in accordance with [16], who observed deleterious effects of chronic and subchronic administration of carbaryl on male reproductive system of animals. These effects included damage to the germinal epithelium of seminiferous tubules and altered spermatogenesis.
Vashakidze [20] reported teratogenecity and decreased reproduction at subchronic intubated dosages of 100 mg/kg body weight/day and higher but not at 50 mg/kg/day. However, a single 50 mg/kg/day in intubated dose on gestation day nine or ten reported to cause teratogenecity. These findings correlate with the present study in which dose related effect is seen on spermatogenesis and loss of sperms of varying degrees in testis of male albino rats.
Kitagawa et al. [15] reported reduced number of spermatogonia and spermatozoa in rats given 3 mg/kg body weight of carbaryl orally for 1 year. These findings are also in accordance with the present study showing depressed spermatogenesis in rats fed with carbaryl orally.
The present study done on male albino rats by giving them carbaryl in dose of 50, 100 and 200 mg/kg body weight in 0.2 ml of groundnut oil orally/6 days week for 60 days showed distorted shape of seminiferous tubules and significant histomorphological changes in testis of albino rats. These findings are contrary to the findings by Dikshith et al. [22], who demonstrated that oral administration of carbaryl (200 mg/kg body weight for 3 days a week) for a period of 90 days did not produce any overt toxicological signs in male albino rats. There were no significant histological changes in testis, liver and brain. The activity of acetylcholine esterase in blood of carbaryl treated rats was however found to be decreased. Carbaryl did not affect the fertility of male albino rats at 200 mg/kg body weight upto 90 days.
Martin [24], Osterloh et al. [25] showed that when carbaryl administered to mice up for 5 days at upto 800 mg/kg/day or by gavage at 150 mg/kg/every 2 days for upto 68 days, did not affect testis weight, histology, sperm count and frequency of sperm abnormalities. These findings are not in accordance with the present study which reveals the distorted shape of seminiferous tubules, disturbed spermatogenesis, loss of sperms in the testis of male albino rats. These findings are much significant with the high dose of carbaryl.
Carpenter et al. [27] reported that carbaryl in the diet of rats for 2 years at 400 ppm (about 20 mg/kg/day) slightly depressed organ weights in males but did not affect mortality, haematology or organ histopathology. No effect levels were at 9 mg/kg/day for males and 21 mg/kg/day for females. In shorter term studies at higher dosages, liver and kidney effects were noted, which were transient and may have been secondary to stress. Though our study does not affect mortality yet it shows significant histopathological changes on testis of albino rats.
Benson and Dorough [28] observed that when rats and gerbils were given carbaryl orally for 70 days at dosages that were increased weekly, all deaths occurred within 24 hours of the first administration of a given dosage. One of 12 rats died at dosage of 120 mg/kg/day, and cumulative mortality was 7/12 at a dosage of 180 mg/kg/day; no further deaths occurred at dosages of upto 200 mg/kg/day. In gerbils, mortality was 2/12 at the initial dosage of 60 mg/kg/day, and the last animal died at a dosage of 100 mg/kg/day. On the contrary there is no mortality of albino rats in the present study at doses of 50, 100 and 200 mg/kg body weight respectively.
The present study shows altered spermatogenesis which can lead to infertility by giving carbaryl to male albino rats. These findings do not correlate with the findings seen by Orlova and Zhalbe [29]. They could not find any change in fertility, gestation and viability of carbaryl (2 mg/kg) treated pups and rats. Similarly, studies of Benson and Dorough [28] showed that carbaryl (10 and 30 mg/kg body weight) induced no reproductive or teratogenic effects in mice. Weil et al. [30] observed no significant effects of carbaryl (10 mg/kg) on fertility, gestation, viability or lactation of rats. Studying non-human primates, Dougherty et al. [31] also could not find any teratogenic effects of carbaryl (2 and 20 mg/kg) in rhesus monkeys.
In the present study there is decreased sperm count. This is supported by decreased sperm number, postulated in the past 50 years in epidemiological studies [8,9].
Considering the effects of carbaryl on the testis in the present study and based on the findings of earlier studies, this compound may be designated as moderately toxic. This may affect spermatogenesis resulting in the production of decrease number of sperms.
Conclusion
Carbaryl is being used extensively as a broad spectrum pesticide. It is known toxicant to the male reproductive system and, is therefore, under focus in the present study. The results of the present study have thrown some light on the toxic effects of carbaryl on testicular functions that are essential for reproductive success.
The present study was conducted on 40 male Wistar albino rats as experimental animals. The rats were procured from the Animals House of the Department of Pharmacology, Government Medical College, Jammu. The rats were divided in four groups as normal control group I, group II, group III and group IV. All the rats were group housed and were fed with standard pellet diet and water for two weeks. After two weeks, the rats of group I were left as such and rats of groups II, III and IV were given 50 mg, 100 mg and 200 mg/kg body weight/day of carbaryl drug in 0.2 ml of groundnut oil orally, 6 days/week for 60 days, respectively. After 60 days, all the rats were anaesthetized by keeping them in an inverted glass jar containing large piece of cotton soaked in anaesthetic ether. The testis were dissected out from each rat and were cut into smaller pieces. These pieces were immediately fixed in 10% formalin. The blocks were prepared for section cutting with a microtome by paraffin wax embedding method. The sections of 7 μ thickness were mounted on glass slides and were stained by H&E and Masson’s trichome stain.
The following findings are drawn from the study:-
(1) There is variation in the shape of seminiferous tubules of testis.
(2) There is sloughing of the germinal cells from the basement membrane.
(3) There is depressed spermatogenesis and loss of sperms.
(4) Some tubules are showing accumulation of cellular masses in the lumen of seminiferous tubules of testis.
(5) Interstitial spaces are showing the oedema.
(6) Degenerated Leydig cells are also seen.
However, since the study was conducted on experimental animals and results may not be exactly the same in humans, suffering from the carbaryl toxicity. But in no case it can be overlooked, while designing a therapy for pesticides, where it becomes necessary to take into consideration the effects of carbaryl on these tissues of vital importance.
It is concluded that the toxic effects are more pronounced in the peripheral parts of the sections of testis. Moreover, intensity of toxic effects both in peripheral and central parts increases with increase in dosage of the carbaryl drug.
References
- Kamrin MA (1997) Pesticides Profiles: Toxicity, Environmental Impact and Fate. Chemical Rubber Company (CRC) Press, USA.
- Stockholm Convention on Persistent Organic Pollutants (POPs), 17th May, 2004
- Ernest H, Patricia EL (1997) A Textbook of Modern Toxicology. (2ndedn), Toxicology Program North Carolina: Appleton and Lange.
- Rani A, Sahai A, Srivastava AK, Rani A (2007) Carbaryl induced histopathological changes in the testis of albino rats. Journal of the Anatomical Society of India 56: 4-6.
- Mattison DR, Bogemil RJ, Chapin R, Hatch M, Hendricks A, et al. (1990) Reproductive effects of pesticides. In "The Effects of Pesticides on Human Health" Published by Princeton Scientific, New Jersey, USA.
- Hileman B (1994) Environmental estrogens linked to reproductive abnormalities and cancer. C&EN 31: 19-23.
- Bendvold E, Gottlieb C, Bygdeman M, Eneroth P (1991) Depressed semen quality in Swedish men from barren couples: a study over three decades. Arch Androl 26: 189-194.
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE (1992) Evidence for decreasing quality of semen during past 50 years. BMJ 305: 609-613.
- Afifi NA, Ramadan A, el-Aziz MI, Saki EE (1991) Influence of dimethoate on testicular and epididymal organs, testosterone plasma level and their tissue residues in rats. Dtsch Tierarztl Wochenschr 98: 419-423.
- Abou Salem ME, El-Mashad AI, Moustafa SA (1997) Pathological male reproductivity and residues of dimethoate toxicity in albino rats. Alex J Vet Sci 13: 119-140.
- Okamura A, Kamijima M, Shibata E, Ohtani K, Takagi K, et al. (2005) A comprehensive evaluation of the testicular toxicity of dichlorvos in Wistar rats. Toxicology 213: 129-137.
- Presibella KM, Kita DH, Carneiro CB, Andrade AJ, Dalsenter PR (2005) Reproductive evaluation of two pesticides combined (deltamethrin and endosulfan) in female rats. Reprod Toxicol 20: 95-101.
- Degraeve N, Moutschen Dahmen M, Houbrechts N, Collizzi A (1976) The hazards of an insecticide: carbaryl used alone and in combination with nitrites. Bull Soc Sci Iiege 45: 46-56.
- Kitagawa K, Wakakura M, Ishikawa S (1977) Light microscopic study of endocrine organs of rats treated by carbamate pesticide. Journal of toxicological sciences 2: 53-60.
- Rybakova MN (1966) On the toxic effect of sevine on animals. Gig Sanit 31: 42-47.
- Vashakidze V.I (1975) Effects of small doses of sevin (NMC) on gonad function following its repeated effect on white rats. Sb–Tr NII Gisieny Truda Profzabolevanii Gruz SSR 14: 253-266.
- Pant N, Srivastava SC, Prasad AK, Shankar R, Srivastava SP (1995) Effects of carbaryl on the rat's male reproductive system. Vet Hum Toxicol 37: 421-425.
- Pant N, Shankar R, Srivastava SP (1996) Spermatotoxic effects of carbaryl in rats. Hum Exp Toxicol 15: 736-738.
- Vashakidze VI (1965) Some questions of the harmful action of sevin on the reproductive functions of experimental animals. Soobshch Akad Nauk Bruz SSR 39: 471-474.
- Robens JF (1969) Teratologic studies of carbaryl, diazinon, norea, disulfiram, and thiram in small laboratory animals. Toxicol Appl Pharmacol 15: 152-163.
- Dikshith TS, Gupta PK, Gaur JS, Datta KK, Mathur AK (1976) Ninety day toxicity of carbaryl in male rats. Environ Res 12: 161-170.
- Murray FJ, Staples RE, Schwetz BA (1979) Teratogenic potential of carbaryl given to rabbits and mice by gavage or by dietary inclusion. Toxicol Appl Pharmacol 51: 81-89.
- Martin AR (1982) Evaluation of pesticide effects on parameters of male reproduction in the mouse. Diss Abstr Int B 42: 4279.
- Osterloh J, Letz G, Pond S, Becker C (1983) An assessment of the potential testicular toxicity of 10 pesticides using the mouse-sperm morphology assay. Mutat Res 116: 407-415.
- Sever LW, Hessol NA (1985) Toxic effects of occupational and environmental chemicals on the testis. In "Endocrine Toxicology" Target Organ Toxicology Series. New York: Raven Press, USA.
- Carpenter C.P, Weil C.S, Palm P.E, Woodside M.W, Nair J.H III (1961) Mammalian toxicity of 1–Naphthyl–N methylcarbamate (sevin insecticide). J. Agric. Food Chem 9: 30-39.
- Benson WH, Dorough W (1984) Comparative ester hydrolysis of carbaryl and ethiofencarb in fourth mammalian species. Pestic Biochem Physiol 21: 199-206.
- Orlova NV, Zhalbé EP (1968) Experimental material contributive to the problem of maximal permissible amounts of sevin in food products. Vopr Pitan 27: 49-55.
- Weil CS, Woodside MD, Carpenter CP, Smyth HF Jr (1972) Current status of tests of carbaryl for reproductive and teratogenic effect. Toxicol Appl Pharmacol 21: 390-404.
- Dougherty WJ, Golberg L, Coulston F (1971) The effect of carbaryl on reproduction in the monkey (Macacca mulatta). Toxicol Appl Pharmacol 19: 365.
Citation: Hamid S, Sharm S, Razdan S (2012) Carbaryl, A Pesticide Causes “Reproductive Toxicity” in Albino Rats. J Clin Exp Pathol 2:126. DOI: 10.4172/2161-0681.1000126
Copyright: © 2012 Hamid S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author and source are credited.