Research Article Open Access
Bromo-DragonFly: Chemistry, Pharmacology and Toxicology of a Benzodifuran Derivative Producing LSD-Like Effects
Coppola M1* and Mondola R21Department of Addiction, ASL CN2, Viale Coppino 46, 12051, Alba (CN), Italy
2Department of Mental Health, ASL CN1, Via Torino 70/B, 12037, Saluzzo (CN), Italy
- *Corresponding Author:
- M. Coppola
Department of Addiction
ASL CN2, Viale Coppino 46
12051, Alba (CN), Italy
Tel: +390173316210
Fax: +390173420344
E-mail: coppolamail@alice.it
Received August 18, 2012; Accepted September 10, 2012; Published September 17, 2012
Citation: Coppola M, Mondola R (2012) Bromo-DragonFly: Chemistry, Pharmacology and Toxicology of a Benzodifuran Derivative Producing LSD-Like Effects. J Addict Res Ther 3:133. doi:10.4172/2155-6105.1000133
Copyright: © 2012 Coppola M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Bromo-DragonFly is a potent and long-acting psychedelic drug producing both LSD-like effects and amphetamine activation. This drug appeared within the recreational drug market in the early 2000s, since then, many cases of severe intoxication and fatalities related with its consumption have been signalled in some countries. The aim of this paper is to summarize the clinical, pharmacological and toxicological information currently available about this new and dangerous hallucinogenic substance of abuse.
Keywords
Bromo-DragonFly; Bromo-benzodifuranil-isopropylamine; Spamfly; Fly-compounds; Phenethylamines
Introduction
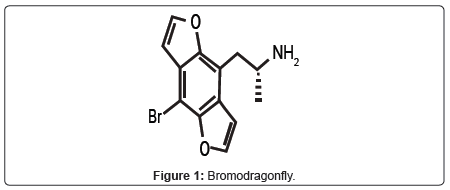
In the last decades, the spread of psychotropic drugs belonging to the phenethylamine family has been continually on the rise [1,2]. These drugs are often synthesized illegally in underground laboratories or marketed via-internet by smart shops and chemicals research suppliers [1,3]. Recently, a phenethylamine-related compound named Bromo- DragonFly (BDF) has received large popularity among young people for its LSD-like effects [4]. BDF is a potent and long-acting synthetic psychedelic substance used as recreational drug since 2001 [4]. This drug, also called ABDF, FLY, DOB-Dragonfly, spamfly, placid, B-fly, 3C-Bromo-Dragonfly, bromo-benzodifuranil-isopropylamine, was first synthesized by Matthew A. Parker in the laboratory of David E. Nichols at Purdue University in 1998 as a new research probe to investigate central nervous system (CNS) serotonin receptor structure and activity [5]. The name of BDF is derived from a resemblance between its molecular skeleton and a dragonfly for the presence of two furan rings on opposing sides of a central phenyl ring forming the wings (Figure 1). No epidemiological study has investigated the spread of BDF among people, but since its appearance within the recreational drug market, several cases of severe intoxication and deaths have been related with its consumption in some countries [3,4,6-10]. Furthermore, 1230 and 7600 blotters of BDF have been seized in Finland in 2009 and 2010, respectively [11]. In addition, the discussion about this hallucinogen among drug users is still lively within the drugs forum [12-16]. Finally, the use of Google Insights for Search, a google application for compare search volume patterns across specific regions, categories, time frames and properties, has shown a shift of interest for this drug from Europe to North America in recent years [17]. Despite being considered a substance that causes concern in terms of its potency and toxicity [9], there is very little information about its acute and chronic human toxicity. The aim of this paper is to summarize the clinical, pharmacological and toxicological information currently available about this new and dangerous hallucinogenic substance of abuse.
Methods
Literature searches were performed using the following electronic databases: PubMed, Embase, PsycINFO, Cochrane database, TOXNET, and MedScape. The keywords used were: Bromo-DragonFly, ABDF, FLY, DOB-Dragonfly, spamfly, placid, B-fly, 3C-Bromo-Dragonfly, bromo-benzodifuranil-isopropylamine. Furthermore, considering the paucity of formal scientific articles and in order to conduct a research of data as extensively as possible, the results were integrated with all the relevant information available within the unconventional references such as drugs forum, web-journals, and chemicals databases. Unconventional references were carried out using google search engine and were considered relevant for our work all reports or self experiences that described psychotropic effects, side effects, modality of consumption, and trend of misuse related with the consumption of BDF. No restriction in language was used in our research.
Chemistry
BDF, IUPAC name 1-(8-bromobenzo [1,2-b;4,5-b’]difuran-4-yl)- 2-aminopropane, is a phenethylamine-related compound belonging to the benzodifuran class [18]. This substance, molecular formula C13H12BrNO2, is generally available as a pink or white crystalline powder. The free base has a molecular weight of 294.14389 g/mol and a melting point of over 240°C. BDF is generally supplied by manufacturers as a water-soluble hydrochloride salt showing a molecular weight of 330.61 g/mol [18-20]. Furthermore, the product used for recreational purpose is also marketed as liquid formulation, in the form of impregnated paper and in tablets [7,18].
Pharmacology
Studies in vitro and in animal models have shown that BDF is the most potent of the dihydrobenzofuran analogues with high affinity binding to the 5HT2A receptor [5,19]. BDF exists in two stereoisomeric forms R and S, and R-enantiomer shows more potency and more affinity to the 5HT2A receptor than S-enantiomer [5]. In drug discrimination studies in LSD-trained rats, used as an initial screen for evaluating the behavioural activity or hallucinogenic potential of new molecules, BDF was slightly more potent than LSD [5,19]. Furthermore, BDF showed to be a very potent ligand for the cloned human 5HT2A and 5HT2C receptors [19]. BDF also acts at 5HT2B receptor, but with an affinity lower than at 5HT2A receptor [21]. In addition, some data suggest that BDF acts as an agonist at α1-adrenergic receptor [4,22]. The action at both α1-adrenergic receptor and serotonin receptor in blood vessels could explain BDF induced severe vasoconstriction [4,22].
Modalities of consumption
Like LSD, BDF is generally used in the form of impregnated paper. Some reports suggest the use of BDF via nasal insufflation and liquid administration, occasionally in tablets. Liquid formulation is sometimes assumed onto sugar cubes. Finally, intravenous administration has also been reported [4,7,10,12]. Users information suggests that BDF is often taken in combination with other psychotropic substances such as: amphetamines, cocaine, synthetic cathinones, ketamine, cannabis, alcohol, benzodiazepines, kratom, LSD, 2C-B [4,7,10].
Toxicology
BDF is used by people for its long lasting LSD-like effects [4]. Dose range reported is between 100-2100 μg, in particular, the more potent European batch is generally active at 200- 500 μg while the less potent American batch” is active at 800-2100 μg [12,23]. Desired effects include: kaleidoscopic hallucinations, altered perception of space and time, high resolution colourful visuals, shimmering lights, increased energy, increased associative thinking, well-being, prolonged sexual pleasure, and mild euphoria. Users reports suggest onset of psychotropic effects within 20-90 minutes of oral ingestion and within 30-60 minutes of nasal insufflation [4,7,10]. Users also reported delayed onset of action for up to 6 hours after oral ingestion, in particular if BDF is taken on a full stomach. In this circumstance, the users can assume another dose or another drug thinking that the first dose was insufficient to cause any psychotropic effects [4,7,10]. The duration of action is between 6-24 hours with a prolonged come down phase up to 2-3 days [4,7,10]. Undesirable effects include: prolonged hallucinations and euphoria, flashback, anxiety, severe insomnia, headache, nausea, diarrhoea, sweating, tightness, twitches, muscle tension, confusion, memory alterations, delusions and paranoid ideation [4,7,10]. In the reported cases that required medical care after recreational consumption of BDF, the most common side effects were: tachycardia, blood hypertension,, hyperpyrexia, mydriasis, psychomotor agitation, hallucinations, generalised seizures, rhabdomyolysis, respiratory problems, liver and kidney failure, and peripheral ischaemia [24-27]. Patients were treated with a variety of vaso-dilating drugs such as ACE inhibitors, nitroprusside, prostacyclin analogs, glyceryl tri-nitrate, calcium channel blockers, but none of them was reported to be effective [26,27]. Kidney failure was treated with veno-venous hemodiafiltration while complications such as aspiration pneumonia and respiratory problems were treated with intravenous antibiotics and respiratory assistance [25-27]. When were present, agitation and psychotic symptoms were treated with large doses of intravenous benzodiazepines [24,25].
Fatalities
Some deaths related with the consumption of BDF have been signalled in different countries such as Sweden, Norway, Finland, Denmark and USA [3,6,7,26-28]. However, very little information have been published about the post-mortem toxicological analysis. In the case reported in Denmark, a 18-year-old woman was found dead after consumption of BDF in liquid form. BDF was detected in femoral blood at the concentration of 4.7 ± 0.7 μg/kg (double determination in two analytical series). The concentration of BDF detected in urine and vitreous humour were 22 ± 2 μg/kg and 0.5 ± 0.1 μg/kg, respectively. The autopsy findings revealed oedema of the lungs, slight oedema of the brain, enlargement of the spleen, irritation of the mucous membranes in the stomach and ischemic changes in the kidney. Blood concentration of BDF found in the deceased woman was 8 times higher than those found in the samples of two men hospitalized after recreational use of BDF [3].
Medical use
To date, there are no approved indications for BDF in human pharmacology, however, it is known the involvement of 5HT receptors in the regulation of intraocular pressure in humans. Some studies have shown that various serotonin 5HT1A and 5HT2A receptor ligands, including BDF and several of its analogs, cause intraocular hypotensive activity in monkeys and rabbits after topical application [28,29]. It has been hypothesized that serotonin 5HT receptor agonists without psychotropic effects could be developed for the treatment of ocular hypertension and glaucoma in humans [29].
Discussion
Hallucinogenic substances have been used by indigenous cultures for millennia, however, the use was generally restricted to sacramental and healing contexts and regulated by ceremonial guidelines [30-32]. In the last decades, these substances have received large popularity among young drug users who experience their effects principally in “rave or party scenes” [33-35]. Recreational use of hallucinogens has favoured the spread of increasingly more powerful and legal molecules capable of satisfying the needs of the users [35]. In particular, the use of internet as a potential source of information on drugs of abuse [36] resulted in the use of several research chemicals as recreational drugs in place of traditional and illicit substances [1,37-42]. BDF is a research chemical synthesized in 1998 to investigate CNS serotonin receptor structure and activity [5]. This molecule is a very potent and long-acting agonist at 5-HT2A receptor used by people for its LSD-like effects [4,18,35]. In fact, as showed from several evidence, 5HT2A receptor is the main site for hallucinogen action and the entire most common hallucinogenic drugs act as agonist on this receptor [43]. It is considered the first arylethylamine derivative to surpass LSD in potency in a behavioural assay and the first molecule with LSD-like activity to have an aromatic nucleus other than benzene or indole [19]. This molecule appears to be typically sold by online research chemicals suppliers because the synthesis is complicated for the usual clandestine chemist and requires sophisticate instrumentations [44]. Pattern of acute toxicity emerged by both the cases of intoxication signalled in some countries and the users reports present within the drugs forum include psychedelic effects combined with an amphetamine-like activation [4,7,10,25,27-29]. Furthermore, the capability of over stimulating the 5HT2A and α1-adrenergic receptor could explain the contraction of vessels smooth muscle cells and consequently the severe vasoconstriction [4,23,44]. BDF is considered a substance that causes concern in terms of its potency and toxicity and many countries have published warnings alert about its toxicity [9,44] Vulnerable people could be encouraged to use BDF by online comments and videos emphasizing its powerful and the long lasting hallucinogenic activity [35]. Furthermore, in so called “rave or party scene”, some users could unknowingly assume this hallucinogen because sold in substitution of LSD [4,18]. Considering the high clinical toxicity, the alertness of medical community is of great importance in order to track and monitoring the spread of this powerful serotonergic hallucinogen.
Conclusion
BDF is a potent and long-acting psychedelic drug producing both LSD-like effects and amphetamine activation. Information currently available suggests that this drug can produce severe intoxications with serious medical complications including rhabdomyolysis, respiratory problems, liver and kidney failure, peripheral ischaemia and psychosis. Pharmacological potency and clinical toxicity associated with the consumption of this substance are reason of concern within the medical community. A better international cooperation is indispensable in order to monitoring and preventing the spread of this dangerous recreational substance.
References
- European Monitoring Centre for Drugs and Drug Abuse (2012) EMCDDA-Europol 2011 Annual Report on the implementation of Council Decision 2005/387/JHA.
- Ramsey J (2011) Detecting and monitoring new psychoactive substances in wastewater.
- Andreasen MA, Telving R, Birkler RI, Schumacher B, Johannsen M (2009) A fatal poisoning involving Bromo-Dragonfly. Forensic Sci Int 183: 91-96.
- Psychonaut Web Mapping Project Research Group.
- Monte AP, Marona-Lewicka D, Parker MA, Wainscott DB, Nichols DE (1996) Dihydrobenzofuran analogues of hallucinogens. 3. Models of 4-substituted (2,5-dimethoxyphenyl)alkylamine derivatives with rigidified methoxy groups. J Med Chem 19: 2953-2961.
- MacDonald AM, Kinniburgh D, Lyon AW (2011) A Novel Designer Drug-Bromo-Dragonfly. Therapeutics & Toxins News.
- Parr S (2011) Substance Abuse in the 21st Century: A New Look.
- Second Victim Dies After Taking Designer Drug In Konawa.
- European Monitoring Centre for Drugs and Drug Abuse. EMCDDA-Europol 2009 Annual Report on the implementation of Council Decision 2005/387/JHA.
- Belgian Early Warning System on Drugs (BEWSD) (2011) Warning about lethal batch of 2C-E.
- European Monitoring Centre for Drugs and Drug (2012) Abuse Statistical bulletin 2012 Other substances seized, 2004 to 2010.
- Erowid Experience Vaults.
- Drugs-Forum (2012).
- Drugs, Booze (2012).
- Bluelight (2012).
- Zoklet (2012) Self experiences.
- Google Insights for Search 2012.
- Dargan P, Wood DM (2010) Technical profile of bromo-dragonfly. European Monitoring centre for Drugs and Drug Addiction.
- Parker MA, Marona-Lewicka D, Lucaites VL, Nelson DL, Nichols DE (1998) A novel (benzodifuranyl) aminoalkene with extremely potent activity at the 5-HT2A receptor. J Med Chem 41: 5148-5149.
- ChemSpider (2012) Bromo-DragonFly.
- Dipartimento Politiche Antidroga (2009) 1-(8-bromobenzo[1,2-b;4,5-b’] difuran-4-yl)-2-aminopropane (Bromo-Dragonfly).
- Brown JS, Davis GB, Kearney TE, Bardin J (1983) Diffuse vascular spasm associated with 4-bromo-2,5-dimethoxyamphetamine ingestion. JAMA 249: 1477-1479.
- Darryl (2008) Bromo-Dragonfly & the DEA Microgram Bulletin. CNS Productions.
- Nielsen VT, Hogberg LC, Behrens JK (2010) Bromo-Dragonfly poisoing of 18-year-old male. Ugeskr Laeger 172: 146-152.
- Wood DM, Looker JJ, Shaikh L, Button J, Puchnarewicz M, et al. (2009) Delayed onset of seizures and toxicity associated with recreational use of bromo-dragonFLY. J Med Toxicol 5: 226-229.
- Personne M, Hulten P (2008) Bromo-Dragonfly a life threatening designer drug. Clin Tox 46: 379-380.
- Thorlacius K, Borna C, Personne M (2008) Bromo-dragon fly-life-threatening drug. Can cause tissue necrosis as demonstrated by the first described case. Lakartidningen 105: 1199-1200.
- Feng Z, Mohapatra S, Klimko PG, Hellberg MR, May JA, et al. (2007) Novel benzodifuran analogs as potent 5-HT2A receptor agonists with ocular hypotensive activity. Bioorg Med Chem Lett 17: 2998-3002.
- May JA, McLaughlin MA, Sharif NA, Hellberg MR, Dean TR (2003) Evaluation of the ocular hypotensive response to serotonin 5-HT1A and 5-HT2A receptor ligands in conscious ocular hypertensive cynomolgues monkey. J Pharmacol Exp Ther 306: 301-309.
- Shepard GH Jr (1998) Psychoactive plants and ethnopsychiatric medicines of the Matsigenka. J Psychoactive Drugs 30: 321-332.
- Lowy B (1971) New records of mushroom stones from Guatemala. Mycologia 63: 983-993.
- Schultes RE (1969) Hallucinogens of plant origin. Science 163: 245-254.
- Klein M, Kramer F (2004) Rave drugs: pharmacological considerations. AANA J 72: 61-67.
- Lassen JF, Ravn HB, Lassen SF (1990) Hallucinogenic psilocybine containing mushrooms. Toxins contained in Danish wild mushrooms. Ugeskr Laeger 152: 314-317.
- Corazza O, Schifano F, Farre M, Deluca P, Davey Z, et al. (2011) Designer drugs on the internet: A phenomenon out-of-control? The emergence of hallucinogenic drug Bromo-Dragonfly. Curr Clin Pharmacol 6: 125-129.
- Eurobarometer (2011) Young people and drugs: Analytical report
- Coppola M, Mondola R (2012) Research chemicals marketed as legal highs: The case of pipradrol derivatives. Toxicol Lett 212: 57-60.
- Coppola M, Mondola R (2012) 3,4-methylenedioxypyrovalerone (MDPV): chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online. Toxicol Lett 208: 12-15.
- Hughes B, Winstock, AR (2012) Controlling new drugs under marketing regulations. Addiction
- Gibbons S (2012) 'Legal highs'--novel and emerging psychoactive drugs: a chemical overview for the toxicologist. Clin Toxicol (Phila) 50: 15-24.
- Sainsbury PD, Kicman AT, Archer RP, King LA, Braithwaite RA (2011) Aminoindanes--the next wave of 'legal highs'? Drug Test Anal 3: 479-482.
- Van Hout MC, Brennan R (2011) 'Heads held high': an exploratory study of legal highs in pre-legislation Ireland. J Ethn Subst Abuse 10: 256-272.
- Nichols DE (2004) Hallucinogens. Pharmacol Ther 101: 131-181.
- Huntington BC (2009) Synthesis and intermediate/by-product analysis of Bromo-dragonfly, a dihydrobenzofuran analogue of phenethylamine hallucinogens.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 28133
- [From(publication date):
October-2012 - Apr 21, 2025] - Breakdown by view type
- HTML page views : 23357
- PDF downloads : 4776